Comparative study on ammonium hydroxide and aqueous ammonia for industrial application
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia Solutions Background and Objectives
Ammonia solutions, including ammonium hydroxide and aqueous ammonia, have played a crucial role in various industrial applications for over a century. These versatile compounds have been instrumental in the development of numerous sectors, including agriculture, pharmaceuticals, and chemical manufacturing. The evolution of ammonia solutions can be traced back to the early 20th century when the Haber-Bosch process revolutionized ammonia production, making it more accessible and cost-effective for industrial use.
The primary objective of this comparative study is to evaluate the efficacy, efficiency, and environmental impact of ammonium hydroxide and aqueous ammonia in industrial applications. By examining their chemical properties, production methods, and practical uses, we aim to provide a comprehensive understanding of these two ammonia solutions and their respective advantages and limitations in various industrial contexts.
Ammonium hydroxide, also known as ammonia water or ammonia solution, is a mixture of ammonia and water. It is typically produced by dissolving anhydrous ammonia gas in water, resulting in a solution with varying concentrations of ammonia. On the other hand, aqueous ammonia refers to a solution of ammonia in water, which can be produced through different methods, including the direct mixing of ammonia gas with water or the dissolution of ammonium salts.
The industrial applications of these ammonia solutions are diverse and far-reaching. In agriculture, they serve as essential components in fertilizer production, contributing to increased crop yields and food security. The pharmaceutical industry utilizes ammonia solutions in the synthesis of various drugs and as pH adjusters in manufacturing processes. In the chemical sector, these compounds are employed as cleaning agents, in the production of plastics, and as catalysts in numerous chemical reactions.
As environmental concerns and sustainability initiatives gain prominence, the industrial use of ammonia solutions has come under scrutiny. This study aims to explore the environmental impact of both ammonium hydroxide and aqueous ammonia, considering factors such as production emissions, transportation, and potential ecological effects. Additionally, we will investigate emerging technologies and alternative production methods that may enhance the sustainability of these ammonia solutions in industrial applications.
By conducting this comparative study, we seek to provide valuable insights into the optimal use of ammonium hydroxide and aqueous ammonia across different industrial sectors. The findings will contribute to informed decision-making processes for industries looking to optimize their ammonia solution usage, improve efficiency, and minimize environmental impact. Furthermore, this research will help identify potential areas for innovation and development in ammonia solution technologies, paving the way for more sustainable and effective industrial practices in the future.
The primary objective of this comparative study is to evaluate the efficacy, efficiency, and environmental impact of ammonium hydroxide and aqueous ammonia in industrial applications. By examining their chemical properties, production methods, and practical uses, we aim to provide a comprehensive understanding of these two ammonia solutions and their respective advantages and limitations in various industrial contexts.
Ammonium hydroxide, also known as ammonia water or ammonia solution, is a mixture of ammonia and water. It is typically produced by dissolving anhydrous ammonia gas in water, resulting in a solution with varying concentrations of ammonia. On the other hand, aqueous ammonia refers to a solution of ammonia in water, which can be produced through different methods, including the direct mixing of ammonia gas with water or the dissolution of ammonium salts.
The industrial applications of these ammonia solutions are diverse and far-reaching. In agriculture, they serve as essential components in fertilizer production, contributing to increased crop yields and food security. The pharmaceutical industry utilizes ammonia solutions in the synthesis of various drugs and as pH adjusters in manufacturing processes. In the chemical sector, these compounds are employed as cleaning agents, in the production of plastics, and as catalysts in numerous chemical reactions.
As environmental concerns and sustainability initiatives gain prominence, the industrial use of ammonia solutions has come under scrutiny. This study aims to explore the environmental impact of both ammonium hydroxide and aqueous ammonia, considering factors such as production emissions, transportation, and potential ecological effects. Additionally, we will investigate emerging technologies and alternative production methods that may enhance the sustainability of these ammonia solutions in industrial applications.
