Comparative Lifecycle Analysis: Lithium Iron Phosphate and Lead-Acid Batteries
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Tech Evolution
The evolution of battery technology has been a critical factor in the advancement of portable electronics, electric vehicles, and renewable energy storage systems. The comparison between Lithium Iron Phosphate (LiFePO4) and Lead-Acid batteries represents a significant milestone in this evolution, showcasing the shift towards more efficient and environmentally friendly energy storage solutions.
Lead-Acid batteries, invented in 1859 by Gaston Planté, have been the dominant rechargeable battery technology for over a century. Their widespread use in automotive applications and uninterruptible power supplies has been due to their low cost, reliability, and established manufacturing processes. However, their limitations in energy density, cycle life, and environmental impact have driven the search for alternative technologies.
The development of Lithium-Ion batteries in the early 1990s marked a revolutionary step in battery technology. Building upon this foundation, Lithium Iron Phosphate batteries emerged in the late 1990s as a safer and more stable variant of Lithium-Ion chemistry. LiFePO4 batteries offer several advantages over traditional Lead-Acid batteries, including higher energy density, longer cycle life, and improved safety characteristics.
The technological progression from Lead-Acid to LiFePO4 batteries has been driven by advancements in materials science, electrochemistry, and manufacturing processes. Key improvements include the development of nanostructured cathode materials, enhanced electrolyte formulations, and sophisticated battery management systems. These innovations have collectively contributed to the superior performance of LiFePO4 batteries in terms of charge/discharge efficiency, thermal stability, and overall lifespan.
In recent years, the focus on sustainability and environmental impact has further accelerated the transition from Lead-Acid to LiFePO4 batteries. The reduced use of toxic materials, lower carbon footprint during production, and improved recyclability of LiFePO4 batteries align well with global efforts to minimize environmental harm and promote circular economy principles in the energy storage sector.
Looking ahead, the battery technology landscape continues to evolve rapidly. While LiFePO4 batteries represent a significant improvement over Lead-Acid batteries, ongoing research is exploring even more advanced chemistries and designs. Solid-state batteries, lithium-sulfur batteries, and other emerging technologies promise to push the boundaries of energy density, safety, and sustainability even further, potentially revolutionizing energy storage across various applications.
Lead-Acid batteries, invented in 1859 by Gaston Planté, have been the dominant rechargeable battery technology for over a century. Their widespread use in automotive applications and uninterruptible power supplies has been due to their low cost, reliability, and established manufacturing processes. However, their limitations in energy density, cycle life, and environmental impact have driven the search for alternative technologies.
The development of Lithium-Ion batteries in the early 1990s marked a revolutionary step in battery technology. Building upon this foundation, Lithium Iron Phosphate batteries emerged in the late 1990s as a safer and more stable variant of Lithium-Ion chemistry. LiFePO4 batteries offer several advantages over traditional Lead-Acid batteries, including higher energy density, longer cycle life, and improved safety characteristics.
The technological progression from Lead-Acid to LiFePO4 batteries has been driven by advancements in materials science, electrochemistry, and manufacturing processes. Key improvements include the development of nanostructured cathode materials, enhanced electrolyte formulations, and sophisticated battery management systems. These innovations have collectively contributed to the superior performance of LiFePO4 batteries in terms of charge/discharge efficiency, thermal stability, and overall lifespan.
In recent years, the focus on sustainability and environmental impact has further accelerated the transition from Lead-Acid to LiFePO4 batteries. The reduced use of toxic materials, lower carbon footprint during production, and improved recyclability of LiFePO4 batteries align well with global efforts to minimize environmental harm and promote circular economy principles in the energy storage sector.
Looking ahead, the battery technology landscape continues to evolve rapidly. While LiFePO4 batteries represent a significant improvement over Lead-Acid batteries, ongoing research is exploring even more advanced chemistries and designs. Solid-state batteries, lithium-sulfur batteries, and other emerging technologies promise to push the boundaries of energy density, safety, and sustainability even further, potentially revolutionizing energy storage across various applications.
