Influence of Particle Size on Lithium Iron Phosphate Battery Performance
AUG 8, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LFP Battery Evolution and Objectives
Lithium iron phosphate (LFP) batteries have undergone significant evolution since their inception in the late 1990s. Initially developed as a safer alternative to lithium cobalt oxide (LCO) batteries, LFP technology has steadily improved in performance and cost-effectiveness. The journey of LFP batteries is marked by continuous advancements in material science, manufacturing processes, and cell design.
In the early stages, LFP batteries faced challenges related to low energy density and poor electrical conductivity. However, researchers and engineers have made substantial progress in addressing these limitations. One of the key areas of focus has been the optimization of particle size and morphology of LFP cathode materials. This aspect has proven crucial in enhancing the overall performance of LFP batteries.
The evolution of LFP batteries has been driven by the growing demand for safer, more sustainable, and longer-lasting energy storage solutions. As the automotive industry shifts towards electrification, LFP batteries have gained prominence due to their thermal stability, long cycle life, and lower cost compared to other lithium-ion chemistries. This has led to increased research and development efforts to further improve LFP technology.
The primary objectives in LFP battery development revolve around several key areas. Firstly, there is a continuous push to increase energy density, allowing for greater storage capacity in a given volume or weight. This is particularly important for electric vehicle applications where range anxiety remains a concern. Secondly, researchers aim to enhance the power density of LFP batteries, enabling faster charging and higher discharge rates.
Another critical objective is to improve the low-temperature performance of LFP batteries, as their capacity and power output tend to decrease significantly in cold conditions. Additionally, efforts are being made to extend the cycle life and calendar life of LFP batteries, making them even more attractive for long-term energy storage applications.
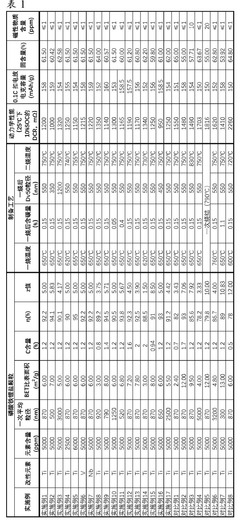
The influence of particle size on LFP battery performance has emerged as a focal point in recent years. Researchers have discovered that by carefully controlling the size and distribution of LFP particles in the cathode, they can significantly impact key performance metrics such as capacity, rate capability, and cycling stability. This has led to a surge in studies exploring nano-sized and hierarchically structured LFP materials.
As the technology continues to mature, the objectives for LFP battery development are expanding to include sustainability and environmental considerations. This includes developing more eco-friendly synthesis methods, improving recycling processes, and reducing the overall carbon footprint of LFP battery production. The ultimate goal is to create a high-performance, cost-effective, and environmentally sustainable energy storage solution that can meet the growing demands of various industries, from transportation to grid storage.
In the early stages, LFP batteries faced challenges related to low energy density and poor electrical conductivity. However, researchers and engineers have made substantial progress in addressing these limitations. One of the key areas of focus has been the optimization of particle size and morphology of LFP cathode materials. This aspect has proven crucial in enhancing the overall performance of LFP batteries.
The evolution of LFP batteries has been driven by the growing demand for safer, more sustainable, and longer-lasting energy storage solutions. As the automotive industry shifts towards electrification, LFP batteries have gained prominence due to their thermal stability, long cycle life, and lower cost compared to other lithium-ion chemistries. This has led to increased research and development efforts to further improve LFP technology.
The primary objectives in LFP battery development revolve around several key areas. Firstly, there is a continuous push to increase energy density, allowing for greater storage capacity in a given volume or weight. This is particularly important for electric vehicle applications where range anxiety remains a concern. Secondly, researchers aim to enhance the power density of LFP batteries, enabling faster charging and higher discharge rates.
Another critical objective is to improve the low-temperature performance of LFP batteries, as their capacity and power output tend to decrease significantly in cold conditions. Additionally, efforts are being made to extend the cycle life and calendar life of LFP batteries, making them even more attractive for long-term energy storage applications.
