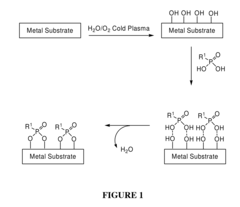
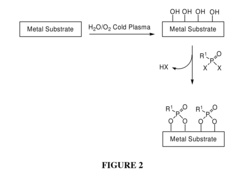
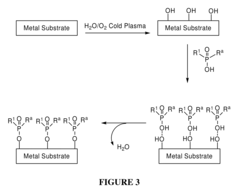
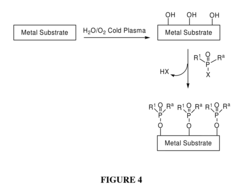
Chemical Functionalization To Improve Metal Polymer Bonding
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal-Polymer Interface Technology Background and Objectives
The interface between metals and polymers represents one of the most challenging yet critical areas in materials science and engineering. Since the mid-20th century, the bonding of these dissimilar materials has been pursued to combine the mechanical strength and electrical conductivity of metals with the lightweight, corrosion-resistant properties of polymers. The evolution of this technology has progressed from simple mechanical interlocking methods to sophisticated chemical functionalization approaches that create covalent bonds across the interface.
The fundamental challenge in metal-polymer bonding stems from their inherently different chemical natures - metals being electron donors with metallic bonding, while polymers feature covalent bonds with limited surface energy. This incompatibility manifests as poor adhesion, delamination under stress, and interface degradation over time, particularly in harsh environments. Traditional joining methods like adhesives, while functional, often fail to provide the durability and strength required for advanced applications.
Chemical functionalization has emerged as a promising solution pathway, offering molecular-level modification of surfaces to create strong, durable bonds. This approach began gaining significant traction in the 1990s with the development of silane coupling agents and has since expanded to include a diverse array of surface treatments including plasma modification, self-assembled monolayers, and grafting techniques. The field has seen accelerated growth with the advent of nanotechnology, enabling unprecedented control over interface properties.
Current technological objectives in this domain focus on developing functionalization methods that provide not only strong adhesion but also address specific performance requirements such as electrical conductivity, thermal management, and resistance to environmental factors. There is particular interest in creating "smart" interfaces that can respond to external stimuli or self-heal when damaged. Additionally, sustainability concerns are driving research toward environmentally friendly functionalization processes that eliminate toxic chemicals and reduce energy consumption.
The strategic importance of metal-polymer bonding technology extends across multiple industries. In aerospace and automotive sectors, lightweight metal-polymer composites offer fuel efficiency and emissions reduction. Medical devices benefit from biocompatible interfaces between metallic components and polymer housings. Electronics manufacturers seek reliable metal-polymer connections for flexible circuits and wearable devices. The packaging industry requires effective barrier properties at metal-polymer junctions.
Looking forward, the technology trajectory points toward multifunctional interfaces that simultaneously address multiple performance criteria while being amenable to high-volume manufacturing processes. The ultimate goal remains the development of predictable, reproducible functionalization methods that can be tailored to specific metal-polymer combinations and application requirements.
The fundamental challenge in metal-polymer bonding stems from their inherently different chemical natures - metals being electron donors with metallic bonding, while polymers feature covalent bonds with limited surface energy. This incompatibility manifests as poor adhesion, delamination under stress, and interface degradation over time, particularly in harsh environments. Traditional joining methods like adhesives, while functional, often fail to provide the durability and strength required for advanced applications.
Chemical functionalization has emerged as a promising solution pathway, offering molecular-level modification of surfaces to create strong, durable bonds. This approach began gaining significant traction in the 1990s with the development of silane coupling agents and has since expanded to include a diverse array of surface treatments including plasma modification, self-assembled monolayers, and grafting techniques. The field has seen accelerated growth with the advent of nanotechnology, enabling unprecedented control over interface properties.
Current technological objectives in this domain focus on developing functionalization methods that provide not only strong adhesion but also address specific performance requirements such as electrical conductivity, thermal management, and resistance to environmental factors. There is particular interest in creating "smart" interfaces that can respond to external stimuli or self-heal when damaged. Additionally, sustainability concerns are driving research toward environmentally friendly functionalization processes that eliminate toxic chemicals and reduce energy consumption.