Market Demand Analysis
The market demand for both Lithium Iron Phosphate (LFP) and Lead-Acid batteries has been experiencing significant shifts in recent years, driven by various factors including technological advancements, environmental concerns, and changing consumer preferences.
LFP batteries have seen a surge in demand, particularly in the electric vehicle (EV) sector. This growth is attributed to their superior safety profile, longer cycle life, and improved energy density compared to traditional lithium-ion batteries. The EV market's rapid expansion, coupled with increasing government initiatives to promote clean energy, has further bolstered the demand for LFP batteries.
In contrast, the market for lead-acid batteries has been relatively stable, with moderate growth primarily in the automotive and industrial sectors. These batteries continue to dominate in applications such as starter batteries for conventional vehicles, uninterruptible power supplies (UPS), and backup power systems due to their reliability and cost-effectiveness.
The renewable energy sector has emerged as a significant driver for both battery types. As the world transitions towards cleaner energy sources, the need for efficient energy storage solutions has intensified. LFP batteries are gaining traction in this space due to their scalability and performance characteristics, while lead-acid batteries remain relevant for smaller-scale and cost-sensitive applications.
In the automotive industry, the shift towards electric and hybrid vehicles has created a dichotomy in battery demand. While LFP batteries are increasingly preferred for EVs due to their higher energy density and longer lifespan, lead-acid batteries continue to be the go-to choice for conventional vehicles and as auxiliary batteries in some hybrid models.
The industrial sector presents a mixed picture. LFP batteries are making inroads in applications requiring high-performance and frequent cycling, such as material handling equipment and telecom backup power. However, lead-acid batteries maintain their stronghold in applications where initial cost is a primary concern and where their proven reliability is valued.
Consumer electronics and portable devices represent another area of divergence. LFP batteries are gaining popularity in high-end applications requiring long life and safety, while lead-acid batteries are rarely used in this sector due to their weight and size limitations.
Geographically, the demand patterns for these batteries vary. Developed markets are showing a stronger inclination towards LFP batteries, driven by stringent environmental regulations and a push for advanced technologies. Emerging markets, however, continue to see robust demand for lead-acid batteries, particularly in automotive and backup power applications, due to their lower cost and established infrastructure.
LFP batteries have seen a surge in demand, particularly in the electric vehicle (EV) sector. This growth is attributed to their superior safety profile, longer cycle life, and improved energy density compared to traditional lithium-ion batteries. The EV market's rapid expansion, coupled with increasing government initiatives to promote clean energy, has further bolstered the demand for LFP batteries.
In contrast, the market for lead-acid batteries has been relatively stable, with moderate growth primarily in the automotive and industrial sectors. These batteries continue to dominate in applications such as starter batteries for conventional vehicles, uninterruptible power supplies (UPS), and backup power systems due to their reliability and cost-effectiveness.
The renewable energy sector has emerged as a significant driver for both battery types. As the world transitions towards cleaner energy sources, the need for efficient energy storage solutions has intensified. LFP batteries are gaining traction in this space due to their scalability and performance characteristics, while lead-acid batteries remain relevant for smaller-scale and cost-sensitive applications.
In the automotive industry, the shift towards electric and hybrid vehicles has created a dichotomy in battery demand. While LFP batteries are increasingly preferred for EVs due to their higher energy density and longer lifespan, lead-acid batteries continue to be the go-to choice for conventional vehicles and as auxiliary batteries in some hybrid models.
The industrial sector presents a mixed picture. LFP batteries are making inroads in applications requiring high-performance and frequent cycling, such as material handling equipment and telecom backup power. However, lead-acid batteries maintain their stronghold in applications where initial cost is a primary concern and where their proven reliability is valued.
Consumer electronics and portable devices represent another area of divergence. LFP batteries are gaining popularity in high-end applications requiring long life and safety, while lead-acid batteries are rarely used in this sector due to their weight and size limitations.
