Exploring the Effects of Additives on Lithium Iron Phosphate Battery Stability
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LFP Battery Additives Background and Objectives
Lithium iron phosphate (LFP) batteries have emerged as a prominent technology in the energy storage landscape, particularly in electric vehicles and renewable energy systems. The development of LFP batteries can be traced back to the late 1990s when researchers sought to create a safer and more stable alternative to traditional lithium-ion batteries. Over the past two decades, LFP batteries have undergone significant improvements in performance, cost-effectiveness, and manufacturing processes.
The evolution of LFP battery technology has been driven by the increasing demand for sustainable energy solutions and the push for electrification in various industries. As the technology matured, researchers and manufacturers have focused on enhancing the energy density, cycle life, and overall stability of LFP batteries. This ongoing pursuit of improvement has led to the exploration of various additives as a means to address the inherent limitations of LFP chemistry.
Additives play a crucial role in modifying the properties of LFP batteries, potentially enhancing their performance, longevity, and safety. These chemical compounds, when introduced in small quantities, can significantly impact the electrochemical processes within the battery, influencing factors such as the formation of the solid electrolyte interphase (SEI), electrode stability, and electrolyte conductivity.
The primary objective of investigating additives in LFP batteries is to overcome the challenges associated with this chemistry, such as relatively lower energy density compared to other lithium-ion chemistries and capacity fading over extended cycling. Researchers aim to develop additives that can improve the ionic conductivity of the electrolyte, enhance the structural stability of the cathode material, and mitigate unwanted side reactions at the electrode-electrolyte interface.
Furthermore, the exploration of additives seeks to address specific performance metrics, including increased capacity retention, improved rate capability, enhanced thermal stability, and extended cycle life. By carefully selecting and optimizing additive combinations, scientists and engineers strive to push the boundaries of LFP battery technology, making it more competitive with other energy storage solutions.
As the global focus on sustainable energy intensifies, the development of advanced LFP batteries with tailored additives aligns with broader technological trends and environmental goals. This research direction not only aims to improve the performance of existing LFP batteries but also to expand their potential applications across various sectors, from transportation to grid-scale energy storage.
The evolution of LFP battery technology has been driven by the increasing demand for sustainable energy solutions and the push for electrification in various industries. As the technology matured, researchers and manufacturers have focused on enhancing the energy density, cycle life, and overall stability of LFP batteries. This ongoing pursuit of improvement has led to the exploration of various additives as a means to address the inherent limitations of LFP chemistry.
Additives play a crucial role in modifying the properties of LFP batteries, potentially enhancing their performance, longevity, and safety. These chemical compounds, when introduced in small quantities, can significantly impact the electrochemical processes within the battery, influencing factors such as the formation of the solid electrolyte interphase (SEI), electrode stability, and electrolyte conductivity.
The primary objective of investigating additives in LFP batteries is to overcome the challenges associated with this chemistry, such as relatively lower energy density compared to other lithium-ion chemistries and capacity fading over extended cycling. Researchers aim to develop additives that can improve the ionic conductivity of the electrolyte, enhance the structural stability of the cathode material, and mitigate unwanted side reactions at the electrode-electrolyte interface.
Furthermore, the exploration of additives seeks to address specific performance metrics, including increased capacity retention, improved rate capability, enhanced thermal stability, and extended cycle life. By carefully selecting and optimizing additive combinations, scientists and engineers strive to push the boundaries of LFP battery technology, making it more competitive with other energy storage solutions.
As the global focus on sustainable energy intensifies, the development of advanced LFP batteries with tailored additives aligns with broader technological trends and environmental goals. This research direction not only aims to improve the performance of existing LFP batteries but also to expand their potential applications across various sectors, from transportation to grid-scale energy storage.
Market Analysis for Enhanced LFP Batteries
The market for enhanced Lithium Iron Phosphate (LFP) batteries is experiencing significant growth, driven by the increasing demand for electric vehicles (EVs) and renewable energy storage solutions. LFP batteries have gained popularity due to their inherent safety, long cycle life, and cost-effectiveness compared to other lithium-ion chemistries. The global LFP battery market was valued at $10.2 billion in 2021 and is projected to reach $25.7 billion by 2028, growing at a CAGR of 14.3% during the forecast period.