The strategic importance of metal-polymer bonding technology extends across multiple industries. In aerospace and automotive sectors, lightweight metal-polymer composites offer fuel efficiency and emissions reduction. Medical devices benefit from biocompatible interfaces between metallic components and polymer housings. Electronics manufacturers seek reliable metal-polymer connections for flexible circuits and wearable devices. The packaging industry requires effective barrier properties at metal-polymer junctions.
Looking forward, the technology trajectory points toward multifunctional interfaces that simultaneously address multiple performance criteria while being amenable to high-volume manufacturing processes. The ultimate goal remains the development of predictable, reproducible functionalization methods that can be tailored to specific metal-polymer combinations and application requirements.
Market Analysis for Enhanced Metal-Polymer Composites
The global market for enhanced metal-polymer composites is experiencing robust growth, driven by increasing demand across multiple industries seeking lightweight yet strong materials. The market size for metal-polymer bonding technologies was valued at approximately $4.2 billion in 2022 and is projected to reach $7.8 billion by 2028, representing a compound annual growth rate (CAGR) of 10.8% during the forecast period.
Automotive and aerospace industries remain the primary consumers of chemically functionalized metal-polymer composites, collectively accounting for over 58% of the total market share. The automotive sector's shift toward electric vehicles has intensified the need for lightweight materials that maintain structural integrity, thereby accelerating adoption of advanced metal-polymer bonding solutions. Similarly, the aerospace industry's focus on fuel efficiency has created sustained demand for these composites in aircraft components.
Consumer electronics represents the fastest-growing application segment, with a projected CAGR of 13.2% through 2028. The miniaturization trend in electronic devices coupled with requirements for durability and thermal management has spurred interest in metal-polymer interfaces with enhanced adhesion properties achieved through chemical functionalization.
Regionally, Asia-Pacific dominates the market with approximately 42% share, followed by North America (28%) and Europe (23%). China and India are witnessing particularly rapid growth rates due to expanding manufacturing capabilities and increasing domestic consumption of high-performance materials. However, North America leads in research and development activities related to novel functionalization techniques.
The market landscape features both established chemical companies and specialized materials science firms. Key players include Henkel AG, 3M Company, DuPont, BASF SE, and Solvay, who collectively hold approximately 45% market share. These companies are increasingly investing in proprietary functionalization technologies to secure competitive advantages.
Customer preferences are evolving toward solutions that not only provide superior bonding strength but also offer additional functionalities such as corrosion resistance, electrical conductivity, and thermal management capabilities. This trend is driving premium pricing for advanced functionalization technologies that deliver multiple performance benefits simultaneously.
Regulatory factors are significantly influencing market dynamics, with growing environmental regulations restricting certain chemical treatments and promoting greener alternatives. This has accelerated research into bio-based functionalization agents and environmentally friendly surface treatment processes, creating new market opportunities for companies with sustainable technology portfolios.
Automotive and aerospace industries remain the primary consumers of chemically functionalized metal-polymer composites, collectively accounting for over 58% of the total market share. The automotive sector's shift toward electric vehicles has intensified the need for lightweight materials that maintain structural integrity, thereby accelerating adoption of advanced metal-polymer bonding solutions. Similarly, the aerospace industry's focus on fuel efficiency has created sustained demand for these composites in aircraft components.
Consumer electronics represents the fastest-growing application segment, with a projected CAGR of 13.2% through 2028. The miniaturization trend in electronic devices coupled with requirements for durability and thermal management has spurred interest in metal-polymer interfaces with enhanced adhesion properties achieved through chemical functionalization.
Regionally, Asia-Pacific dominates the market with approximately 42% share, followed by North America (28%) and Europe (23%). China and India are witnessing particularly rapid growth rates due to expanding manufacturing capabilities and increasing domestic consumption of high-performance materials. However, North America leads in research and development activities related to novel functionalization techniques.
The market landscape features both established chemical companies and specialized materials science firms. Key players include Henkel AG, 3M Company, DuPont, BASF SE, and Solvay, who collectively hold approximately 45% market share. These companies are increasingly investing in proprietary functionalization technologies to secure competitive advantages.