Geographically, the demand patterns for these batteries vary. Developed markets are showing a stronger inclination towards LFP batteries, driven by stringent environmental regulations and a push for advanced technologies. Emerging markets, however, continue to see robust demand for lead-acid batteries, particularly in automotive and backup power applications, due to their lower cost and established infrastructure.
Current Tech Challenges
The comparative lifecycle analysis of Lithium Iron Phosphate (LFP) and Lead-Acid batteries reveals several significant technological challenges that researchers and manufacturers are currently grappling with. One of the primary hurdles lies in accurately quantifying and comparing the environmental impacts of these two battery technologies throughout their entire lifecycle, from raw material extraction to end-of-life disposal or recycling.
For LFP batteries, a major challenge is the energy-intensive production process, particularly in the synthesis of cathode materials. The high temperatures required for this process contribute significantly to the overall carbon footprint of LFP batteries. Additionally, the extraction and processing of lithium and other rare earth elements pose environmental concerns and potential supply chain vulnerabilities.
Lead-Acid batteries, while more established and easier to recycle, face challenges in improving their energy density and cycle life to compete with newer technologies. The toxicity of lead remains a significant environmental and health concern, necessitating stringent safety measures during production, use, and disposal.
Both battery types face challenges in optimizing their performance under various operating conditions. Temperature sensitivity, particularly in extreme climates, affects battery efficiency and lifespan. For LFP batteries, low-temperature performance is a notable area for improvement, while Lead-Acid batteries struggle with high-temperature degradation.
The recycling processes for both technologies present unique challenges. While Lead-Acid batteries benefit from a well-established recycling infrastructure, the process still involves handling hazardous materials. LFP batteries, being relatively newer, lack a comprehensive recycling ecosystem. Developing efficient and economically viable recycling methods for LFP batteries, particularly in separating and recovering valuable materials, remains a significant technological hurdle.
Energy efficiency during the use phase is another area of focus. Improving charge-discharge efficiency, reducing self-discharge rates, and extending cycle life are ongoing challenges for both battery types. These improvements are crucial for enhancing overall lifecycle performance and reducing long-term environmental impacts.
Lastly, the comparative analysis is complicated by the rapid pace of technological advancements, particularly in the LFP battery sector. As new manufacturing techniques and materials emerge, the lifecycle impacts are continually evolving, making it challenging to conduct up-to-date and accurate comparisons. This dynamic nature of battery technology necessitates ongoing research and analysis to provide relevant insights for decision-making in various applications, from electric vehicles to grid energy storage.
For LFP batteries, a major challenge is the energy-intensive production process, particularly in the synthesis of cathode materials. The high temperatures required for this process contribute significantly to the overall carbon footprint of LFP batteries. Additionally, the extraction and processing of lithium and other rare earth elements pose environmental concerns and potential supply chain vulnerabilities.
Lead-Acid batteries, while more established and easier to recycle, face challenges in improving their energy density and cycle life to compete with newer technologies. The toxicity of lead remains a significant environmental and health concern, necessitating stringent safety measures during production, use, and disposal.
Both battery types face challenges in optimizing their performance under various operating conditions. Temperature sensitivity, particularly in extreme climates, affects battery efficiency and lifespan. For LFP batteries, low-temperature performance is a notable area for improvement, while Lead-Acid batteries struggle with high-temperature degradation.
The recycling processes for both technologies present unique challenges. While Lead-Acid batteries benefit from a well-established recycling infrastructure, the process still involves handling hazardous materials. LFP batteries, being relatively newer, lack a comprehensive recycling ecosystem. Developing efficient and economically viable recycling methods for LFP batteries, particularly in separating and recovering valuable materials, remains a significant technological hurdle.
Energy efficiency during the use phase is another area of focus. Improving charge-discharge efficiency, reducing self-discharge rates, and extending cycle life are ongoing challenges for both battery types. These improvements are crucial for enhancing overall lifecycle performance and reducing long-term environmental impacts.