The automotive sector is the primary driver of this market expansion, with major EV manufacturers adopting LFP batteries for their entry-level and mid-range models. Tesla, for instance, has shifted to LFP batteries for its standard-range vehicles, citing improved cost efficiency and supply chain stability. This trend is expected to continue as automakers seek to reduce production costs and improve the affordability of electric vehicles.
In the energy storage sector, LFP batteries are gaining traction for both residential and utility-scale applications. The technology's safety profile and long cycle life make it particularly attractive for stationary storage systems, where energy density is less critical than in mobile applications. Countries with ambitious renewable energy targets are driving demand for large-scale energy storage solutions, further boosting the LFP battery market.
Geographically, China dominates the LFP battery market, accounting for over 60% of global production capacity. However, other regions are rapidly expanding their manufacturing capabilities to reduce dependence on Chinese suppliers and meet local demand. Europe and North America are investing heavily in domestic LFP battery production, with several gigafactories under construction or in the planning stages.
The market for enhanced LFP batteries, specifically those with improved stability through additives, is a growing niche within the broader LFP market. These advanced formulations address some of the traditional limitations of LFP technology, such as lower energy density and poor performance at low temperatures. By enhancing stability and performance, these batteries can expand their application range and compete more effectively with other lithium-ion chemistries in high-performance sectors.
Key market players in the enhanced LFP battery segment include established battery manufacturers like CATL, BYD, and A123 Systems, as well as emerging companies focused on advanced LFP formulations. These companies are investing heavily in research and development to create proprietary additive technologies that can differentiate their products in an increasingly competitive market.
The automotive sector is the primary driver of this market expansion, with major EV manufacturers adopting LFP batteries for their entry-level and mid-range models. Tesla, for instance, has shifted to LFP batteries for its standard-range vehicles, citing improved cost efficiency and supply chain stability. This trend is expected to continue as automakers seek to reduce production costs and improve the affordability of electric vehicles.
In the energy storage sector, LFP batteries are gaining traction for both residential and utility-scale applications. The technology's safety profile and long cycle life make it particularly attractive for stationary storage systems, where energy density is less critical than in mobile applications. Countries with ambitious renewable energy targets are driving demand for large-scale energy storage solutions, further boosting the LFP battery market.
Geographically, China dominates the LFP battery market, accounting for over 60% of global production capacity. However, other regions are rapidly expanding their manufacturing capabilities to reduce dependence on Chinese suppliers and meet local demand. Europe and North America are investing heavily in domestic LFP battery production, with several gigafactories under construction or in the planning stages.
The market for enhanced LFP batteries, specifically those with improved stability through additives, is a growing niche within the broader LFP market. These advanced formulations address some of the traditional limitations of LFP technology, such as lower energy density and poor performance at low temperatures. By enhancing stability and performance, these batteries can expand their application range and compete more effectively with other lithium-ion chemistries in high-performance sectors.
Key market players in the enhanced LFP battery segment include established battery manufacturers like CATL, BYD, and A123 Systems, as well as emerging companies focused on advanced LFP formulations. These companies are investing heavily in research and development to create proprietary additive technologies that can differentiate their products in an increasingly competitive market.
Current Challenges in LFP Battery Stability
Despite the numerous advantages of Lithium Iron Phosphate (LFP) batteries, including their high safety profile, long cycle life, and cost-effectiveness, they still face several challenges in terms of stability. One of the primary issues is capacity fading over extended cycling, which is often attributed to the degradation of the cathode material and the formation of a resistive surface film on both electrodes.
The formation and growth of this surface film, known as the Solid Electrolyte Interphase (SEI), is a major contributor to capacity loss and increased internal resistance. While the SEI is essential for protecting the electrode from further decomposition, its continuous growth consumes active lithium and electrolyte, leading to decreased battery performance over time.
Another significant challenge is the poor rate capability of LFP batteries, particularly at low temperatures. This limitation is primarily due to the low electronic conductivity of LFP material and the sluggish kinetics of lithium ion insertion/extraction at the LFP/electrolyte interface. As a result, LFP batteries often struggle to deliver high power output in cold environments, which is a critical concern for applications such as electric vehicles.
The stability of LFP batteries is also compromised by side reactions between the electrolyte and electrode materials. These parasitic reactions can lead to gas evolution, electrolyte decomposition, and the formation of insoluble products that clog the pores of the electrodes, further impeding ion transport and reducing overall battery performance.