By conducting this comparative study, we seek to provide valuable insights into the optimal use of ammonium hydroxide and aqueous ammonia across different industrial sectors. The findings will contribute to informed decision-making processes for industries looking to optimize their ammonia solution usage, improve efficiency, and minimize environmental impact. Furthermore, this research will help identify potential areas for innovation and development in ammonia solution technologies, paving the way for more sustainable and effective industrial practices in the future.
Industrial Demand Analysis
The industrial demand for ammonium hydroxide and aqueous ammonia has been steadily growing due to their versatile applications across various sectors. These compounds play crucial roles in numerous industrial processes, driving their market demand and shaping industry trends.
In the agricultural sector, both ammonium hydroxide and aqueous ammonia are widely used as nitrogen fertilizers. The increasing global population and the subsequent need for higher crop yields have led to a surge in demand for these compounds. Farmers rely on these nitrogen-rich solutions to enhance soil fertility and promote plant growth, contributing significantly to the overall market demand.
The chemical industry represents another major consumer of ammonium hydroxide and aqueous ammonia. These compounds serve as essential raw materials in the production of various chemicals, including plastics, synthetic fibers, and pharmaceuticals. The expanding chemical manufacturing sector, particularly in emerging economies, has been a key driver of demand growth for these ammonia-based solutions.
Environmental applications have also contributed to the rising industrial demand for ammonium hydroxide and aqueous ammonia. These compounds are utilized in flue gas treatment systems to reduce nitrogen oxide emissions from power plants and industrial facilities. As environmental regulations become more stringent worldwide, the demand for these solutions in emission control applications is expected to increase further.
The semiconductor industry relies on high-purity ammonium hydroxide for cleaning and etching processes in the production of electronic components. With the ongoing digital transformation and the growing demand for electronic devices, this sector has become a significant consumer of ultra-pure ammonium hydroxide, driving market growth in the high-tech segment.
In the mining industry, aqueous ammonia is used in the extraction of various metals, particularly in the leaching process of copper, nickel, and uranium ores. The global mining sector's expansion and the increasing demand for metals in manufacturing and construction have contributed to the growing industrial demand for aqueous ammonia.
The textile industry utilizes ammonium hydroxide in dyeing processes and as a neutralizing agent. The growth of the global textile market, driven by fast fashion and increasing disposable incomes in developing countries, has led to a rise in demand for ammonia-based solutions in this sector.
Market analysis indicates that the Asia-Pacific region dominates the industrial demand for ammonium hydroxide and aqueous ammonia, primarily due to its robust agricultural sector and rapidly expanding industrial base. North America and Europe follow, with significant consumption in chemical manufacturing and environmental applications.
Future market trends suggest a continued growth in demand, driven by emerging applications in renewable energy storage, where ammonia is being explored as a potential hydrogen carrier. Additionally, the development of green ammonia production methods is expected to open new market opportunities and potentially reshape the industrial landscape for these compounds.
In the agricultural sector, both ammonium hydroxide and aqueous ammonia are widely used as nitrogen fertilizers. The increasing global population and the subsequent need for higher crop yields have led to a surge in demand for these compounds. Farmers rely on these nitrogen-rich solutions to enhance soil fertility and promote plant growth, contributing significantly to the overall market demand.
The chemical industry represents another major consumer of ammonium hydroxide and aqueous ammonia. These compounds serve as essential raw materials in the production of various chemicals, including plastics, synthetic fibers, and pharmaceuticals. The expanding chemical manufacturing sector, particularly in emerging economies, has been a key driver of demand growth for these ammonia-based solutions.
Environmental applications have also contributed to the rising industrial demand for ammonium hydroxide and aqueous ammonia. These compounds are utilized in flue gas treatment systems to reduce nitrogen oxide emissions from power plants and industrial facilities. As environmental regulations become more stringent worldwide, the demand for these solutions in emission control applications is expected to increase further.
The semiconductor industry relies on high-purity ammonium hydroxide for cleaning and etching processes in the production of electronic components. With the ongoing digital transformation and the growing demand for electronic devices, this sector has become a significant consumer of ultra-pure ammonium hydroxide, driving market growth in the high-tech segment.
In the mining industry, aqueous ammonia is used in the extraction of various metals, particularly in the leaching process of copper, nickel, and uranium ores. The global mining sector's expansion and the increasing demand for metals in manufacturing and construction have contributed to the growing industrial demand for aqueous ammonia.