Customer preferences are evolving toward solutions that not only provide superior bonding strength but also offer additional functionalities such as corrosion resistance, electrical conductivity, and thermal management capabilities. This trend is driving premium pricing for advanced functionalization technologies that deliver multiple performance benefits simultaneously.
Regulatory factors are significantly influencing market dynamics, with growing environmental regulations restricting certain chemical treatments and promoting greener alternatives. This has accelerated research into bio-based functionalization agents and environmentally friendly surface treatment processes, creating new market opportunities for companies with sustainable technology portfolios.
Current Challenges in Metal-Polymer Adhesion
Despite significant advancements in metal-polymer joining technologies, several persistent challenges continue to impede optimal adhesion performance in industrial applications. The fundamental issue stems from the inherent physicochemical incompatibility between metals and polymers. Metals exhibit high surface energy and hydrophilic properties, while most engineering polymers are hydrophobic with low surface energy, creating an intrinsic adhesion barrier that conventional bonding methods struggle to overcome.
Surface contamination presents another significant obstacle. Metal surfaces frequently contain oils, oxides, and processing residues that interfere with chemical functionalization processes. These contaminants create an unstable interfacial layer that compromises long-term bond durability, particularly in harsh environmental conditions. Similarly, polymer surfaces often contain processing additives, release agents, and low molecular weight components that migrate to the interface over time, undermining adhesion stability.
Thermal expansion mismatch between metals and polymers introduces substantial mechanical stress at the bonding interface. During temperature fluctuations, the significant difference in coefficient of thermal expansion (CTE) generates interfacial strain that can initiate crack propagation and eventual bond failure. This challenge becomes particularly acute in applications experiencing thermal cycling or extreme temperature environments.
Durability under environmental exposure remains problematic for metal-polymer bonds. Moisture ingress at the interface leads to hydrolytic degradation of adhesion promoters and the formation of weak boundary layers. Additionally, UV radiation, chemical exposure, and mechanical fatigue accelerate bond deterioration through multiple degradation pathways that are difficult to predict and mitigate simultaneously.
Current chemical functionalization approaches face scalability and manufacturing integration challenges. Many laboratory-demonstrated surface treatments require specialized equipment, precise process control, or hazardous chemicals that prove difficult to implement in high-volume production environments. The trade-off between adhesion performance and manufacturing practicality often results in suboptimal solutions.
The lack of standardized testing protocols specifically designed for evaluating metal-polymer interfaces further complicates advancement in this field. Existing test methods frequently fail to replicate real-world stress conditions or accelerated aging scenarios, leading to poor correlation between laboratory results and actual performance in applications. This testing gap hinders the systematic development and validation of improved functionalization strategies.
Emerging sustainability requirements add another dimension to the challenge landscape. Traditional surface treatments often involve environmentally problematic chemicals such as hexavalent chromium compounds or solvent-based primers. Developing eco-friendly alternatives that maintain equivalent performance while meeting increasingly stringent environmental regulations represents a significant hurdle for researchers and manufacturers alike.
Surface contamination presents another significant obstacle. Metal surfaces frequently contain oils, oxides, and processing residues that interfere with chemical functionalization processes. These contaminants create an unstable interfacial layer that compromises long-term bond durability, particularly in harsh environmental conditions. Similarly, polymer surfaces often contain processing additives, release agents, and low molecular weight components that migrate to the interface over time, undermining adhesion stability.
Thermal expansion mismatch between metals and polymers introduces substantial mechanical stress at the bonding interface. During temperature fluctuations, the significant difference in coefficient of thermal expansion (CTE) generates interfacial strain that can initiate crack propagation and eventual bond failure. This challenge becomes particularly acute in applications experiencing thermal cycling or extreme temperature environments.
Durability under environmental exposure remains problematic for metal-polymer bonds. Moisture ingress at the interface leads to hydrolytic degradation of adhesion promoters and the formation of weak boundary layers. Additionally, UV radiation, chemical exposure, and mechanical fatigue accelerate bond deterioration through multiple degradation pathways that are difficult to predict and mitigate simultaneously.
Current chemical functionalization approaches face scalability and manufacturing integration challenges. Many laboratory-demonstrated surface treatments require specialized equipment, precise process control, or hazardous chemicals that prove difficult to implement in high-volume production environments. The trade-off between adhesion performance and manufacturing practicality often results in suboptimal solutions.