Furthermore, LFP batteries are susceptible to iron dissolution from the cathode, especially at elevated temperatures or during overcharging. The dissolved iron ions can migrate to the anode and deposit on its surface, causing irreversible capacity loss and potential safety issues.
Thermal stability is another area of concern, particularly in large-format batteries used in electric vehicles or grid storage systems. While LFP is generally considered more thermally stable than other cathode materials, thermal runaway can still occur under extreme conditions, posing safety risks and accelerating battery degradation.
Addressing these stability challenges is crucial for the widespread adoption of LFP batteries in various applications. Current research efforts are focused on developing novel additives, surface coatings, and electrolyte formulations to mitigate these issues and enhance the overall stability and performance of LFP batteries.
The formation and growth of this surface film, known as the Solid Electrolyte Interphase (SEI), is a major contributor to capacity loss and increased internal resistance. While the SEI is essential for protecting the electrode from further decomposition, its continuous growth consumes active lithium and electrolyte, leading to decreased battery performance over time.
Another significant challenge is the poor rate capability of LFP batteries, particularly at low temperatures. This limitation is primarily due to the low electronic conductivity of LFP material and the sluggish kinetics of lithium ion insertion/extraction at the LFP/electrolyte interface. As a result, LFP batteries often struggle to deliver high power output in cold environments, which is a critical concern for applications such as electric vehicles.
The stability of LFP batteries is also compromised by side reactions between the electrolyte and electrode materials. These parasitic reactions can lead to gas evolution, electrolyte decomposition, and the formation of insoluble products that clog the pores of the electrodes, further impeding ion transport and reducing overall battery performance.
Furthermore, LFP batteries are susceptible to iron dissolution from the cathode, especially at elevated temperatures or during overcharging. The dissolved iron ions can migrate to the anode and deposit on its surface, causing irreversible capacity loss and potential safety issues.
Thermal stability is another area of concern, particularly in large-format batteries used in electric vehicles or grid storage systems. While LFP is generally considered more thermally stable than other cathode materials, thermal runaway can still occur under extreme conditions, posing safety risks and accelerating battery degradation.
Addressing these stability challenges is crucial for the widespread adoption of LFP batteries in various applications. Current research efforts are focused on developing novel additives, surface coatings, and electrolyte formulations to mitigate these issues and enhance the overall stability and performance of LFP batteries.
Existing Additive Solutions for LFP Stability
01 Electrolyte composition for improved stability
Optimizing the electrolyte composition can enhance the stability of lithium iron phosphate batteries. This may involve using specific additives, adjusting salt concentrations, or incorporating novel electrolyte formulations to improve the electrochemical performance and longevity of the battery.- Electrolyte composition for improved stability: Optimizing the electrolyte composition can significantly enhance the stability of lithium iron phosphate batteries. This includes using additives, adjusting salt concentrations, or incorporating novel electrolyte formulations to improve the solid electrolyte interphase (SEI) formation, reduce side reactions, and enhance overall battery performance and longevity.
- Surface modification of cathode materials: Modifying the surface of lithium iron phosphate cathode materials can improve their stability and performance. Techniques such as coating with conductive materials, doping with other elements, or creating core-shell structures can enhance the material's conductivity, reduce side reactions with the electrolyte, and improve cycling stability.
- Advanced manufacturing processes: Implementing advanced manufacturing processes can lead to more stable lithium iron phosphate batteries. This includes optimizing particle size and morphology, improving material synthesis methods, and enhancing electrode preparation techniques to create more uniform and defect-free battery components.
- Thermal management systems: Developing effective thermal management systems is crucial for maintaining the stability of lithium iron phosphate batteries. This involves designing cooling systems, implementing heat dissipation strategies, and optimizing battery pack configurations to prevent overheating and ensure consistent performance across various operating conditions.
- Battery management system optimization: Enhancing battery management systems (BMS) can significantly improve the stability and longevity of lithium iron phosphate batteries. This includes developing advanced algorithms for state-of-charge and state-of-health estimation, implementing more precise voltage and current control, and optimizing charging and discharging strategies to prevent overcharging and deep discharging.
