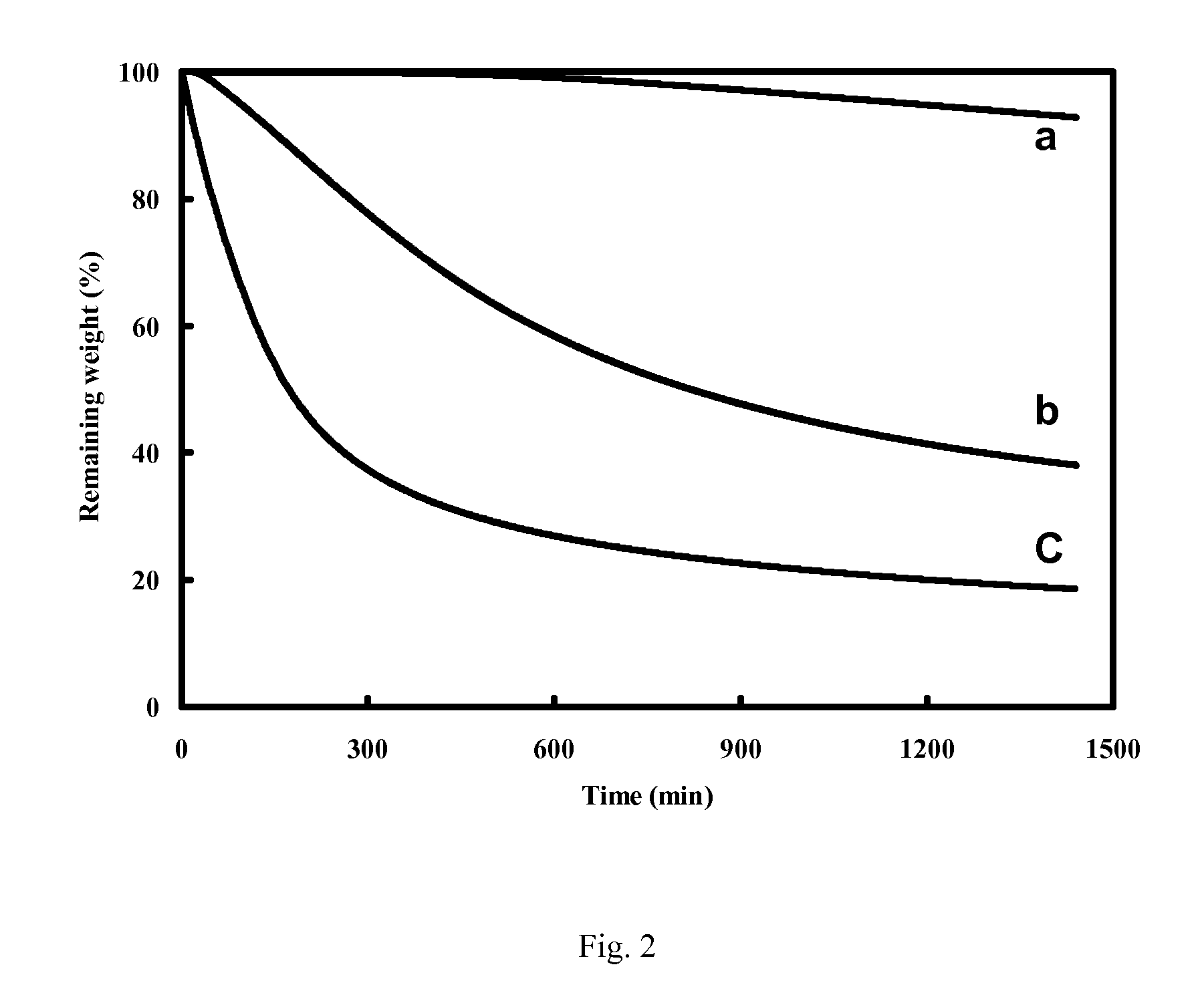
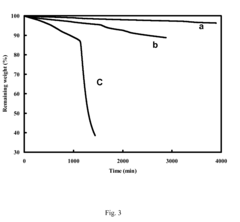
Lithium Chloride vs Silver Chloride: Thermal Stability
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Thermal Stability Background and Research Objectives
The thermal stability of inorganic compounds has been a critical area of research in materials science for decades, with significant implications for various industrial applications. Lithium chloride (LiCl) and silver chloride (AgCl) represent two distinct metal halides with contrasting thermal behaviors that warrant comprehensive investigation. This research aims to establish a thorough understanding of the thermal stability characteristics of these compounds under various environmental conditions and temperature ranges.