The influence of particle size on LFP battery performance has emerged as a focal point in recent years. Researchers have discovered that by carefully controlling the size and distribution of LFP particles in the cathode, they can significantly impact key performance metrics such as capacity, rate capability, and cycling stability. This has led to a surge in studies exploring nano-sized and hierarchically structured LFP materials.
As the technology continues to mature, the objectives for LFP battery development are expanding to include sustainability and environmental considerations. This includes developing more eco-friendly synthesis methods, improving recycling processes, and reducing the overall carbon footprint of LFP battery production. The ultimate goal is to create a high-performance, cost-effective, and environmentally sustainable energy storage solution that can meet the growing demands of various industries, from transportation to grid storage.
Market Analysis for LFP Batteries
The market for Lithium Iron Phosphate (LFP) batteries has experienced significant growth in recent years, driven by their superior safety, longer cycle life, and lower cost compared to other lithium-ion battery chemistries. The global LFP battery market size was valued at approximately $10 billion in 2020 and is projected to reach $25 billion by 2026, growing at a CAGR of around 15% during the forecast period.
The electric vehicle (EV) sector is the primary driver of LFP battery demand, accounting for over 60% of the market share. Major automakers, including Tesla, Volkswagen, and BYD, have increasingly adopted LFP batteries in their electric vehicles, particularly in mass-market models. This trend is expected to continue as manufacturers seek to reduce costs and improve the affordability of EVs.
Energy storage systems (ESS) represent another significant market segment for LFP batteries, with applications in grid-scale storage, residential solar systems, and commercial buildings. The ESS market for LFP batteries is projected to grow at a CAGR of over 20% in the coming years, driven by the increasing integration of renewable energy sources and the need for grid stabilization.
Geographically, China dominates the LFP battery market, accounting for over 70% of global production capacity. However, other regions, including Europe and North America, are rapidly expanding their LFP battery manufacturing capabilities to reduce dependence on Chinese suppliers and meet growing domestic demand.
The influence of particle size on LFP battery performance is a critical factor in market competitiveness. Manufacturers are investing heavily in research and development to optimize particle size distribution, aiming to enhance energy density, power output, and cycling stability. This focus on particle engineering is expected to drive further improvements in LFP battery performance and expand their market applications.
Despite the positive market outlook, challenges remain. The limited energy density of LFP batteries compared to nickel-based chemistries may restrict their adoption in certain high-end EV applications. Additionally, the availability and cost of raw materials, particularly iron and phosphorus, could impact market growth and pricing dynamics.
In conclusion, the LFP battery market is poised for substantial growth, driven by increasing demand in the EV and energy storage sectors. Ongoing research into particle size optimization and other performance enhancements will likely expand the market potential of LFP batteries, solidifying their position as a key player in the global energy transition.
The electric vehicle (EV) sector is the primary driver of LFP battery demand, accounting for over 60% of the market share. Major automakers, including Tesla, Volkswagen, and BYD, have increasingly adopted LFP batteries in their electric vehicles, particularly in mass-market models. This trend is expected to continue as manufacturers seek to reduce costs and improve the affordability of EVs.
Energy storage systems (ESS) represent another significant market segment for LFP batteries, with applications in grid-scale storage, residential solar systems, and commercial buildings. The ESS market for LFP batteries is projected to grow at a CAGR of over 20% in the coming years, driven by the increasing integration of renewable energy sources and the need for grid stabilization.
Geographically, China dominates the LFP battery market, accounting for over 70% of global production capacity. However, other regions, including Europe and North America, are rapidly expanding their LFP battery manufacturing capabilities to reduce dependence on Chinese suppliers and meet growing domestic demand.
The influence of particle size on LFP battery performance is a critical factor in market competitiveness. Manufacturers are investing heavily in research and development to optimize particle size distribution, aiming to enhance energy density, power output, and cycling stability. This focus on particle engineering is expected to drive further improvements in LFP battery performance and expand their market applications.
Despite the positive market outlook, challenges remain. The limited energy density of LFP batteries compared to nickel-based chemistries may restrict their adoption in certain high-end EV applications. Additionally, the availability and cost of raw materials, particularly iron and phosphorus, could impact market growth and pricing dynamics.