02 Surface modification of cathode materials
Modifying the surface of lithium iron phosphate cathode materials can improve their stability and performance. Techniques such as coating, doping, or surface treatment can enhance the material's resistance to degradation, improve conductivity, and increase overall battery stability.Expand Specific Solutions03 Nanostructured electrode materials
Developing nanostructured lithium iron phosphate materials for electrodes can significantly improve battery stability. Nanostructures can enhance ion diffusion, increase surface area for reactions, and improve the material's structural integrity during charge-discharge cycles.Expand Specific Solutions04 Advanced battery management systems
Implementing sophisticated battery management systems can enhance the stability of lithium iron phosphate batteries. These systems can monitor and control various parameters such as temperature, voltage, and current to optimize battery performance and prevent conditions that could lead to instability or degradation.Expand Specific Solutions05 Thermal management strategies
Developing effective thermal management strategies is crucial for maintaining the stability of lithium iron phosphate batteries. This can include innovative cooling systems, heat dissipation techniques, or thermal insulation methods to prevent overheating and ensure optimal operating temperatures for enhanced stability and performance.Expand Specific Solutions
Key Players in LFP Battery Additive Research
The exploration of additives' effects on lithium iron phosphate battery stability is at a mature stage, with significant market growth driven by the increasing demand for electric vehicles and energy storage systems. The global market for lithium iron phosphate batteries is expanding rapidly, with key players like BYD Co., Ltd., Ningde Amperex Technology Ltd., and LG Energy Solution Ltd. leading the charge. These companies are investing heavily in research and development to enhance battery performance and stability through innovative additives. The technology's maturity is evident in the widespread adoption by major automotive manufacturers and energy storage providers, indicating a competitive landscape where companies are focusing on incremental improvements and cost reduction strategies to gain market share.
Ningde Amperex Technology Ltd.
Technical Solution: CATL has developed a novel additive strategy for lithium iron phosphate (LFP) batteries, focusing on electrolyte optimization. Their approach involves using a combination of fluoroethylene carbonate (FEC) and vinylene carbonate (VC) as electrolyte additives[1]. This formulation has shown to significantly enhance the formation of a stable solid electrolyte interphase (SEI) layer on the electrode surface, which is crucial for battery longevity and performance. The company has also explored the use of lithium difluoro(oxalato)borate (LiDFOB) as an additive, which has demonstrated improved cycling stability and rate capability in LFP cells[3]. CATL's research indicates that these additives can effectively mitigate the dissolution of iron from the cathode, a common issue in LFP batteries that leads to capacity fade.
Strengths: Enhanced SEI formation, improved cycling stability, and mitigation of iron dissolution. Weaknesses: Potential increase in production costs due to specialized additives, and possible trade-offs between different performance aspects (e.g., power vs. energy density).
BYD Co., Ltd.
Technical Solution: BYD has pioneered the Blade Battery technology, which incorporates innovative additives to enhance LFP battery stability. Their approach focuses on a proprietary electrolyte formulation that includes functional additives such as fluorinated compounds and boron-based materials[2]. These additives work synergistically to form a more robust and flexible SEI layer, which better accommodates the volume changes during cycling. BYD's research has shown that their additive package can significantly reduce gas generation within the cell, a common issue in LFP batteries that can lead to swelling and degradation[4]. Additionally, they have developed nano-scale carbon coating techniques for LFP particles, which, when combined with their electrolyte additives, result in improved electronic conductivity and rate performance.
Strengths: Enhanced safety due to reduced gas generation, improved rate performance, and better cycle life. Weaknesses: Potentially higher manufacturing complexity and costs associated with specialized additives and coating processes.
Core Innovations in LFP Battery Additives
Lithium supplement additive, preparation method therefor, and application thereof
PatentPendingEP4492502A1
Innovation
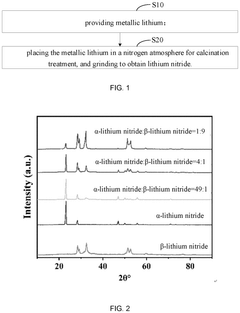
- A lithium-supplementing additive comprising pure α-phase, β-phase, or mixed-phase lithium nitride, prepared through calcination and grinding of metallic lithium in a nitrogen atmosphere, with a graphene coating to enhance stability and conductivity, is used to supplement lithium ions during the first charging cycle, ensuring a smooth voltage change and improved electrochemical performance.
A phosphazene derivative, composition, and an electrochemical device comprising the same
PatentPendingEP4446329A1
Innovation
- A phosphazene derivative with a specific structure is introduced as an electrolyte additive, which improves safety and cycle performance without increasing process complexity, using a composition that includes the derivative, a non-aqueous solvent, and common electrolytes, with optimized concentrations to enhance electrochemical stability and fire retardance.