The lack of standardized testing protocols specifically designed for evaluating metal-polymer interfaces further complicates advancement in this field. Existing test methods frequently fail to replicate real-world stress conditions or accelerated aging scenarios, leading to poor correlation between laboratory results and actual performance in applications. This testing gap hinders the systematic development and validation of improved functionalization strategies.
Emerging sustainability requirements add another dimension to the challenge landscape. Traditional surface treatments often involve environmentally problematic chemicals such as hexavalent chromium compounds or solvent-based primers. Developing eco-friendly alternatives that maintain equivalent performance while meeting increasingly stringent environmental regulations represents a significant hurdle for researchers and manufacturers alike.
State-of-the-Art Chemical Functionalization Methods
01 Surface modification techniques for metal-polymer bonding
Various surface modification techniques can be employed to enhance the adhesion between metals and polymers. These techniques include plasma treatment, chemical etching, and surface functionalization with coupling agents. By modifying the surface properties of either the metal or polymer substrate, reactive sites are created that facilitate stronger chemical bonding at the interface, resulting in improved adhesion strength and durability of the metal-polymer composite.- Surface modification techniques for metal-polymer bonding: Various surface modification techniques can be employed to enhance the adhesion between metals and polymers. These techniques include plasma treatment, chemical etching, and surface functionalization with coupling agents. By modifying the surface properties of either the metal or polymer substrate, stronger interfacial bonds can be formed, resulting in improved adhesion strength and durability of the metal-polymer composite.
- Functionalized polymer composites for metal adhesion: Polymers can be chemically functionalized to introduce specific groups that have affinity for metal surfaces. These functionalized polymers contain reactive moieties such as carboxylic acids, amines, or silanes that can form chemical bonds with metal substrates. The incorporation of these functional groups into the polymer matrix enhances the interfacial adhesion between the polymer and metal components, leading to stronger and more durable composite materials.
- Metal surface treatments for improved polymer bonding: Metal surfaces can be treated with various chemical agents to improve their compatibility with polymers. These treatments include the application of silane coupling agents, phosphate conversion coatings, and organometallic compounds. Such treatments create reactive sites on the metal surface that can form chemical bonds with polymer molecules, thereby enhancing the adhesion strength at the metal-polymer interface and improving the overall performance of the composite structure.
- Nanostructured interfaces for enhanced metal-polymer bonding: Nanostructured interfaces can significantly improve the bonding between metals and polymers. These interfaces can be created through the deposition of nanoparticles, growth of nanowires, or formation of nanoporous structures on the metal surface. The increased surface area and mechanical interlocking provided by these nanostructures enhance the adhesion strength between the metal and polymer components, resulting in superior mechanical properties of the composite material.
- Covalent bonding strategies for metal-polymer interfaces: Covalent bonding strategies involve the formation of strong chemical bonds between metal surfaces and polymer matrices. These strategies often utilize bifunctional molecules or reactive intermediates that can simultaneously bond to both the metal and polymer components. The establishment of covalent bonds across the interface results in exceptionally strong adhesion, improved durability, and enhanced resistance to environmental degradation, making these approaches particularly valuable for demanding applications.