In conclusion, the LFP battery market is poised for substantial growth, driven by increasing demand in the EV and energy storage sectors. Ongoing research into particle size optimization and other performance enhancements will likely expand the market potential of LFP batteries, solidifying their position as a key player in the global energy transition.
Current Challenges in LFP Particle Size Control
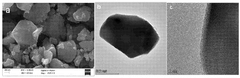
Despite significant advancements in lithium iron phosphate (LFP) battery technology, controlling particle size remains a critical challenge in optimizing battery performance. The size of LFP particles directly influences key battery characteristics such as energy density, power output, and cycle life. However, achieving precise control over particle size during synthesis and maintaining uniformity across large-scale production presents several obstacles.
One of the primary challenges is the inherent trade-off between particle size and battery performance metrics. Smaller particles generally offer improved lithium-ion diffusion and electron transfer rates, leading to enhanced power capabilities. However, they also result in lower tap density and volumetric energy density. Conversely, larger particles can increase energy density but may compromise rate capability and cycle life. Striking the optimal balance between these competing factors remains a significant hurdle.
The synthesis process itself poses challenges in controlling particle size. Traditional solid-state methods often result in non-uniform particle sizes and agglomeration. While hydrothermal and solvothermal methods offer better control, they face scalability issues and can be costly for large-scale production. Achieving consistent particle size distribution across different batches and maintaining this consistency during scale-up are ongoing challenges for manufacturers.
Another critical issue is the tendency of LFP particles to agglomerate during synthesis and subsequent processing steps. Agglomeration can lead to uneven particle size distribution and reduced active surface area, negatively impacting battery performance. Developing effective dispersion techniques and surface modification methods to prevent agglomeration without compromising the material's electrochemical properties is an area of active research.
The stability of particle size during battery operation also presents challenges. LFP particles can undergo size changes due to lithium insertion/extraction cycles, leading to structural degradation and capacity fade over time. Mitigating these effects through advanced particle engineering and protective coatings is crucial for long-term battery performance.
Furthermore, the relationship between particle size and electrolyte interactions adds another layer of complexity. Smaller particles with higher surface area can lead to increased side reactions with the electrolyte, potentially compromising battery safety and longevity. Balancing the benefits of smaller particles with their potential drawbacks in terms of electrolyte stability is an ongoing challenge in LFP battery design.
Lastly, the characterization and quality control of LFP particle size in industrial settings pose significant challenges. Developing rapid, accurate, and cost-effective methods for particle size analysis that can be integrated into production lines is essential for maintaining consistent quality in large-scale manufacturing.
One of the primary challenges is the inherent trade-off between particle size and battery performance metrics. Smaller particles generally offer improved lithium-ion diffusion and electron transfer rates, leading to enhanced power capabilities. However, they also result in lower tap density and volumetric energy density. Conversely, larger particles can increase energy density but may compromise rate capability and cycle life. Striking the optimal balance between these competing factors remains a significant hurdle.
The synthesis process itself poses challenges in controlling particle size. Traditional solid-state methods often result in non-uniform particle sizes and agglomeration. While hydrothermal and solvothermal methods offer better control, they face scalability issues and can be costly for large-scale production. Achieving consistent particle size distribution across different batches and maintaining this consistency during scale-up are ongoing challenges for manufacturers.
Another critical issue is the tendency of LFP particles to agglomerate during synthesis and subsequent processing steps. Agglomeration can lead to uneven particle size distribution and reduced active surface area, negatively impacting battery performance. Developing effective dispersion techniques and surface modification methods to prevent agglomeration without compromising the material's electrochemical properties is an area of active research.
The stability of particle size during battery operation also presents challenges. LFP particles can undergo size changes due to lithium insertion/extraction cycles, leading to structural degradation and capacity fade over time. Mitigating these effects through advanced particle engineering and protective coatings is crucial for long-term battery performance.
Furthermore, the relationship between particle size and electrolyte interactions adds another layer of complexity. Smaller particles with higher surface area can lead to increased side reactions with the electrolyte, potentially compromising battery safety and longevity. Balancing the benefits of smaller particles with their potential drawbacks in terms of electrolyte stability is an ongoing challenge in LFP battery design.