Lastly, the comparative analysis is complicated by the rapid pace of technological advancements, particularly in the LFP battery sector. As new manufacturing techniques and materials emerge, the lifecycle impacts are continually evolving, making it challenging to conduct up-to-date and accurate comparisons. This dynamic nature of battery technology necessitates ongoing research and analysis to provide relevant insights for decision-making in various applications, from electric vehicles to grid energy storage.
LFP vs Lead-Acid Tech
01 Lifecycle comparison between Lithium Iron Phosphate and Lead-Acid Batteries
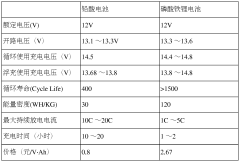
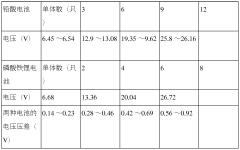
Lithium Iron Phosphate (LiFePO4) batteries generally have a longer lifecycle compared to Lead-Acid batteries. LiFePO4 batteries can typically withstand more charge-discharge cycles, have a higher depth of discharge, and maintain better performance over time. This results in a longer overall lifespan and potentially lower total cost of ownership for applications requiring frequent cycling.- Lifecycle comparison between Lithium Iron Phosphate and Lead-Acid Batteries: Lithium Iron Phosphate (LiFePO4) batteries generally have a longer lifecycle compared to Lead-Acid batteries. LiFePO4 batteries can typically withstand more charge-discharge cycles and have a longer calendar life. This results in reduced replacement frequency and lower long-term costs for applications requiring frequent cycling.
- Environmental impact and recycling of battery materials: The lifecycle assessment of both battery types includes their environmental impact and recyclability. LiFePO4 batteries are generally considered more environmentally friendly due to the absence of lead and acid. Both types can be recycled, but the processes differ. Lead-Acid batteries have a well-established recycling infrastructure, while LiFePO4 recycling technologies are still evolving.
- Performance characteristics affecting lifecycle: Various performance characteristics influence the lifecycle of both battery types. LiFePO4 batteries typically offer higher energy density, faster charging rates, and better performance in extreme temperatures. Lead-Acid batteries, while having lower energy density, are known for their reliability and lower initial cost. These factors affect the overall lifecycle and suitability for different applications.
- Maintenance requirements and operational lifecycle: The operational lifecycle of batteries is influenced by their maintenance requirements. Lead-Acid batteries often require regular maintenance, including water level checks and equalization charges. LiFePO4 batteries are generally maintenance-free, which can lead to lower operational costs and extended usable life in certain applications.
- Advancements in battery technology affecting lifecycle: Ongoing research and development in battery technology are continuously improving the lifecycle of both LiFePO4 and Lead-Acid batteries. This includes enhancements in electrode materials, electrolyte compositions, and battery management systems. These advancements aim to extend cycle life, improve performance, and reduce degradation over time for both battery types.