Historically, the study of thermal stability in metal halides began in the early 20th century, gaining momentum with the advancement of thermal analysis techniques in the 1950s and 1960s. The evolution of differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and differential thermal analysis (DTA) has significantly enhanced our ability to quantify and compare thermal behaviors with precision.
The thermal properties of LiCl have attracted particular attention due to its applications in molten salt reactors, battery technologies, and desiccation systems. With a melting point of approximately 605°C, LiCl exhibits notable stability at moderate temperatures but presents hygroscopic challenges that affect its thermal performance in practical applications. Conversely, AgCl, with a melting point of around 455°C, has been extensively studied for its photosensitive properties and applications in photography, electrochemistry, and as a reference electrode material.
Recent technological advancements in renewable energy storage, nuclear energy, and advanced materials manufacturing have renewed interest in understanding the comparative thermal stability of these compounds. The increasing demand for high-temperature operational materials in these sectors necessitates a more nuanced understanding of thermal degradation mechanisms, phase transitions, and stability limits.
This research specifically aims to investigate the thermal decomposition pathways, phase transition behaviors, and stability thresholds of LiCl and AgCl under controlled heating conditions. We will examine how factors such as heating rate, atmosphere composition, pressure conditions, and the presence of impurities influence their thermal stability profiles.
Additionally, this study seeks to develop predictive models for thermal behavior that can inform material selection decisions in high-temperature applications. By establishing quantitative relationships between structural characteristics and thermal stability parameters, we aim to contribute to the broader understanding of structure-property relationships in metal halides.
The ultimate objective is to provide comprehensive thermal stability data that can guide engineering decisions in applications where these materials might be employed, particularly in energy storage systems, catalysis, and advanced manufacturing processes where thermal resilience is paramount.
Historically, the study of thermal stability in metal halides began in the early 20th century, gaining momentum with the advancement of thermal analysis techniques in the 1950s and 1960s. The evolution of differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and differential thermal analysis (DTA) has significantly enhanced our ability to quantify and compare thermal behaviors with precision.
The thermal properties of LiCl have attracted particular attention due to its applications in molten salt reactors, battery technologies, and desiccation systems. With a melting point of approximately 605°C, LiCl exhibits notable stability at moderate temperatures but presents hygroscopic challenges that affect its thermal performance in practical applications. Conversely, AgCl, with a melting point of around 455°C, has been extensively studied for its photosensitive properties and applications in photography, electrochemistry, and as a reference electrode material.
Recent technological advancements in renewable energy storage, nuclear energy, and advanced materials manufacturing have renewed interest in understanding the comparative thermal stability of these compounds. The increasing demand for high-temperature operational materials in these sectors necessitates a more nuanced understanding of thermal degradation mechanisms, phase transitions, and stability limits.
This research specifically aims to investigate the thermal decomposition pathways, phase transition behaviors, and stability thresholds of LiCl and AgCl under controlled heating conditions. We will examine how factors such as heating rate, atmosphere composition, pressure conditions, and the presence of impurities influence their thermal stability profiles.
Additionally, this study seeks to develop predictive models for thermal behavior that can inform material selection decisions in high-temperature applications. By establishing quantitative relationships between structural characteristics and thermal stability parameters, we aim to contribute to the broader understanding of structure-property relationships in metal halides.
The ultimate objective is to provide comprehensive thermal stability data that can guide engineering decisions in applications where these materials might be employed, particularly in energy storage systems, catalysis, and advanced manufacturing processes where thermal resilience is paramount.
Market Applications and Demand Analysis for Thermally Stable Chlorides
The global market for thermally stable chlorides has witnessed significant growth in recent years, driven primarily by expanding applications in energy storage, chemical processing, and advanced materials manufacturing. Lithium chloride and silver chloride, with their distinct thermal stability profiles, serve different market segments with varying demand patterns and growth trajectories.
In the energy sector, lithium chloride's thermal stability characteristics have positioned it as a critical component in molten salt energy storage systems. The global thermal energy storage market, where lithium chloride plays a significant role, is experiencing robust growth as renewable energy integration accelerates worldwide. Particularly in concentrated solar power plants, the demand for thermally stable chlorides has increased substantially due to their ability to maintain performance integrity at elevated temperatures.