The textile industry utilizes ammonium hydroxide in dyeing processes and as a neutralizing agent. The growth of the global textile market, driven by fast fashion and increasing disposable incomes in developing countries, has led to a rise in demand for ammonia-based solutions in this sector.
Market analysis indicates that the Asia-Pacific region dominates the industrial demand for ammonium hydroxide and aqueous ammonia, primarily due to its robust agricultural sector and rapidly expanding industrial base. North America and Europe follow, with significant consumption in chemical manufacturing and environmental applications.
Future market trends suggest a continued growth in demand, driven by emerging applications in renewable energy storage, where ammonia is being explored as a potential hydrogen carrier. Additionally, the development of green ammonia production methods is expected to open new market opportunities and potentially reshape the industrial landscape for these compounds.
Technical Challenges and Limitations
The comparative study of ammonium hydroxide and aqueous ammonia for industrial applications reveals several technical challenges and limitations that need to be addressed. One of the primary concerns is the stability of these compounds during storage and transportation. Both substances are prone to decomposition, especially at higher temperatures, which can lead to pressure build-up in containers and potential safety hazards.
The corrosive nature of ammonium hydroxide and aqueous ammonia poses significant challenges in material selection for storage tanks, pipelines, and processing equipment. This necessitates the use of specialized materials that can withstand prolonged exposure, increasing overall costs and maintenance requirements. Additionally, the volatility of ammonia presents difficulties in handling and containment, requiring robust safety measures and specialized ventilation systems to prevent worker exposure and environmental contamination.
Another technical limitation lies in the concentration control of these solutions. Maintaining precise concentrations is crucial for many industrial processes, but the tendency of ammonia to evaporate can lead to fluctuations in concentration over time. This variability can impact process efficiency and product quality, necessitating frequent monitoring and adjustment.
The environmental impact of ammonia emissions is a growing concern in industrial applications. Both ammonium hydroxide and aqueous ammonia can contribute to air pollution and eutrophication of water bodies if not properly managed. Developing effective emission control technologies and implementing stringent handling protocols are ongoing challenges for industries utilizing these compounds.
In terms of reactivity, while the high alkalinity of these solutions is beneficial for many applications, it can also lead to unwanted side reactions in certain processes. This reactivity can result in product contamination or reduced efficiency, requiring careful process design and control measures. Furthermore, the potential for ammonia to form explosive mixtures with air under certain conditions presents additional safety challenges that must be carefully managed in industrial settings.
The energy-intensive nature of ammonia production, primarily through the Haber-Bosch process, presents sustainability challenges. Efforts to develop more energy-efficient and environmentally friendly production methods are ongoing but have yet to achieve widespread industrial implementation. This limitation impacts the overall sustainability and cost-effectiveness of ammonium hydroxide and aqueous ammonia in various applications.
Lastly, regulatory compliance and safety standards for handling and using these compounds are becoming increasingly stringent. Industries must navigate complex regulatory landscapes, which can vary significantly across different regions, adding to the operational challenges and costs associated with their use.
The corrosive nature of ammonium hydroxide and aqueous ammonia poses significant challenges in material selection for storage tanks, pipelines, and processing equipment. This necessitates the use of specialized materials that can withstand prolonged exposure, increasing overall costs and maintenance requirements. Additionally, the volatility of ammonia presents difficulties in handling and containment, requiring robust safety measures and specialized ventilation systems to prevent worker exposure and environmental contamination.
Another technical limitation lies in the concentration control of these solutions. Maintaining precise concentrations is crucial for many industrial processes, but the tendency of ammonia to evaporate can lead to fluctuations in concentration over time. This variability can impact process efficiency and product quality, necessitating frequent monitoring and adjustment.
The environmental impact of ammonia emissions is a growing concern in industrial applications. Both ammonium hydroxide and aqueous ammonia can contribute to air pollution and eutrophication of water bodies if not properly managed. Developing effective emission control technologies and implementing stringent handling protocols are ongoing challenges for industries utilizing these compounds.