02 Silane coupling agents for metal-polymer interfaces
Silane coupling agents play a crucial role in improving the adhesion between metal surfaces and polymeric materials. These bifunctional molecules contain functional groups that can react with both the metal oxide layer and the polymer matrix, creating a chemical bridge between the two dissimilar materials. The silane treatment creates a covalently bonded interfacial layer that significantly enhances the adhesion strength, durability, and resistance to environmental degradation of metal-polymer composites.Expand Specific Solutions03 Polymer grafting on metal surfaces
Polymer grafting involves the covalent attachment of polymer chains directly onto metal surfaces to create strong interfacial bonding. This can be achieved through various methods including 'grafting-from' approaches where polymerization is initiated from the metal surface, or 'grafting-to' approaches where pre-formed polymers with reactive end groups are attached to the metal. These techniques create a robust interface with improved mechanical properties and resistance to delamination in metal-polymer composite materials.Expand Specific Solutions04 Metal nanoparticle functionalization for polymer composites
Metal nanoparticles can be chemically functionalized to enhance their compatibility and bonding with polymer matrices. Surface modification of these nanoparticles with organic ligands, polymers, or coupling agents creates strong interfacial interactions with the polymer matrix. This functionalization prevents nanoparticle aggregation and improves dispersion throughout the polymer, resulting in enhanced mechanical, electrical, and thermal properties of the resulting nanocomposite materials.Expand Specific Solutions05 Adhesion promotion in electronic and semiconductor applications
Chemical functionalization techniques are particularly important for metal-polymer bonding in electronic and semiconductor applications. These methods include the use of adhesion promoters, surface activation treatments, and specialized coupling agents designed for microelectronic materials. The enhanced interfacial bonding improves device reliability by preventing delamination under thermal cycling, enhancing moisture resistance, and ensuring long-term stability of the metal-polymer interfaces in electronic components and packaging.Expand Specific Solutions
Leading Companies in Metal-Polymer Bonding Industry
The metal-polymer bonding technology market is currently in a growth phase, characterized by increasing demand for advanced materials in automotive, aerospace, and electronics industries. The global market size for chemical functionalization technologies to improve metal-polymer bonding is estimated to exceed $3 billion, with projected annual growth of 6-8%. Major players demonstrate varying levels of technical maturity, with established corporations like 3M, Dow Global Technologies, and Henkel leading with comprehensive patent portfolios and commercial solutions. Tire manufacturers including Bridgestone, Goodyear, and Michelin have developed specialized bonding technologies for rubber-metal interfaces. Research institutions such as Beijing University of Chemical Technology and University of Michigan are advancing fundamental science in this field, while emerging companies like Wanhua Chemical and Intezyne Technologies are introducing innovative approaches to chemical functionalization, creating a competitive landscape balanced between established players and new entrants.
3M Innovative Properties Co.
Technical Solution: 3M has pioneered multi-functional surface modification technologies for metal-polymer interfaces using proprietary organophosphonic acid derivatives and zirconate coupling agents. Their approach creates nanoscale chemical bridges between metal oxides and various polymer systems through carefully engineered molecular architectures. The technology employs a gradient functionalization strategy where the chemical composition gradually transitions from metal-philic to polymer-philic across a controlled thickness. 3M's process includes vapor-phase deposition of functional molecules followed by controlled crosslinking to create stable interfaces resistant to hydrolytic degradation. Their research demonstrates lap shear strengths exceeding 35 MPa for aluminum-epoxy bonds with exceptional durability under thermal cycling (-40°C to 120°C). 3M has also developed self-healing functionalization systems incorporating dynamic covalent chemistry that can reform bonds after mechanical stress, significantly extending the service life of metal-polymer composite structures.
Strengths: Exceptional bond durability under thermal cycling and mechanical stress; proprietary vapor deposition technology enabling uniform treatment of complex geometries; self-healing capabilities extending service life. Weaknesses: Specialized equipment requirements for vapor deposition processes; higher implementation costs compared to conventional primers; process sensitivity to ambient humidity during application requiring controlled environments.
Dow Global Technologies LLC
Technical Solution: Dow has developed proprietary silane-based coupling agents that create covalent bonds between metal substrates and polymer matrices. Their technology involves a two-step process: first, applying organofunctional silanes to create reactive sites on metal surfaces through hydrolysis and condensation reactions; second, these functionalized surfaces form strong chemical bonds with polymers during processing. Dow's approach includes specialized surface preparation techniques that remove contaminants and oxide layers before functionalization, significantly enhancing adhesion strength. Their research has demonstrated up to 180% improvement in peel strength compared to untreated interfaces, with notable resistance to environmental degradation under high humidity and temperature cycling conditions. Dow has also pioneered plasma-assisted deposition methods that create nanoscale functionalized layers optimized for specific metal-polymer combinations.
Strengths: Exceptional bond durability in harsh environments; scalable manufacturing processes suitable for industrial implementation; comprehensive portfolio of coupling agents for different metal-polymer combinations. Weaknesses: Some solutions require specialized application equipment; higher implementation costs compared to mechanical bonding methods; process sensitivity to surface contamination requiring stringent pre-treatment protocols.