Silver chloride, conversely, dominates specialized optical and electronic applications where its unique thermal properties provide competitive advantages. The photochromic lens industry represents a major consumer of silver chloride, with market research indicating steady growth in premium eyewear segments. Additionally, silver chloride's application in specialized sensors has expanded with the proliferation of IoT devices and advanced monitoring systems.
The pharmaceutical and chemical processing industries represent another significant market for thermally stable chlorides. Lithium chloride's hygroscopic properties make it valuable in dehumidification systems and certain catalytic processes, while silver chloride finds applications in high-purity chemical synthesis where thermal stability during reactions is paramount.
Regional market analysis reveals distinct consumption patterns, with North America and Europe leading in high-tech applications of both compounds, while Asia-Pacific demonstrates the fastest growth rate, particularly in energy storage applications utilizing lithium chloride. This regional disparity reflects differences in industrial development priorities and technological adoption rates.
Price sensitivity varies significantly between application sectors. Energy storage applications demonstrate moderate price elasticity, with purchasing decisions balanced between cost considerations and performance requirements. In contrast, specialized electronic and optical applications show lower price sensitivity, prioritizing the unique thermal stability characteristics of silver chloride over cost factors.
Future market growth appears promising, with emerging applications in next-generation batteries, advanced ceramics, and specialized catalysts expected to create new demand streams. The increasing focus on sustainable energy solutions particularly benefits lithium chloride markets, while silver chloride may see expanded applications in advanced sensing technologies and specialized medical devices.
Customer requirements are evolving toward higher purity grades and more precise thermal stability specifications, reflecting the increasingly sophisticated applications of these materials in precision industries. This trend necessitates ongoing refinement of production processes to meet stringent performance criteria while maintaining economic viability.
In the energy sector, lithium chloride's thermal stability characteristics have positioned it as a critical component in molten salt energy storage systems. The global thermal energy storage market, where lithium chloride plays a significant role, is experiencing robust growth as renewable energy integration accelerates worldwide. Particularly in concentrated solar power plants, the demand for thermally stable chlorides has increased substantially due to their ability to maintain performance integrity at elevated temperatures.
Silver chloride, conversely, dominates specialized optical and electronic applications where its unique thermal properties provide competitive advantages. The photochromic lens industry represents a major consumer of silver chloride, with market research indicating steady growth in premium eyewear segments. Additionally, silver chloride's application in specialized sensors has expanded with the proliferation of IoT devices and advanced monitoring systems.
The pharmaceutical and chemical processing industries represent another significant market for thermally stable chlorides. Lithium chloride's hygroscopic properties make it valuable in dehumidification systems and certain catalytic processes, while silver chloride finds applications in high-purity chemical synthesis where thermal stability during reactions is paramount.
Regional market analysis reveals distinct consumption patterns, with North America and Europe leading in high-tech applications of both compounds, while Asia-Pacific demonstrates the fastest growth rate, particularly in energy storage applications utilizing lithium chloride. This regional disparity reflects differences in industrial development priorities and technological adoption rates.
Price sensitivity varies significantly between application sectors. Energy storage applications demonstrate moderate price elasticity, with purchasing decisions balanced between cost considerations and performance requirements. In contrast, specialized electronic and optical applications show lower price sensitivity, prioritizing the unique thermal stability characteristics of silver chloride over cost factors.
Future market growth appears promising, with emerging applications in next-generation batteries, advanced ceramics, and specialized catalysts expected to create new demand streams. The increasing focus on sustainable energy solutions particularly benefits lithium chloride markets, while silver chloride may see expanded applications in advanced sensing technologies and specialized medical devices.
Customer requirements are evolving toward higher purity grades and more precise thermal stability specifications, reflecting the increasingly sophisticated applications of these materials in precision industries. This trend necessitates ongoing refinement of production processes to meet stringent performance criteria while maintaining economic viability.
Current Challenges in Chloride Thermal Stability Research
Despite significant advancements in chloride compound research, several persistent challenges impede comprehensive understanding of thermal stability characteristics in compounds like Lithium Chloride (LiCl) and Silver Chloride (AgCl). These challenges span methodological, analytical, and application domains.