In terms of reactivity, while the high alkalinity of these solutions is beneficial for many applications, it can also lead to unwanted side reactions in certain processes. This reactivity can result in product contamination or reduced efficiency, requiring careful process design and control measures. Furthermore, the potential for ammonia to form explosive mixtures with air under certain conditions presents additional safety challenges that must be carefully managed in industrial settings.
The energy-intensive nature of ammonia production, primarily through the Haber-Bosch process, presents sustainability challenges. Efforts to develop more energy-efficient and environmentally friendly production methods are ongoing but have yet to achieve widespread industrial implementation. This limitation impacts the overall sustainability and cost-effectiveness of ammonium hydroxide and aqueous ammonia in various applications.
Lastly, regulatory compliance and safety standards for handling and using these compounds are becoming increasingly stringent. Industries must navigate complex regulatory landscapes, which can vary significantly across different regions, adding to the operational challenges and costs associated with their use.
Current Application Methods
01 Chemical properties and applications
Ammonium hydroxide and aqueous ammonia are essentially the same substance, consisting of ammonia dissolved in water. They are alkaline solutions with various industrial and household applications, including cleaning, neutralization of acids, and as a source of nitrogen in chemical processes.- Chemical properties and applications: Ammonium hydroxide and aqueous ammonia are essentially the same substance, consisting of ammonia dissolved in water. They are widely used in various industrial processes, including cleaning, neutralization, and as a source of nitrogen. The solution is alkaline and can react with acids to form ammonium salts.
- Production methods: Various methods are employed to produce ammonium hydroxide and aqueous ammonia. These include the reaction of ammonia gas with water, as well as the recovery of ammonia from industrial processes. Some techniques involve the use of specialized equipment to control the concentration and purity of the resulting solution.
- Environmental and safety considerations: The use and handling of ammonium hydroxide and aqueous ammonia require careful attention to environmental and safety protocols. This includes proper storage, transportation, and disposal methods to prevent environmental contamination and ensure worker safety. Specialized equipment and procedures are often necessary to manage the potential hazards associated with these substances.
- Industrial applications: Ammonium hydroxide and aqueous ammonia find extensive use in various industries. They are employed in the production of fertilizers, textiles, and pharmaceuticals. Additionally, these substances play a role in water treatment, metal processing, and as cleaning agents in industrial settings. Their versatility makes them valuable in numerous manufacturing processes.
- Analytical methods and quality control: Accurate analysis and quality control of ammonium hydroxide and aqueous ammonia solutions are crucial for their effective use. Various analytical techniques are employed to determine concentration, purity, and other important parameters. These methods may include titration, spectroscopy, and chromatography, ensuring that the solutions meet the required specifications for different applications.
02 Use in semiconductor manufacturing
Ammonium hydroxide is widely used in the semiconductor industry for cleaning and etching processes. It is often combined with hydrogen peroxide and water to form a cleaning solution known as SC-1 or RCA-1, which is effective in removing organic contaminants and particles from silicon wafers.Expand Specific Solutions03 Environmental and safety considerations
Handling and storage of ammonium hydroxide require careful attention to safety measures due to its corrosive nature and potential to release ammonia gas. Proper ventilation, personal protective equipment, and spill containment procedures are essential when working with this chemical.Expand Specific Solutions04 Production methods
Aqueous ammonia can be produced through various methods, including the reaction of ammonia gas with water, the Haber-Bosch process followed by dissolution in water, or as a byproduct of other industrial processes. The concentration of ammonia in the solution can be adjusted for different applications.Expand Specific Solutions05 Analytical and purification techniques
Various analytical methods are used to determine the concentration and purity of ammonium hydroxide solutions. These include titration, spectrophotometry, and ion chromatography. Purification techniques may involve distillation or ion exchange processes to remove impurities and achieve the desired concentration.Expand Specific Solutions
Key Industry Players
The comparative study of ammonium hydroxide and aqueous ammonia for industrial applications is situated in a mature market with established players. The industry is in a stable growth phase, driven by diverse applications in sectors such as agriculture, chemicals, and water treatment. Key players like FMC Corp., BASF, and DuPont de Nemours, Inc. have advanced the technology, leading to high technical maturity. These companies, along with research institutions like MIT and CSIR, continue to innovate, focusing on improving efficiency and environmental sustainability. The market size is substantial, with global demand for ammonia-based products steadily increasing, particularly in emerging economies.