Lastly, the characterization and quality control of LFP particle size in industrial settings pose significant challenges. Developing rapid, accurate, and cost-effective methods for particle size analysis that can be integrated into production lines is essential for maintaining consistent quality in large-scale manufacturing.
Existing Particle Size Optimization Techniques
01 Electrode material composition and structure
Improving the composition and structure of electrode materials, particularly the cathode, can enhance the performance of lithium iron phosphate batteries. This includes optimizing particle size, morphology, and doping with other elements to increase conductivity and stability.- Electrode material composition and structure: Optimizing the composition and structure of electrode materials, particularly the cathode, can significantly improve the performance of lithium iron phosphate batteries. This includes modifying the particle size, morphology, and doping of LiFePO4 to enhance conductivity, capacity, and cycling stability.
- Electrolyte formulation and additives: Developing advanced electrolyte formulations and incorporating functional additives can enhance the ionic conductivity, interfacial stability, and overall performance of lithium iron phosphate batteries. This includes the use of novel solvents, salts, and additives to improve the electrochemical properties and safety of the battery.
- Battery management and control systems: Implementing sophisticated battery management and control systems can optimize the performance and longevity of lithium iron phosphate batteries. This includes advanced algorithms for state-of-charge estimation, thermal management, and charge-discharge control to maximize efficiency and prevent degradation.
- Nanostructured materials and coatings: Utilizing nanostructured materials and surface coatings can enhance the electrochemical properties of lithium iron phosphate batteries. This approach can improve the electron and ion transport, increase the active surface area, and protect against side reactions, leading to better overall battery performance.
- Fast-charging techniques: Developing and implementing fast-charging techniques specific to lithium iron phosphate batteries can significantly improve their practicality and appeal. This includes optimizing charging protocols, current densities, and temperature control to achieve rapid charging without compromising battery life or safety.
02 Electrolyte formulation and additives
Developing advanced electrolyte formulations and incorporating specific additives can improve the ionic conductivity, stability, and overall performance of lithium iron phosphate batteries. This includes the use of novel solvents, salts, and functional additives to enhance battery life and safety.Expand Specific Solutions03 Battery management and control systems
Implementing sophisticated battery management and control systems can optimize the performance of lithium iron phosphate batteries. This includes advanced algorithms for charge-discharge control, thermal management, and state-of-charge estimation to improve efficiency and longevity.Expand Specific Solutions04 Manufacturing processes and techniques
Enhancing manufacturing processes and techniques can lead to improved lithium iron phosphate battery performance. This includes innovations in electrode coating, cell assembly, and quality control measures to ensure consistent and high-quality battery production.Expand Specific Solutions05 Battery pack design and thermal management
Optimizing battery pack design and implementing effective thermal management strategies can significantly enhance the overall performance of lithium iron phosphate batteries. This includes innovative cooling systems, cell arrangement, and pack architecture to improve energy density and safety.Expand Specific Solutions
Key Players in LFP Battery Industry
The influence of particle size on lithium iron phosphate (LFP) battery performance is a critical area of research in the energy storage industry, currently in a growth phase. The market for LFP batteries is expanding rapidly, driven by demand in electric vehicles and grid storage applications. Technologically, the field is advancing but still evolving, with companies like CATL, BYD, and LG Energy Solution leading innovation. These firms are investing heavily in R&D to optimize particle size for enhanced battery performance, focusing on improving energy density, charge/discharge rates, and cycle life. Smaller players like Blue Horizon Innovations and Shenzhen Dynanonic are also contributing to technological advancements, indicating a competitive and dynamic landscape.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has focused on developing LFP cathode materials with optimized particle size and morphology. They employ a two-step synthesis process that results in plate-like particles with an average size of 300-500 nm[4]. This particle shape and size allow for better packing density and increased surface area for lithium-ion exchange. LG has also implemented a proprietary doping technique to improve the electrical conductivity of LFP particles[5]. Their approach includes a surface modification process that creates a nanoscale conductive layer on the LFP particles, enhancing overall battery performance[6].