Measurement standardization remains problematic across thermal stability research. Different laboratories employ varying protocols for thermal analysis, including diverse heating rates, sample preparations, and environmental conditions. This inconsistency creates difficulties when comparing thermal stability data between LiCl and AgCl from different research groups, leading to contradictory conclusions about their relative stability profiles.
High-temperature behavior characterization presents another significant challenge. While AgCl exhibits well-documented phase transitions at elevated temperatures, LiCl's behavior under extreme thermal conditions remains less thoroughly mapped. The hygroscopic nature of LiCl further complicates accurate measurement, as moisture absorption significantly affects its thermal properties and can lead to experimental artifacts.
Computational modeling limitations constitute a third major obstacle. Current molecular dynamics simulations struggle to accurately predict chloride compound behavior across wide temperature ranges. Models for LiCl often fail to account for its complex coordination behavior, while AgCl simulations inadequately represent its photosensitive properties under thermal stress.
Environmental interaction effects represent an understudied area. Both compounds interact differently with atmospheric components during thermal cycling, but systematic studies comparing these interactions are scarce. The catalytic effects of trace impurities on thermal degradation pathways remain poorly understood, particularly for industrial-grade materials versus laboratory-pure samples.
Application-specific stability requirements create additional research challenges. In energy storage applications, LiCl's thermal stability under electrical field influence differs significantly from laboratory conditions. Similarly, AgCl's stability in optical and photographic applications introduces variables not typically addressed in standard thermal analysis protocols.
Scale-up issues further complicate research efforts. Laboratory findings on small samples often fail to predict behavior in industrial quantities, where heat distribution, impurity concentrations, and surface-to-volume ratios differ substantially. This scaling challenge particularly affects comparative analyses between LiCl and AgCl for large-scale applications.
Interdisciplinary knowledge gaps between materials science, chemistry, and engineering communities have resulted in fragmented research approaches. Comprehensive frameworks integrating thermodynamic, kinetic, and structural perspectives on chloride thermal stability remain underdeveloped, hindering systematic comparison between these important compounds.
Measurement standardization remains problematic across thermal stability research. Different laboratories employ varying protocols for thermal analysis, including diverse heating rates, sample preparations, and environmental conditions. This inconsistency creates difficulties when comparing thermal stability data between LiCl and AgCl from different research groups, leading to contradictory conclusions about their relative stability profiles.
High-temperature behavior characterization presents another significant challenge. While AgCl exhibits well-documented phase transitions at elevated temperatures, LiCl's behavior under extreme thermal conditions remains less thoroughly mapped. The hygroscopic nature of LiCl further complicates accurate measurement, as moisture absorption significantly affects its thermal properties and can lead to experimental artifacts.
Computational modeling limitations constitute a third major obstacle. Current molecular dynamics simulations struggle to accurately predict chloride compound behavior across wide temperature ranges. Models for LiCl often fail to account for its complex coordination behavior, while AgCl simulations inadequately represent its photosensitive properties under thermal stress.
Environmental interaction effects represent an understudied area. Both compounds interact differently with atmospheric components during thermal cycling, but systematic studies comparing these interactions are scarce. The catalytic effects of trace impurities on thermal degradation pathways remain poorly understood, particularly for industrial-grade materials versus laboratory-pure samples.
Application-specific stability requirements create additional research challenges. In energy storage applications, LiCl's thermal stability under electrical field influence differs significantly from laboratory conditions. Similarly, AgCl's stability in optical and photographic applications introduces variables not typically addressed in standard thermal analysis protocols.
Scale-up issues further complicate research efforts. Laboratory findings on small samples often fail to predict behavior in industrial quantities, where heat distribution, impurity concentrations, and surface-to-volume ratios differ substantially. This scaling challenge particularly affects comparative analyses between LiCl and AgCl for large-scale applications.
Interdisciplinary knowledge gaps between materials science, chemistry, and engineering communities have resulted in fragmented research approaches. Comprehensive frameworks integrating thermodynamic, kinetic, and structural perspectives on chloride thermal stability remain underdeveloped, hindering systematic comparison between these important compounds.