FMC Corp.
Technical Solution: FMC Corp. has developed a proprietary technology for the production of aqueous ammonia focused on industrial applications. Their process utilizes a novel absorption system that allows for the efficient dissolution of gaseous ammonia into water, resulting in high-concentration aqueous ammonia solutions. FMC's technology incorporates advanced heat exchange systems to manage the exothermic reaction, ensuring optimal temperature control and product stability. The company has also implemented a sophisticated purification process to remove potential contaminants, resulting in a high-purity product suitable for various industrial applications[2]. FMC's system is designed with modularity in mind, allowing for scalable production capacities to meet diverse industrial needs[4].
Strengths: Scalable production, high-purity product, efficient absorption process. Weaknesses: Potential safety concerns due to high-pressure systems, energy-intensive cooling requirements.
SABIC Global Technologies BV
Technical Solution: SABIC has developed an advanced process for the production of both ammonium hydroxide and aqueous ammonia, focusing on large-scale industrial applications. Their technology utilizes a dual-stream approach, allowing for simultaneous production of both chemicals. SABIC's process incorporates a novel catalytic system that enhances the reaction efficiency between ammonia and water, resulting in higher yields and reduced energy consumption. The company has also implemented advanced process control systems, including real-time monitoring and predictive maintenance, to ensure consistent product quality and minimize downtime[6]. SABIC's technology includes a sophisticated waste heat recovery system, significantly improving overall energy efficiency[8].
Strengths: Dual-product capability, high energy efficiency, advanced process control. Weaknesses: Complex system design, high initial capital investment.
Core Technologies and Patents
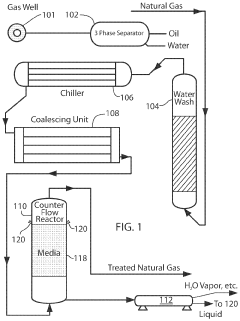
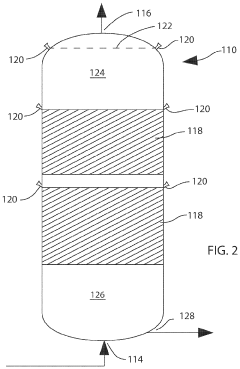
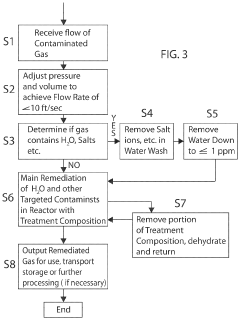
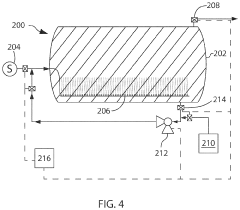
Chemical compositions and treatment systems and treatment methods using same for remediating h2s and other contaminants in fluids, including liquids,gasses and mixtures thereof
PatentActiveUS20210230487A1
Innovation
- A novel treatment system and composition that involves a water wash flow cell to remove Na and Cl ions, dehydration of the gas to reduce water content, and a counter-flow reactor with a non-reactive media to regulate gas flow and ensure sufficient contact with a concentrated aqueous hydroxide solution, organic acids, and chelating agents, which effectively remediate H2S and CO2 without generating precipitates.
Synthesis of amonia by NANO catalytic process
PatentInactiveIN1226CHE2014A
Innovation
- The method involves using zeolites to separate nitrogen from air and manganese dioxide catalysts for hydrogen production from water, followed by purification and subsequent reaction with nano catalysts like osmium, ruthenium, and nickel at reduced temperatures and pressures to synthesize ammonia with high efficiency.
Environmental Impact Assessment
The environmental impact assessment of ammonium hydroxide and aqueous ammonia for industrial applications is a critical consideration in their comparative study. Both compounds have significant environmental implications that must be carefully evaluated.