Strengths: Improved packing density, enhanced conductivity, and better rate performance. Weaknesses: Potential challenges in scaling up the complex synthesis process.
BYD Co., Ltd.
Technical Solution: BYD has developed a unique "blade battery" technology that incorporates optimized LFP particle design. Their approach focuses on creating elongated LFP particles with a length-to-width ratio of 5:1 to 10:1[7]. These blade-like particles are typically 2-5 μm in length and 0.2-0.5 μm in width. BYD's manufacturing process includes a controlled crystallization step that ensures uniform particle growth and size distribution[8]. They have also implemented a carbon-nitrogen co-doping technique to enhance the electronic conductivity of LFP particles, resulting in improved overall battery performance[9].
Strengths: High energy density, improved safety, and better thermal management. Weaknesses: Specialized manufacturing equipment required, potentially limiting production flexibility.
Innovative Approaches to LFP Particle Engineering
Positive active material, positive electrode plate, lithiumion battery, and electrical device
PatentPendingEP4512770A1
Innovation
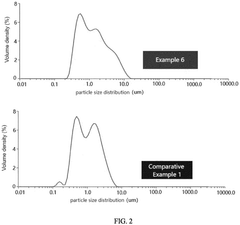
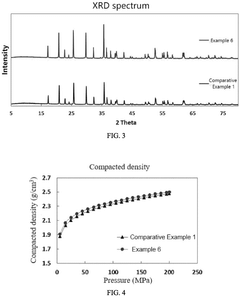
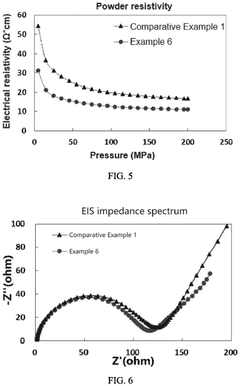
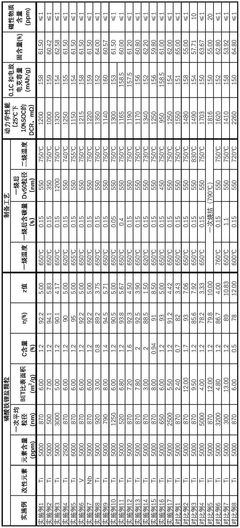
- A positive active material with a controlled particle size distribution, where the volume percentage of lithium iron phosphate particles smaller than 1 µm (x) and greater than 5 µm (z) is optimized within a range of 0.5 to 2.8, resulting in a higher compacted density, reduced impedance, improved conductivity, and enhanced capacity.
Lithium iron phosphate particles and preparation method therefor, positive electrode sheet, secondary battery and electric device
PatentWO2025148364A1
Innovation
- By preparing large-particle lithium iron phosphate particles, the particle size is 500nm to 3000nm, the BET specific surface area is 3m²/g to 8m²/g, the carbon content is 0.8 to 2.0%, and doping modification is carried out to form a uniform and dense carbon coating layer to enhance the surface conductivity of the particles.
Environmental Impact of LFP Manufacturing
The manufacturing process of Lithium Iron Phosphate (LFP) batteries has significant environmental implications that warrant careful consideration. The production of LFP cathode materials involves energy-intensive processes and the use of various chemicals, which can contribute to environmental pollution if not properly managed.
One of the primary environmental concerns in LFP manufacturing is the energy consumption associated with high-temperature synthesis methods. Traditional solid-state reactions require temperatures up to 700°C, resulting in substantial energy use and associated greenhouse gas emissions. However, recent advancements in low-temperature synthesis techniques, such as hydrothermal and sol-gel methods, have shown promise in reducing energy requirements and minimizing environmental impact.
Water usage and wastewater management are critical aspects of LFP production. The synthesis process often involves aqueous solutions, and proper treatment of wastewater is essential to prevent the release of harmful chemicals into the environment. Implementing closed-loop water recycling systems and advanced wastewater treatment technologies can significantly reduce water consumption and minimize the discharge of pollutants.
The use of raw materials in LFP manufacturing also has environmental implications. While iron and phosphate are relatively abundant and less environmentally problematic, the extraction and processing of lithium can have substantial environmental impacts. Sustainable sourcing practices and the development of efficient recycling technologies for lithium are crucial for mitigating these effects.