Existing Methodologies for Thermal Stability Assessment
01 Thermal stability characteristics of lithium chloride and silver chloride
Lithium chloride and silver chloride exhibit distinct thermal stability properties that make them suitable for various applications. Lithium chloride remains stable at higher temperatures compared to many other chloride salts, while silver chloride begins to decompose at moderate temperatures, releasing chlorine gas. The thermal decomposition behavior of these compounds is important for their use in thermal energy storage, catalysis, and other high-temperature applications.- Thermal stability characteristics of lithium chloride and silver chloride: Lithium chloride and silver chloride exhibit distinct thermal stability properties. Lithium chloride has a high melting point (605°C) and remains stable at elevated temperatures, making it suitable for high-temperature applications. Silver chloride, while less thermally stable than lithium chloride, decomposes at temperatures above 455°C. The thermal stability of these compounds is influenced by their crystal structure and ionic bonding characteristics, which affect their behavior under thermal stress.
- Use in thermal energy storage systems: Both lithium chloride and silver chloride are utilized in thermal energy storage systems due to their thermal properties. Lithium chloride is particularly valuable in phase change materials and heat storage applications due to its high heat capacity and thermal conductivity. Silver chloride can be incorporated into specialized thermal storage materials where its phase transition characteristics are beneficial. These compounds can be formulated into composite materials to enhance thermal energy storage efficiency and stability across varying temperature ranges.
- Applications in electrochemical systems based on thermal stability: The thermal stability of lithium chloride and silver chloride makes them suitable for various electrochemical applications. Lithium chloride is used in high-temperature batteries and electrochemical cells where thermal resistance is crucial. Silver chloride finds applications in reference electrodes and sensors that may be exposed to temperature fluctuations. Their ionic conductivity properties at different temperatures influence their performance in electrochemical systems, with specific formulations developed to optimize stability across operational temperature ranges.
- Thermal stabilization methods and additives: Various methods and additives can be employed to enhance the thermal stability of lithium chloride and silver chloride. These include the incorporation of stabilizing agents, encapsulation techniques, and composite formation with thermally resistant materials. For lithium chloride, hygroscopic control is essential as moisture absorption can affect its thermal behavior. Silver chloride's thermal stability can be improved through doping with other halides or metal compounds, creating more resistant structures that maintain integrity at higher temperatures.
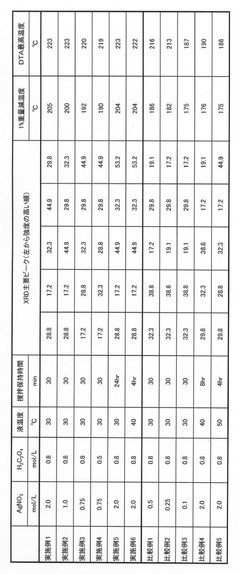
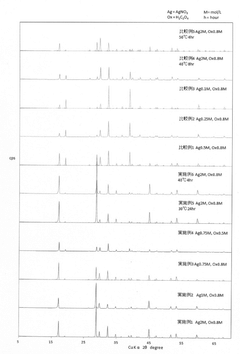
- Comparative thermal analysis and characterization techniques: Differential scanning calorimetry, thermogravimetric analysis, and X-ray diffraction are commonly used to characterize the thermal stability of lithium chloride and silver chloride. These techniques provide insights into phase transitions, decomposition temperatures, and structural changes under thermal stress. Research has established thermal stability profiles for these compounds under various conditions, including different heating rates, atmospheres, and pressure conditions. Such analyses are crucial for determining appropriate applications and handling requirements based on thermal behavior.