Key Patents in Metal-Polymer Interface Chemistry
Covalent modification of metal surfaces
PatentInactiveUS20130310912A1
Innovation
- Introducing hydroxyl groups onto metal surfaces to enable covalent bonding with polymers or small molecules through dehydration or condensation reactions, forming stable and robust linkages without the need for additional reagents or catalysts, allowing for a wide range of substrates and functionalities.
Nanocoupling for improvement of coating adhesion of polymer on metal substrates
PatentInactiveUS8734911B2
Innovation
- A method involving the introduction of hydroxyl groups on metal substrates followed by polymerization of monomers to chemically bond polymers onto the surface through oxygen atoms, enhancing adhesion and stability, and optionally coating additional polymer layers for improved biocompatibility and mechanical properties.
Material Compatibility and Selection Guidelines
The selection of compatible materials is critical for achieving optimal metal-polymer bonding through chemical functionalization. When considering material pairings, engineers must evaluate both the inherent properties of the substrate materials and their potential for chemical modification. Metals commonly used in bonding applications include aluminum, titanium, steel, and copper alloys, each presenting unique surface characteristics that influence functionalization strategies. For polymers, thermoplastics such as polyamides, polyolefins, and engineering plastics like PEEK or PPS are frequently employed in industrial applications requiring metal-polymer interfaces.
Material selection should prioritize thermal expansion coefficient compatibility to minimize stress at the bonding interface during temperature fluctuations. The thermal expansion coefficient of metals (typically 10-23 × 10^-6/K) differs significantly from polymers (50-200 × 10^-6/K), creating potential for delamination under thermal cycling. Materials with closer expansion coefficients generally form more durable bonds when properly functionalized.
Surface energy compatibility represents another crucial factor, as it directly impacts wettability and adhesion potential. Metals typically exhibit high surface energy (250-500 mJ/m²), while polymers demonstrate considerably lower values (20-50 mJ/m²). Chemical functionalization bridges this gap by introducing compatible functional groups that enhance interfacial interactions. For instance, silane coupling agents effectively connect inorganic metal surfaces with organic polymer matrices.
Chemical resistance of both materials must be evaluated against the intended operating environment. The functionalization chemistry should not compromise the corrosion resistance of metals or accelerate polymer degradation. Galvanic compatibility requires special attention when bonding dissimilar metals to polymers containing conductive fillers, as electrochemical reactions can undermine bond integrity over time.
Processing parameters significantly influence material selection decisions. The temperature requirements for polymer processing must align with the thermal stability of the selected functionalization agents. Similarly, the mechanical properties of the final assembly depend on appropriate material pairing—the modulus mismatch between metals (70-200 GPa) and polymers (0.1-3.5 GPa) necessitates careful interface design to distribute stress effectively.
A systematic selection approach involves characterizing material surface chemistry before functionalization, conducting compatibility testing with proposed functionalization agents, and performing accelerated aging tests to predict long-term performance. Documentation of successful material combinations for specific functionalization protocols provides valuable reference data for future applications, ultimately establishing reliable material selection guidelines for enhanced metal-polymer bonding.
Material selection should prioritize thermal expansion coefficient compatibility to minimize stress at the bonding interface during temperature fluctuations. The thermal expansion coefficient of metals (typically 10-23 × 10^-6/K) differs significantly from polymers (50-200 × 10^-6/K), creating potential for delamination under thermal cycling. Materials with closer expansion coefficients generally form more durable bonds when properly functionalized.
Surface energy compatibility represents another crucial factor, as it directly impacts wettability and adhesion potential. Metals typically exhibit high surface energy (250-500 mJ/m²), while polymers demonstrate considerably lower values (20-50 mJ/m²). Chemical functionalization bridges this gap by introducing compatible functional groups that enhance interfacial interactions. For instance, silane coupling agents effectively connect inorganic metal surfaces with organic polymer matrices.
Chemical resistance of both materials must be evaluated against the intended operating environment. The functionalization chemistry should not compromise the corrosion resistance of metals or accelerate polymer degradation. Galvanic compatibility requires special attention when bonding dissimilar metals to polymers containing conductive fillers, as electrochemical reactions can undermine bond integrity over time.