Ammonium hydroxide, when released into the environment, can have detrimental effects on aquatic ecosystems. It can cause pH changes in water bodies, potentially leading to fish kills and disruption of aquatic flora. The compound's high solubility in water means it can easily contaminate groundwater sources if not properly managed. Additionally, ammonium hydroxide can contribute to eutrophication in water bodies, promoting excessive algal growth and oxygen depletion.
Aqueous ammonia, while similar in composition, presents slightly different environmental challenges. Its volatility means it can easily evaporate into the atmosphere, contributing to air pollution. Ammonia in the air can react with other pollutants to form fine particulate matter, which has negative impacts on air quality and human health. Moreover, atmospheric ammonia can be deposited back onto land and water surfaces through precipitation, contributing to soil acidification and nitrogen loading in ecosystems.
Both compounds have the potential to impact soil quality when released. They can alter soil pH, affecting plant growth and microbial communities. Excessive nitrogen input from these compounds can lead to imbalances in soil nutrient composition, potentially harming native plant species and promoting the growth of invasive plants.
In terms of greenhouse gas emissions, the production and use of both compounds can contribute to climate change. The manufacturing processes often involve energy-intensive steps, resulting in carbon dioxide emissions. Furthermore, ammonia itself is a potent greenhouse gas, and its release into the atmosphere can have direct climate impacts.
Occupational health and safety considerations are also crucial in the environmental assessment. Both compounds pose risks to workers through inhalation or skin contact, necessitating strict safety protocols and protective equipment in industrial settings.
Waste management and disposal of these compounds require careful attention to prevent environmental contamination. Proper treatment and neutralization processes must be implemented to mitigate potential harm to ecosystems and human health.
In conclusion, while both ammonium hydroxide and aqueous ammonia have important industrial applications, their environmental impacts are significant and multifaceted. A comprehensive environmental impact assessment must consider their effects on air, water, soil, and ecosystems, as well as their contributions to climate change and potential risks to human health. This assessment is crucial for developing sustainable industrial practices and implementing effective mitigation strategies.
Ammonium hydroxide, when released into the environment, can have detrimental effects on aquatic ecosystems. It can cause pH changes in water bodies, potentially leading to fish kills and disruption of aquatic flora. The compound's high solubility in water means it can easily contaminate groundwater sources if not properly managed. Additionally, ammonium hydroxide can contribute to eutrophication in water bodies, promoting excessive algal growth and oxygen depletion.
Aqueous ammonia, while similar in composition, presents slightly different environmental challenges. Its volatility means it can easily evaporate into the atmosphere, contributing to air pollution. Ammonia in the air can react with other pollutants to form fine particulate matter, which has negative impacts on air quality and human health. Moreover, atmospheric ammonia can be deposited back onto land and water surfaces through precipitation, contributing to soil acidification and nitrogen loading in ecosystems.
Both compounds have the potential to impact soil quality when released. They can alter soil pH, affecting plant growth and microbial communities. Excessive nitrogen input from these compounds can lead to imbalances in soil nutrient composition, potentially harming native plant species and promoting the growth of invasive plants.
In terms of greenhouse gas emissions, the production and use of both compounds can contribute to climate change. The manufacturing processes often involve energy-intensive steps, resulting in carbon dioxide emissions. Furthermore, ammonia itself is a potent greenhouse gas, and its release into the atmosphere can have direct climate impacts.
Occupational health and safety considerations are also crucial in the environmental assessment. Both compounds pose risks to workers through inhalation or skin contact, necessitating strict safety protocols and protective equipment in industrial settings.
Waste management and disposal of these compounds require careful attention to prevent environmental contamination. Proper treatment and neutralization processes must be implemented to mitigate potential harm to ecosystems and human health.
In conclusion, while both ammonium hydroxide and aqueous ammonia have important industrial applications, their environmental impacts are significant and multifaceted. A comprehensive environmental impact assessment must consider their effects on air, water, soil, and ecosystems, as well as their contributions to climate change and potential risks to human health. This assessment is crucial for developing sustainable industrial practices and implementing effective mitigation strategies.