Particle size control in LFP production, which is central to battery performance, can influence environmental impact. Nano-sized particles often require more energy-intensive processes and may pose potential risks if released into the environment. Balancing the benefits of improved battery performance with the environmental considerations of nanoparticle production is an ongoing challenge for manufacturers.
Air emissions from LFP manufacturing facilities are another environmental concern. The release of particulate matter and volatile organic compounds during production processes can contribute to air pollution. Implementing effective air filtration systems and adopting cleaner production technologies are essential for minimizing these emissions and protecting air quality in surrounding areas.
The environmental footprint of LFP manufacturing extends beyond the production phase to include packaging and transportation. Optimizing packaging materials and logistics can help reduce waste and transportation-related emissions. Additionally, the development of localized supply chains can decrease the overall carbon footprint associated with the global movement of raw materials and finished products.
As the demand for LFP batteries continues to grow, addressing these environmental challenges becomes increasingly important. Ongoing research and development efforts are focused on creating more sustainable manufacturing processes, improving material efficiency, and enhancing recycling capabilities. By prioritizing environmental considerations in LFP production, the industry can work towards minimizing its ecological impact while meeting the rising demand for energy storage solutions.
One of the primary environmental concerns in LFP manufacturing is the energy consumption associated with high-temperature synthesis methods. Traditional solid-state reactions require temperatures up to 700°C, resulting in substantial energy use and associated greenhouse gas emissions. However, recent advancements in low-temperature synthesis techniques, such as hydrothermal and sol-gel methods, have shown promise in reducing energy requirements and minimizing environmental impact.
Water usage and wastewater management are critical aspects of LFP production. The synthesis process often involves aqueous solutions, and proper treatment of wastewater is essential to prevent the release of harmful chemicals into the environment. Implementing closed-loop water recycling systems and advanced wastewater treatment technologies can significantly reduce water consumption and minimize the discharge of pollutants.
The use of raw materials in LFP manufacturing also has environmental implications. While iron and phosphate are relatively abundant and less environmentally problematic, the extraction and processing of lithium can have substantial environmental impacts. Sustainable sourcing practices and the development of efficient recycling technologies for lithium are crucial for mitigating these effects.
Particle size control in LFP production, which is central to battery performance, can influence environmental impact. Nano-sized particles often require more energy-intensive processes and may pose potential risks if released into the environment. Balancing the benefits of improved battery performance with the environmental considerations of nanoparticle production is an ongoing challenge for manufacturers.
Air emissions from LFP manufacturing facilities are another environmental concern. The release of particulate matter and volatile organic compounds during production processes can contribute to air pollution. Implementing effective air filtration systems and adopting cleaner production technologies are essential for minimizing these emissions and protecting air quality in surrounding areas.
The environmental footprint of LFP manufacturing extends beyond the production phase to include packaging and transportation. Optimizing packaging materials and logistics can help reduce waste and transportation-related emissions. Additionally, the development of localized supply chains can decrease the overall carbon footprint associated with the global movement of raw materials and finished products.
As the demand for LFP batteries continues to grow, addressing these environmental challenges becomes increasingly important. Ongoing research and development efforts are focused on creating more sustainable manufacturing processes, improving material efficiency, and enhancing recycling capabilities. By prioritizing environmental considerations in LFP production, the industry can work towards minimizing its ecological impact while meeting the rising demand for energy storage solutions.
Safety Considerations for LFP Batteries
Safety considerations are paramount in the development and application of Lithium Iron Phosphate (LFP) batteries, especially when considering the influence of particle size on battery performance. The inherent stability of LFP cathode materials contributes significantly to the overall safety profile of these batteries, making them a preferred choice in many applications where safety is a critical factor.
The particle size of LFP materials plays a crucial role in determining the safety characteristics of the battery. Smaller particle sizes generally lead to improved thermal stability, which is a key safety feature. This enhanced thermal stability results from the increased surface area-to-volume ratio, allowing for more efficient heat dissipation and reducing the risk of thermal runaway events.