Processing parameters significantly influence material selection decisions. The temperature requirements for polymer processing must align with the thermal stability of the selected functionalization agents. Similarly, the mechanical properties of the final assembly depend on appropriate material pairing—the modulus mismatch between metals (70-200 GPa) and polymers (0.1-3.5 GPa) necessitates careful interface design to distribute stress effectively.
A systematic selection approach involves characterizing material surface chemistry before functionalization, conducting compatibility testing with proposed functionalization agents, and performing accelerated aging tests to predict long-term performance. Documentation of successful material combinations for specific functionalization protocols provides valuable reference data for future applications, ultimately establishing reliable material selection guidelines for enhanced metal-polymer bonding.
Environmental Impact and Sustainability Considerations
The chemical functionalization processes used to enhance metal-polymer bonding carry significant environmental implications that must be carefully considered in industrial applications. Traditional surface treatment methods often involve hazardous chemicals such as chromates, strong acids, and organic solvents that pose substantial environmental and health risks. These processes generate toxic waste streams requiring specialized disposal procedures and contribute to air and water pollution when not properly managed.
Recent advancements in green chemistry approaches have led to the development of more environmentally benign functionalization methods. Water-based treatments, solvent-free processes, and bio-inspired surface modifications represent promising alternatives that significantly reduce environmental footprint while maintaining effective bonding performance. For instance, plasma treatments operating at atmospheric pressure consume less energy than vacuum-based systems and eliminate the need for chemical etchants.
Life cycle assessment (LCA) studies indicate that optimized chemical functionalization techniques can reduce overall environmental impact by extending product lifespan through improved bond durability. This longevity effect often outweighs the initial environmental costs of the treatment processes, particularly in transportation and aerospace applications where component failure can lead to catastrophic consequences.
Regulatory frameworks worldwide are increasingly restricting the use of hazardous substances in manufacturing processes. The European Union's REACH regulation, RoHS directive, and similar legislation in other regions have accelerated the transition toward more sustainable functionalization methods. Companies developing metal-polymer bonding solutions must navigate this evolving regulatory landscape to ensure long-term market viability.
Circular economy considerations are becoming increasingly relevant in bonding technology development. Designing metal-polymer interfaces that facilitate end-of-life separation enables more effective recycling of both materials. Some innovative functionalization approaches incorporate reversible bonding mechanisms that respond to specific stimuli, allowing for disassembly without degrading the component materials.
Energy consumption during surface preparation and functionalization represents another critical sustainability factor. Emerging technologies such as UV-activated functionalization and room-temperature curing adhesion promoters offer significant energy savings compared to conventional thermal processing methods, reducing the carbon footprint associated with metal-polymer bonding applications.
Recent advancements in green chemistry approaches have led to the development of more environmentally benign functionalization methods. Water-based treatments, solvent-free processes, and bio-inspired surface modifications represent promising alternatives that significantly reduce environmental footprint while maintaining effective bonding performance. For instance, plasma treatments operating at atmospheric pressure consume less energy than vacuum-based systems and eliminate the need for chemical etchants.
Life cycle assessment (LCA) studies indicate that optimized chemical functionalization techniques can reduce overall environmental impact by extending product lifespan through improved bond durability. This longevity effect often outweighs the initial environmental costs of the treatment processes, particularly in transportation and aerospace applications where component failure can lead to catastrophic consequences.
Regulatory frameworks worldwide are increasingly restricting the use of hazardous substances in manufacturing processes. The European Union's REACH regulation, RoHS directive, and similar legislation in other regions have accelerated the transition toward more sustainable functionalization methods. Companies developing metal-polymer bonding solutions must navigate this evolving regulatory landscape to ensure long-term market viability.
Circular economy considerations are becoming increasingly relevant in bonding technology development. Designing metal-polymer interfaces that facilitate end-of-life separation enables more effective recycling of both materials. Some innovative functionalization approaches incorporate reversible bonding mechanisms that respond to specific stimuli, allowing for disassembly without degrading the component materials.
Energy consumption during surface preparation and functionalization represents another critical sustainability factor. Emerging technologies such as UV-activated functionalization and room-temperature curing adhesion promoters offer significant energy savings compared to conventional thermal processing methods, reducing the carbon footprint associated with metal-polymer bonding applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!