Environmental Impact of LFP Battery Additives
The environmental impact of additives used in Lithium Iron Phosphate (LFP) batteries is a critical consideration in the ongoing development and widespread adoption of this energy storage technology. As the demand for LFP batteries continues to grow, particularly in electric vehicles and renewable energy systems, it is essential to assess the ecological footprint of these additives throughout their lifecycle.
Additives play a crucial role in enhancing the performance and stability of LFP batteries. However, their production, use, and disposal can have significant environmental implications. The manufacturing process of these additives often involves energy-intensive procedures and the use of potentially hazardous chemicals. This can lead to increased carbon emissions and the release of pollutants into the environment if not properly managed.
During the operational life of LFP batteries, the additives contribute to improved efficiency and longevity. This extended lifespan can indirectly reduce the environmental impact by decreasing the frequency of battery replacements and the associated resource consumption. However, the long-term effects of these additives on the environment, particularly in cases of battery leakage or improper disposal, remain a concern that requires ongoing research and monitoring.
The end-of-life phase of LFP batteries presents another set of environmental challenges related to additives. Recycling processes must be designed to effectively recover and safely handle these additives, preventing their release into ecosystems. Some additives may complicate the recycling process, potentially reducing the overall recyclability of LFP batteries if not addressed through innovative recycling technologies.
Water pollution is a specific area of concern regarding LFP battery additives. Certain additives, if released into aquatic environments, could have detrimental effects on marine life and water quality. This underscores the importance of proper containment and disposal practices throughout the battery lifecycle.
On the positive side, some additives are being developed with environmental sustainability in mind. These eco-friendly alternatives aim to maintain or improve battery performance while minimizing negative environmental impacts. Research into biodegradable additives and those derived from renewable sources is showing promise in reducing the ecological footprint of LFP batteries.
The regulatory landscape surrounding LFP battery additives is evolving to address these environmental concerns. Stricter guidelines for the production, use, and disposal of battery additives are being implemented in many regions. These regulations aim to promote the development of more environmentally benign additives and ensure responsible practices throughout the battery industry.
As the LFP battery market expands, ongoing research and development efforts are crucial to mitigate the environmental impact of additives. This includes exploring alternative materials, optimizing additive formulations for reduced toxicity, and improving recycling technologies. The goal is to strike a balance between enhancing battery performance and minimizing ecological harm, ensuring that the widespread adoption of LFP batteries contributes positively to global sustainability efforts.
Additives play a crucial role in enhancing the performance and stability of LFP batteries. However, their production, use, and disposal can have significant environmental implications. The manufacturing process of these additives often involves energy-intensive procedures and the use of potentially hazardous chemicals. This can lead to increased carbon emissions and the release of pollutants into the environment if not properly managed.
During the operational life of LFP batteries, the additives contribute to improved efficiency and longevity. This extended lifespan can indirectly reduce the environmental impact by decreasing the frequency of battery replacements and the associated resource consumption. However, the long-term effects of these additives on the environment, particularly in cases of battery leakage or improper disposal, remain a concern that requires ongoing research and monitoring.
The end-of-life phase of LFP batteries presents another set of environmental challenges related to additives. Recycling processes must be designed to effectively recover and safely handle these additives, preventing their release into ecosystems. Some additives may complicate the recycling process, potentially reducing the overall recyclability of LFP batteries if not addressed through innovative recycling technologies.
Water pollution is a specific area of concern regarding LFP battery additives. Certain additives, if released into aquatic environments, could have detrimental effects on marine life and water quality. This underscores the importance of proper containment and disposal practices throughout the battery lifecycle.
On the positive side, some additives are being developed with environmental sustainability in mind. These eco-friendly alternatives aim to maintain or improve battery performance while minimizing negative environmental impacts. Research into biodegradable additives and those derived from renewable sources is showing promise in reducing the ecological footprint of LFP batteries.
The regulatory landscape surrounding LFP battery additives is evolving to address these environmental concerns. Stricter guidelines for the production, use, and disposal of battery additives are being implemented in many regions. These regulations aim to promote the development of more environmentally benign additives and ensure responsible practices throughout the battery industry.