02 Environmental impact and recycling of battery materials
The lifecycle assessment of both battery types includes their environmental impact and recyclability. LiFePO4 batteries are generally considered more environmentally friendly due to their non-toxic materials and higher recycling efficiency. Lead-Acid batteries, while highly recyclable, pose environmental concerns due to the toxic nature of lead. Advancements in recycling technologies are being developed to improve the sustainability of both battery types.Expand Specific Solutions03 Performance characteristics affecting lifecycle
Various factors influence the lifecycle of both battery types, including temperature tolerance, charge-discharge efficiency, and self-discharge rates. LiFePO4 batteries typically offer better performance in these areas, contributing to their longer lifecycle. However, recent advancements in Lead-Acid battery technology have improved their performance characteristics, potentially extending their useful life in certain applications.Expand Specific Solutions04 Application-specific lifecycle considerations
The choice between LiFePO4 and Lead-Acid batteries often depends on the specific application requirements. For high-cycle applications like renewable energy storage or electric vehicles, LiFePO4 batteries are often preferred due to their longer lifecycle. Lead-Acid batteries may still be favored in applications with less frequent cycling or where initial cost is a primary concern, despite potentially shorter overall lifecycles.Expand Specific Solutions05 Maintenance and lifecycle management strategies
Proper maintenance and management strategies can significantly impact the lifecycle of both battery types. LiFePO4 batteries generally require less maintenance and have more flexible charging regimes. Lead-Acid batteries often need more frequent maintenance, including water level checks and equalization charges. Advanced battery management systems and charging technologies are being developed to optimize the lifecycle of both battery types in various applications.Expand Specific Solutions
Key Industry Players
The comparative lifecycle analysis of Lithium Iron Phosphate (LFP) and Lead-Acid batteries is at a mature stage, with significant market growth and technological advancements. The global market for these battery technologies is expanding rapidly, driven by increasing demand for energy storage solutions in various sectors. Companies like GS Yuasa, Furukawa Battery, and Shenzhen Center Power Tech are leading players in the lead-acid battery segment, while firms such as CATL (through Guangdong Bangpu Recycling) and Hefei Guoxuan High-Tech Power Energy are prominent in the LFP battery market. The technology maturity is evident from the involvement of major automotive manufacturers like Nissan, BMW, and Mercedes-Benz, indicating widespread adoption and ongoing research for further improvements in both battery types.
Nissan Motor Co., Ltd.
Technical Solution: Nissan has conducted extensive research on both LFP and lead-acid batteries for automotive applications. Their LFP technology focuses on improving energy density and fast-charging capabilities. Nissan's latest LFP cells achieve a volumetric energy density of 430 Wh/L[5], making them competitive with other lithium-ion chemistries. For lead-acid batteries, Nissan has developed an advanced valve-regulated design with enhanced cycle life for start-stop vehicle applications. Their lifecycle analysis compares the environmental impact of LFP and lead-acid batteries in various vehicle types, considering factors such as raw material sourcing, manufacturing energy, use-phase efficiency, and end-of-life recycling. Results indicate that LFP batteries in electric vehicles can reduce lifecycle CO2 emissions by up to 40% compared to lead-acid batteries in conventional vehicles[6].
Strengths: High volumetric energy density for LFP, improved lead-acid design for specific applications, and comprehensive vehicle-focused lifecycle analysis. Weaknesses: Limited data on long-term performance in extreme climates.
Robert Bosch GmbH
Technical Solution: Bosch has developed a comprehensive lifecycle analysis approach for both LFP and lead-acid batteries, focusing on their application in automotive and energy storage systems. Their analysis encompasses raw material extraction, manufacturing, use phase, and end-of-life recycling. Bosch's LFP batteries utilize a proprietary cathode material synthesis process that reduces energy consumption by up to 30% during manufacturing[3]. For lead-acid batteries, Bosch has implemented advanced grid designs and carbon additives to enhance cycle life and charge acceptance. Their comparative analysis shows that while LFP batteries have a higher initial environmental impact due to energy-intensive production, they outperform lead-acid batteries in long-term sustainability due to longer lifespan and higher efficiency[4].
Strengths: Comprehensive lifecycle analysis, improved manufacturing efficiency for LFP, and enhanced lead-acid battery performance. Weaknesses: Higher upfront environmental impact for LFP production.
Core Battery Innovations
Self-adjusting hybrid battery composed of lead acid batteries and lithium iron phosphate batteries
PatentWO2010091583A1
Innovation
- By connecting the lead-acid battery and the lithium iron phosphate battery in parallel, the electrolyte density of the lead-acid battery is adjusted to make the open circuit voltage consistent, the current is automatically adjusted, and complex control circuits are avoided. The high-power discharge capability of the lithium iron phosphate battery is prioritized, and the When charging, priority is given to lead-acid batteries.
Power supply assembly
PatentInactiveUS20110064994A1
Innovation
- A power supply assembly comprising an electric storage device with two electrodes and an electric energy conversion device featuring lithium iron phosphate batteries and a supercapacitor, which provides denser storage and higher discharge rates, enhancing stability and longevity.