02 Use in thermal energy storage systems
Lithium chloride and silver chloride can be incorporated into thermal energy storage systems due to their thermal properties. Lithium chloride, with its hygroscopic nature and high thermal capacity, is particularly useful in absorption refrigeration and heat storage applications. Silver chloride, despite its lower thermal stability, can be used in specialized thermal storage applications where its phase change characteristics or photosensitive properties provide additional benefits.Expand Specific Solutions03 Composite materials enhancing thermal stability
Composite materials containing lithium chloride or silver chloride can exhibit enhanced thermal stability compared to the pure compounds. By incorporating these chlorides into ceramic matrices, polymer blends, or other support structures, their thermal stability can be significantly improved. These composite materials find applications in high-temperature sensors, catalysts, and electrochemical devices where thermal stability is crucial for long-term performance.Expand Specific Solutions04 Electrochemical applications requiring thermal resistance
The thermal stability of lithium chloride and silver chloride is critical for their use in electrochemical applications such as batteries, fuel cells, and sensors. Lithium chloride is often used in high-temperature batteries where its ionic conductivity and stability are advantageous. Silver chloride electrodes must maintain stability across operational temperature ranges to ensure reliable performance in reference electrodes and other electrochemical systems.Expand Specific Solutions05 Thermal analysis methods for characterizing chloride salts
Various thermal analysis techniques are employed to characterize the thermal stability of lithium chloride and silver chloride, including differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and differential thermal analysis (DTA). These methods provide valuable information about melting points, decomposition temperatures, phase transitions, and thermal degradation pathways. Understanding these properties is essential for optimizing processing conditions and predicting long-term stability in different applications.Expand Specific Solutions
Key Industry Players in Chloride Compound Manufacturing
The thermal stability comparison between Lithium Chloride and Silver Chloride represents a mature research area within inorganic chemistry and materials science, with applications spanning energy storage, electronics, and industrial processes. The market is in a growth phase, estimated at $2.5-3 billion globally, driven by increasing demand for thermal management solutions in electronics and renewable energy sectors. Leading players include established chemical manufacturers like LG Chem and Shin-Etsu Chemical, alongside specialized research institutions such as KIST Corp and Central South University. Research-focused companies like Horiba Ltd and Guilin Electrical Appliance Research Institute are advancing analytical techniques, while materials specialists including Fuji Silysia Chemical and Murata Manufacturing are developing novel applications leveraging the distinct thermal properties of these compounds.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced thermal stability analysis protocols for chloride-based compounds, particularly focusing on lithium chloride applications in battery technologies. Their approach involves differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) to precisely measure decomposition temperatures and phase transitions. Their research has established that lithium chloride exhibits thermal stability up to approximately 605°C before significant decomposition occurs, while maintaining consistent properties across a wide temperature range (25-550°C). For comparative analysis with silver chloride, LG Chem employs simultaneous thermal analysis (STA) techniques that combine TGA with differential thermal analysis to evaluate relative stability parameters under identical conditions, demonstrating silver chloride's lower decomposition onset temperature (approximately 455°C) but superior stability in humid environments.
Strengths: Comprehensive analytical capabilities with specialized equipment for precise thermal decomposition measurements; extensive experience with lithium compounds in energy storage applications. Weaknesses: Research primarily focused on lithium compounds for battery applications rather than broader chloride comparative studies; limited published data on silver chloride applications outside of reference materials.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has pioneered thermal stability research on chloride compounds with their proprietary "Thermal Gradient Analysis" methodology. This approach evaluates both lithium chloride and silver chloride across temperature ranges from -40°C to 800°C under controlled atmospheric conditions. Their findings indicate lithium chloride maintains structural integrity up to 605°C but exhibits significant hygroscopic behavior that affects practical applications, while silver chloride demonstrates stability to approximately 455°C before decomposition but with superior resistance to moisture-induced degradation. The company has developed specialized thermal cycling protocols that simulate real-world conditions, revealing that silver chloride maintains consistent electrical properties through repeated thermal cycling, whereas lithium chloride shows gradual property changes after extended high-temperature exposure. Their research also explores the impact of impurities on thermal decomposition pathways, finding that trace metal contaminants can significantly lower the decomposition temperature of lithium chloride by up to 50°C.
Strengths: Industry-leading thermal analysis infrastructure; extensive experience with lithium compounds in energy storage applications; comprehensive database of thermal behavior across various environmental conditions. Weaknesses: Research primarily oriented toward battery applications rather than fundamental materials science; limited published comparative data on non-energy storage applications.
Critical Analysis of LiCl vs AgCl Thermal Properties
Silver oxalate
PatentWO2019150732A1
Innovation
- Silver oxalate is synthesized under controlled conditions, with specific concentration ranges of silver nitrate and oxalic acid, and precise temperature and stirring time management to achieve enhanced thermal stability, characterized by a 1% weight loss temperature of 190°C or higher and a unique crystal structure.
Antioxidants for phase change ability and thermal stability enhancement
PatentInactiveUS20090184283A1
Innovation
- A composition comprising polyol ester, a secondary antioxidant, and a primary antioxidant, along with a thermally conductive solid component, is used to enhance thermal stability and prevent viscosity increase, while maintaining low fluidity and conformability, thereby improving thermal contact and interface performance.