As the LFP battery market expands, ongoing research and development efforts are crucial to mitigate the environmental impact of additives. This includes exploring alternative materials, optimizing additive formulations for reduced toxicity, and improving recycling technologies. The goal is to strike a balance between enhancing battery performance and minimizing ecological harm, ensuring that the widespread adoption of LFP batteries contributes positively to global sustainability efforts.
Safety Regulations for LFP Battery Additives
Safety regulations for LFP battery additives play a crucial role in ensuring the stability and reliability of lithium iron phosphate (LFP) batteries. These regulations are designed to address potential risks associated with the use of additives and to maintain the overall safety of battery systems.
One of the primary concerns in LFP battery additive safety is the potential for thermal runaway. Regulatory bodies require manufacturers to conduct extensive thermal stability tests on batteries containing additives. These tests typically involve subjecting the batteries to extreme temperature conditions and monitoring their behavior. Additives must not compromise the thermal stability of the battery or increase the likelihood of thermal runaway events.
Chemical compatibility is another key aspect of safety regulations. Additives must be thoroughly evaluated for their interactions with other battery components, including the electrolyte, separator, and electrode materials. Regulatory standards often mandate long-term compatibility testing to ensure that additives do not degrade or react adversely with other battery materials over time.
Toxicity and environmental impact are also significant considerations in safety regulations. Additives must comply with strict guidelines regarding their potential health and environmental effects. This includes assessing the toxicity of the additives themselves, as well as any byproducts that may form during battery operation or disposal. Manufacturers are required to provide detailed safety data sheets and handling instructions for additives.
Performance impact is another area of regulatory scrutiny. While additives are often used to enhance battery performance, they must not compromise safety in the pursuit of improved efficiency. Regulatory bodies typically require extensive cycling tests and performance evaluations to ensure that additives do not negatively affect the battery's long-term stability or safety characteristics.
Transportation safety is a critical aspect of LFP battery additive regulations. Additives must comply with international transportation standards, such as those set by the United Nations for the transport of dangerous goods. This includes specific packaging requirements, labeling standards, and restrictions on the quantity of additives that can be transported.
Regulatory bodies also focus on quality control and manufacturing processes. Manufacturers must demonstrate robust quality assurance systems to ensure consistent production of safe additives. This often involves regular audits, batch testing, and documentation of manufacturing processes.
Lastly, safety regulations for LFP battery additives are continually evolving as new research emerges and technology advances. Manufacturers and researchers must stay informed about the latest regulatory updates and adapt their practices accordingly to maintain compliance and ensure the ongoing safety of LFP batteries.
One of the primary concerns in LFP battery additive safety is the potential for thermal runaway. Regulatory bodies require manufacturers to conduct extensive thermal stability tests on batteries containing additives. These tests typically involve subjecting the batteries to extreme temperature conditions and monitoring their behavior. Additives must not compromise the thermal stability of the battery or increase the likelihood of thermal runaway events.
Chemical compatibility is another key aspect of safety regulations. Additives must be thoroughly evaluated for their interactions with other battery components, including the electrolyte, separator, and electrode materials. Regulatory standards often mandate long-term compatibility testing to ensure that additives do not degrade or react adversely with other battery materials over time.
Toxicity and environmental impact are also significant considerations in safety regulations. Additives must comply with strict guidelines regarding their potential health and environmental effects. This includes assessing the toxicity of the additives themselves, as well as any byproducts that may form during battery operation or disposal. Manufacturers are required to provide detailed safety data sheets and handling instructions for additives.
Performance impact is another area of regulatory scrutiny. While additives are often used to enhance battery performance, they must not compromise safety in the pursuit of improved efficiency. Regulatory bodies typically require extensive cycling tests and performance evaluations to ensure that additives do not negatively affect the battery's long-term stability or safety characteristics.
Transportation safety is a critical aspect of LFP battery additive regulations. Additives must comply with international transportation standards, such as those set by the United Nations for the transport of dangerous goods. This includes specific packaging requirements, labeling standards, and restrictions on the quantity of additives that can be transported.
Regulatory bodies also focus on quality control and manufacturing processes. Manufacturers must demonstrate robust quality assurance systems to ensure consistent production of safe additives. This often involves regular audits, batch testing, and documentation of manufacturing processes.
Lastly, safety regulations for LFP battery additives are continually evolving as new research emerges and technology advances. Manufacturers and researchers must stay informed about the latest regulatory updates and adapt their practices accordingly to maintain compliance and ensure the ongoing safety of LFP batteries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!