Safety and Handling Protocols
Safety and handling protocols are crucial aspects when comparing ammonium hydroxide and aqueous ammonia for industrial applications. Both substances require careful management due to their corrosive and toxic nature.
For ammonium hydroxide, proper ventilation is essential in storage and handling areas to prevent the accumulation of ammonia vapors. Workers must wear appropriate personal protective equipment (PPE), including chemical-resistant gloves, goggles, and respiratory protection. Storage tanks should be equipped with pressure relief valves to prevent over-pressurization.
Aqueous ammonia demands similar precautions, with additional emphasis on containment measures. Double-walled tanks or secondary containment systems are recommended to mitigate the risk of leaks. Regular inspections of storage and transfer equipment are necessary to detect potential issues early.
Both substances require specific emergency response procedures. Eyewash stations and safety showers must be readily accessible in areas where these chemicals are handled. Spill response kits should be available, and personnel must be trained in their proper use. In case of a release, immediate evacuation of the affected area is crucial, followed by proper ventilation before re-entry.
Temperature control is critical for both substances. Ammonium hydroxide and aqueous ammonia should be stored in cool, well-ventilated areas away from direct sunlight and heat sources. This helps maintain stability and reduces the risk of pressure build-up in storage containers.
Transportation of these chemicals requires adherence to specific regulations. Proper labeling, documentation, and use of approved containers are mandatory. Drivers transporting these substances must be trained in hazardous materials handling and emergency response procedures.
Regular safety training for all personnel involved in handling these chemicals is essential. This should cover proper use of PPE, safe handling techniques, emergency procedures, and first aid measures. Maintaining up-to-date safety data sheets (SDS) and ensuring their accessibility to all relevant personnel is also crucial.
Implementing a comprehensive monitoring system for both substances is advisable. This may include ammonia detectors in storage and handling areas, as well as regular air quality checks to ensure worker safety and environmental compliance.
When comparing the two, aqueous ammonia generally presents a lower risk profile due to its lower vapor pressure, potentially making it easier to handle in some industrial settings. However, both substances require stringent safety protocols, and the choice between them should consider the specific requirements of the industrial application and the facility's ability to implement necessary safety measures.
For ammonium hydroxide, proper ventilation is essential in storage and handling areas to prevent the accumulation of ammonia vapors. Workers must wear appropriate personal protective equipment (PPE), including chemical-resistant gloves, goggles, and respiratory protection. Storage tanks should be equipped with pressure relief valves to prevent over-pressurization.
Aqueous ammonia demands similar precautions, with additional emphasis on containment measures. Double-walled tanks or secondary containment systems are recommended to mitigate the risk of leaks. Regular inspections of storage and transfer equipment are necessary to detect potential issues early.
Both substances require specific emergency response procedures. Eyewash stations and safety showers must be readily accessible in areas where these chemicals are handled. Spill response kits should be available, and personnel must be trained in their proper use. In case of a release, immediate evacuation of the affected area is crucial, followed by proper ventilation before re-entry.
Temperature control is critical for both substances. Ammonium hydroxide and aqueous ammonia should be stored in cool, well-ventilated areas away from direct sunlight and heat sources. This helps maintain stability and reduces the risk of pressure build-up in storage containers.
Transportation of these chemicals requires adherence to specific regulations. Proper labeling, documentation, and use of approved containers are mandatory. Drivers transporting these substances must be trained in hazardous materials handling and emergency response procedures.
Regular safety training for all personnel involved in handling these chemicals is essential. This should cover proper use of PPE, safe handling techniques, emergency procedures, and first aid measures. Maintaining up-to-date safety data sheets (SDS) and ensuring their accessibility to all relevant personnel is also crucial.
Implementing a comprehensive monitoring system for both substances is advisable. This may include ammonia detectors in storage and handling areas, as well as regular air quality checks to ensure worker safety and environmental compliance.
When comparing the two, aqueous ammonia generally presents a lower risk profile due to its lower vapor pressure, potentially making it easier to handle in some industrial settings. However, both substances require stringent safety protocols, and the choice between them should consider the specific requirements of the industrial application and the facility's ability to implement necessary safety measures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!