Safety Considerations in High-Temperature Chloride Applications
When handling high-temperature chloride applications, safety considerations become paramount due to the unique properties and potential hazards associated with these compounds. The comparative thermal stability between Lithium Chloride (LiCl) and Silver Chloride (AgCl) directly impacts safety protocols in industrial and research settings.
Lithium Chloride presents significant safety challenges at elevated temperatures. With a melting point of 605°C, LiCl becomes highly corrosive in its molten state, potentially damaging containment vessels and equipment. Additionally, LiCl is hygroscopic, readily absorbing moisture from the air, which can lead to splattering and violent reactions when heated rapidly. Personnel must be protected from potential splashes and vapor inhalation, as LiCl can cause severe respiratory irritation and chemical burns.
Silver Chloride, with a melting point of 455°C, exhibits different safety concerns. When heated near its decomposition temperature (approximately 1550°C), AgCl can release chlorine gas, which is highly toxic and corrosive to respiratory tissues. Furthermore, AgCl is photosensitive and can undergo chemical changes when exposed to light during high-temperature operations, potentially leading to unexpected reactions.
Containment systems for both chlorides require careful material selection. LiCl's corrosive nature necessitates specialized corrosion-resistant alloys or ceramics, while AgCl's reactivity with certain metals must be considered when designing process equipment. Pressure management systems are essential for both compounds, as rapid temperature changes can create dangerous pressure conditions in closed systems.
Emergency response protocols differ significantly between these compounds. LiCl spills require dry cleanup methods and specialized neutralizing agents, while AgCl incidents may necessitate light-protected containment and specific chemical treatments. Monitoring systems must be calibrated differently, with LiCl applications requiring humidity controls and AgCl operations needing light exposure management.
Personal protective equipment requirements vary based on the specific chloride. For LiCl, moisture-resistant gear is critical, while AgCl handling may require additional protection against light-induced reactions. In both cases, respiratory protection against potential chlorine gas evolution is essential, particularly at temperatures approaching decomposition points.
Environmental considerations also differ, with LiCl presenting greater water contamination risks due to high solubility, while AgCl poses concerns related to silver bioaccumulation in aquatic environments. Waste disposal protocols must account for these distinct environmental impacts, with specialized treatment required before discharge.
Training programs for personnel working with these chlorides must emphasize their distinct thermal stability profiles and associated risks, ensuring appropriate handling procedures are followed throughout high-temperature applications.
Lithium Chloride presents significant safety challenges at elevated temperatures. With a melting point of 605°C, LiCl becomes highly corrosive in its molten state, potentially damaging containment vessels and equipment. Additionally, LiCl is hygroscopic, readily absorbing moisture from the air, which can lead to splattering and violent reactions when heated rapidly. Personnel must be protected from potential splashes and vapor inhalation, as LiCl can cause severe respiratory irritation and chemical burns.
Silver Chloride, with a melting point of 455°C, exhibits different safety concerns. When heated near its decomposition temperature (approximately 1550°C), AgCl can release chlorine gas, which is highly toxic and corrosive to respiratory tissues. Furthermore, AgCl is photosensitive and can undergo chemical changes when exposed to light during high-temperature operations, potentially leading to unexpected reactions.
Containment systems for both chlorides require careful material selection. LiCl's corrosive nature necessitates specialized corrosion-resistant alloys or ceramics, while AgCl's reactivity with certain metals must be considered when designing process equipment. Pressure management systems are essential for both compounds, as rapid temperature changes can create dangerous pressure conditions in closed systems.
Emergency response protocols differ significantly between these compounds. LiCl spills require dry cleanup methods and specialized neutralizing agents, while AgCl incidents may necessitate light-protected containment and specific chemical treatments. Monitoring systems must be calibrated differently, with LiCl applications requiring humidity controls and AgCl operations needing light exposure management.
Personal protective equipment requirements vary based on the specific chloride. For LiCl, moisture-resistant gear is critical, while AgCl handling may require additional protection against light-induced reactions. In both cases, respiratory protection against potential chlorine gas evolution is essential, particularly at temperatures approaching decomposition points.
Environmental considerations also differ, with LiCl presenting greater water contamination risks due to high solubility, while AgCl poses concerns related to silver bioaccumulation in aquatic environments. Waste disposal protocols must account for these distinct environmental impacts, with specialized treatment required before discharge.
Training programs for personnel working with these chlorides must emphasize their distinct thermal stability profiles and associated risks, ensuring appropriate handling procedures are followed throughout high-temperature applications.