Environmental Impact
The environmental impact of batteries is a critical consideration in their lifecycle analysis, particularly when comparing Lithium Iron Phosphate (LFP) and Lead-Acid batteries. Both types have distinct environmental footprints throughout their production, use, and end-of-life stages.
LFP batteries generally have a lower environmental impact during their operational phase due to their higher energy density and longer lifespan. This translates to fewer replacements over time, reducing the overall resource consumption and waste generation. Additionally, LFP batteries have a lower self-discharge rate, which contributes to improved energy efficiency and reduced environmental burden during use.
In contrast, lead-acid batteries have a more significant environmental impact during their production and end-of-life stages. The mining and processing of lead pose substantial environmental risks, including soil and water contamination. However, lead-acid batteries benefit from a well-established recycling infrastructure, with recycling rates reaching up to 99% in some regions. This high recyclability partially offsets their initial environmental impact.
The production of LFP batteries involves the extraction and processing of lithium, iron, and phosphate, which can have localized environmental impacts. However, these materials are generally less toxic than lead, reducing the risk of environmental contamination. The manufacturing process for LFP batteries is also typically more energy-intensive than that of lead-acid batteries, contributing to a higher carbon footprint during production.
End-of-life management is a crucial aspect of environmental impact assessment. While lead-acid batteries have a mature recycling process, the recycling of LFP batteries is still evolving. Improving LFP battery recycling technologies and infrastructure is essential to minimize their long-term environmental impact and recover valuable materials.
Water consumption is another important factor to consider. Lead-acid battery production typically requires more water than LFP battery manufacturing, potentially straining local water resources in production areas. However, the water used in LFP battery production may require more extensive treatment due to the chemicals involved.
Greenhouse gas emissions associated with battery production and use vary between the two technologies. LFP batteries generally result in lower emissions during their operational life due to their higher efficiency and longer lifespan. However, the energy-intensive production process of LFP batteries can lead to higher initial emissions compared to lead-acid batteries.
In conclusion, while both LFP and lead-acid batteries have environmental impacts, LFP batteries tend to offer better overall environmental performance when considering their full lifecycle. However, continued improvements in production efficiency, recycling technologies, and end-of-life management are crucial for minimizing the environmental footprint of both battery types.
LFP batteries generally have a lower environmental impact during their operational phase due to their higher energy density and longer lifespan. This translates to fewer replacements over time, reducing the overall resource consumption and waste generation. Additionally, LFP batteries have a lower self-discharge rate, which contributes to improved energy efficiency and reduced environmental burden during use.
In contrast, lead-acid batteries have a more significant environmental impact during their production and end-of-life stages. The mining and processing of lead pose substantial environmental risks, including soil and water contamination. However, lead-acid batteries benefit from a well-established recycling infrastructure, with recycling rates reaching up to 99% in some regions. This high recyclability partially offsets their initial environmental impact.
The production of LFP batteries involves the extraction and processing of lithium, iron, and phosphate, which can have localized environmental impacts. However, these materials are generally less toxic than lead, reducing the risk of environmental contamination. The manufacturing process for LFP batteries is also typically more energy-intensive than that of lead-acid batteries, contributing to a higher carbon footprint during production.
End-of-life management is a crucial aspect of environmental impact assessment. While lead-acid batteries have a mature recycling process, the recycling of LFP batteries is still evolving. Improving LFP battery recycling technologies and infrastructure is essential to minimize their long-term environmental impact and recover valuable materials.
Water consumption is another important factor to consider. Lead-acid battery production typically requires more water than LFP battery manufacturing, potentially straining local water resources in production areas. However, the water used in LFP battery production may require more extensive treatment due to the chemicals involved.
Greenhouse gas emissions associated with battery production and use vary between the two technologies. LFP batteries generally result in lower emissions during their operational life due to their higher efficiency and longer lifespan. However, the energy-intensive production process of LFP batteries can lead to higher initial emissions compared to lead-acid batteries.