However, the relationship between particle size and safety is not straightforward. While smaller particles can improve thermal stability, they may also increase the reactivity of the material, potentially leading to accelerated aging and capacity fade. This trade-off necessitates careful optimization of particle size to balance safety and performance.
One of the primary safety advantages of LFP batteries is their resistance to oxygen release during thermal events. This characteristic is less dependent on particle size and more on the inherent crystal structure of LFP. Nevertheless, controlling particle size and morphology can further enhance this property by minimizing structural defects that could potentially compromise safety.
The mechanical stability of LFP particles is another critical safety aspect influenced by particle size. Larger particles tend to have better mechanical integrity, reducing the risk of particle fracture during cycling. This is particularly important in preventing the formation of reactive surfaces that could lead to unwanted side reactions and potential safety hazards.
In terms of electrical safety, the particle size affects the internal resistance of the battery. Smaller particles generally result in lower internal resistance, which can improve the battery's ability to handle high current loads safely. However, this must be balanced against the potential for increased self-discharge rates associated with smaller particles.
When considering the manufacturing process, the control of particle size distribution is crucial for ensuring consistent safety performance across batches. Uniform particle size contributes to homogeneous current distribution within the electrode, reducing the likelihood of localized hotspots that could compromise safety.
The interaction between LFP particles and the electrolyte is another safety consideration influenced by particle size. Smaller particles have a larger surface area exposed to the electrolyte, which can enhance the formation of a stable solid electrolyte interphase (SEI) layer. A well-formed SEI layer is critical for preventing unwanted reactions between the electrode and electrolyte, thereby enhancing long-term safety and stability.
In conclusion, while LFP batteries are inherently safer than many other lithium-ion chemistries, the influence of particle size on their safety performance is significant and multifaceted. Optimizing particle size requires a careful balance between thermal stability, mechanical integrity, electrical performance, and long-term reliability to ensure the highest safety standards are met in LFP battery applications.
The particle size of LFP materials plays a crucial role in determining the safety characteristics of the battery. Smaller particle sizes generally lead to improved thermal stability, which is a key safety feature. This enhanced thermal stability results from the increased surface area-to-volume ratio, allowing for more efficient heat dissipation and reducing the risk of thermal runaway events.
However, the relationship between particle size and safety is not straightforward. While smaller particles can improve thermal stability, they may also increase the reactivity of the material, potentially leading to accelerated aging and capacity fade. This trade-off necessitates careful optimization of particle size to balance safety and performance.
One of the primary safety advantages of LFP batteries is their resistance to oxygen release during thermal events. This characteristic is less dependent on particle size and more on the inherent crystal structure of LFP. Nevertheless, controlling particle size and morphology can further enhance this property by minimizing structural defects that could potentially compromise safety.
The mechanical stability of LFP particles is another critical safety aspect influenced by particle size. Larger particles tend to have better mechanical integrity, reducing the risk of particle fracture during cycling. This is particularly important in preventing the formation of reactive surfaces that could lead to unwanted side reactions and potential safety hazards.
In terms of electrical safety, the particle size affects the internal resistance of the battery. Smaller particles generally result in lower internal resistance, which can improve the battery's ability to handle high current loads safely. However, this must be balanced against the potential for increased self-discharge rates associated with smaller particles.
When considering the manufacturing process, the control of particle size distribution is crucial for ensuring consistent safety performance across batches. Uniform particle size contributes to homogeneous current distribution within the electrode, reducing the likelihood of localized hotspots that could compromise safety.
The interaction between LFP particles and the electrolyte is another safety consideration influenced by particle size. Smaller particles have a larger surface area exposed to the electrolyte, which can enhance the formation of a stable solid electrolyte interphase (SEI) layer. A well-formed SEI layer is critical for preventing unwanted reactions between the electrode and electrolyte, thereby enhancing long-term safety and stability.
In conclusion, while LFP batteries are inherently safer than many other lithium-ion chemistries, the influence of particle size on their safety performance is significant and multifaceted. Optimizing particle size requires a careful balance between thermal stability, mechanical integrity, electrical performance, and long-term reliability to ensure the highest safety standards are met in LFP battery applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!