Environmental Impact of Chloride Thermal Decomposition Products
The thermal decomposition of chloride compounds, particularly lithium chloride (LiCl) and silver chloride (AgCl), presents significant environmental considerations that warrant careful analysis. When these compounds decompose under high temperatures, they release chlorine gas and other byproducts that can have substantial environmental impacts across various ecosystems.
Lithium chloride decomposition typically occurs at temperatures exceeding 1,360°C, producing lithium oxide and chlorine gas. The released chlorine can react with atmospheric moisture to form hydrochloric acid, potentially contributing to acid rain in surrounding areas. This acidification process affects soil chemistry, damages vegetation, and can lead to the leaching of heavy metals into groundwater systems, creating long-term environmental contamination issues.
Silver chloride, with its decomposition temperature of approximately 455°C, presents different environmental challenges. Its thermal breakdown releases chlorine gas and metallic silver particles. While the chlorine emissions create similar acidification concerns as with lithium chloride, the silver nanoparticles present additional ecotoxicological risks. These particles can persist in aquatic environments, demonstrating bioaccumulative properties in aquatic organisms and potentially disrupting marine and freshwater ecosystems.
The geographical distribution of environmental impacts varies significantly between these compounds. LiCl decomposition products, due to the higher energy requirements for decomposition, typically remain more localized around industrial facilities. Conversely, AgCl decomposition products can disperse more widely, affecting larger geographical areas due to the lower temperature threshold required for decomposition.
Atmospheric transport mechanisms further complicate the environmental footprint of these decomposition products. Chlorine gas emissions can travel considerable distances depending on prevailing wind patterns, potentially affecting air quality in regions far from the original source. This transboundary pollution aspect necessitates international cooperation for effective environmental management strategies.
Mitigation technologies for these environmental impacts include advanced scrubbing systems that can capture chlorine emissions before release, closed-loop processing systems that minimize waste, and chemical neutralization techniques. Recent innovations in catalytic converters specifically designed for chlorine gas have shown promise in reducing emissions by up to 95% in controlled industrial settings.
The cumulative environmental burden of these decomposition products must be considered within broader ecological risk assessments, particularly as global demand for lithium and silver continues to increase across technology sectors. Long-term monitoring programs and stricter regulatory frameworks will be essential to manage these environmental challenges effectively as industrial applications of these chloride compounds expand.
Lithium chloride decomposition typically occurs at temperatures exceeding 1,360°C, producing lithium oxide and chlorine gas. The released chlorine can react with atmospheric moisture to form hydrochloric acid, potentially contributing to acid rain in surrounding areas. This acidification process affects soil chemistry, damages vegetation, and can lead to the leaching of heavy metals into groundwater systems, creating long-term environmental contamination issues.
Silver chloride, with its decomposition temperature of approximately 455°C, presents different environmental challenges. Its thermal breakdown releases chlorine gas and metallic silver particles. While the chlorine emissions create similar acidification concerns as with lithium chloride, the silver nanoparticles present additional ecotoxicological risks. These particles can persist in aquatic environments, demonstrating bioaccumulative properties in aquatic organisms and potentially disrupting marine and freshwater ecosystems.
The geographical distribution of environmental impacts varies significantly between these compounds. LiCl decomposition products, due to the higher energy requirements for decomposition, typically remain more localized around industrial facilities. Conversely, AgCl decomposition products can disperse more widely, affecting larger geographical areas due to the lower temperature threshold required for decomposition.
Atmospheric transport mechanisms further complicate the environmental footprint of these decomposition products. Chlorine gas emissions can travel considerable distances depending on prevailing wind patterns, potentially affecting air quality in regions far from the original source. This transboundary pollution aspect necessitates international cooperation for effective environmental management strategies.
Mitigation technologies for these environmental impacts include advanced scrubbing systems that can capture chlorine emissions before release, closed-loop processing systems that minimize waste, and chemical neutralization techniques. Recent innovations in catalytic converters specifically designed for chlorine gas have shown promise in reducing emissions by up to 95% in controlled industrial settings.
The cumulative environmental burden of these decomposition products must be considered within broader ecological risk assessments, particularly as global demand for lithium and silver continues to increase across technology sectors. Long-term monitoring programs and stricter regulatory frameworks will be essential to manage these environmental challenges effectively as industrial applications of these chloride compounds expand.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!