In conclusion, while both LFP and lead-acid batteries have environmental impacts, LFP batteries tend to offer better overall environmental performance when considering their full lifecycle. However, continued improvements in production efficiency, recycling technologies, and end-of-life management are crucial for minimizing the environmental footprint of both battery types.
Recycling & Disposal
The recycling and disposal processes for Lithium Iron Phosphate (LFP) and Lead-Acid batteries differ significantly, impacting their overall environmental footprint and lifecycle sustainability. LFP batteries, being a newer technology, have more advanced recycling methods that are still evolving. The recycling process for LFP batteries typically involves pyrometallurgical or hydrometallurgical techniques to recover valuable materials such as lithium, iron, and phosphorus.
These methods can recover up to 95% of the battery materials, significantly reducing the need for new raw material extraction. However, the current recycling infrastructure for LFP batteries is still developing, and the process can be energy-intensive and costly. Despite these challenges, the high recovery rate and the increasing demand for battery materials make LFP recycling economically viable and environmentally beneficial in the long term.
Lead-Acid batteries, on the other hand, have a well-established recycling infrastructure due to their long history of use. The recycling process for Lead-Acid batteries is relatively simple and efficient, with recovery rates of up to 99% for lead and plastic components. This high recycling efficiency is partly due to the homogeneous composition of Lead-Acid batteries and the economic value of recovered lead.
However, the recycling process for Lead-Acid batteries involves potential environmental and health risks due to lead's toxicity. Strict regulations and safety measures are necessary to prevent lead contamination during recycling and disposal. Despite these challenges, the circular economy for Lead-Acid batteries is well-established, with most batteries being recycled at the end of their life.
When comparing the disposal of non-recyclable components, LFP batteries generally have a lower environmental impact. The materials in LFP batteries are less toxic and pose fewer risks to ecosystems if improperly disposed of. In contrast, improper disposal of Lead-Acid batteries can lead to severe environmental contamination due to lead and sulfuric acid leakage.
Both battery types require proper handling and disposal to minimize environmental impact. However, the established recycling infrastructure for Lead-Acid batteries currently gives them an advantage in terms of end-of-life management. As LFP battery use increases and recycling technologies improve, this gap is expected to narrow, potentially leading to LFP batteries having a more favorable lifecycle profile in terms of recycling and disposal.
These methods can recover up to 95% of the battery materials, significantly reducing the need for new raw material extraction. However, the current recycling infrastructure for LFP batteries is still developing, and the process can be energy-intensive and costly. Despite these challenges, the high recovery rate and the increasing demand for battery materials make LFP recycling economically viable and environmentally beneficial in the long term.
Lead-Acid batteries, on the other hand, have a well-established recycling infrastructure due to their long history of use. The recycling process for Lead-Acid batteries is relatively simple and efficient, with recovery rates of up to 99% for lead and plastic components. This high recycling efficiency is partly due to the homogeneous composition of Lead-Acid batteries and the economic value of recovered lead.
However, the recycling process for Lead-Acid batteries involves potential environmental and health risks due to lead's toxicity. Strict regulations and safety measures are necessary to prevent lead contamination during recycling and disposal. Despite these challenges, the circular economy for Lead-Acid batteries is well-established, with most batteries being recycled at the end of their life.
When comparing the disposal of non-recyclable components, LFP batteries generally have a lower environmental impact. The materials in LFP batteries are less toxic and pose fewer risks to ecosystems if improperly disposed of. In contrast, improper disposal of Lead-Acid batteries can lead to severe environmental contamination due to lead and sulfuric acid leakage.
Both battery types require proper handling and disposal to minimize environmental impact. However, the established recycling infrastructure for Lead-Acid batteries currently gives them an advantage in terms of end-of-life management. As LFP battery use increases and recycling technologies improve, this gap is expected to narrow, potentially leading to LFP batteries having a more favorable lifecycle profile in terms of recycling and disposal.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!