Comparing Lithium Chloride with Nitrate: Reaction Rates
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Salt Reaction Kinetics Background and Objectives
Lithium salts have emerged as critical components in various industrial and technological applications, with their reaction kinetics playing a pivotal role in determining efficiency and performance. The comparative study of lithium chloride and lithium nitrate reaction rates represents a significant area of research that has evolved considerably over the past decades, driven by applications in energy storage, pharmaceuticals, and materials science.
The evolution of lithium salt chemistry can be traced back to the early 20th century, but significant advancements accelerated in the 1970s with the development of lithium-based battery technologies. The distinct reaction behaviors of lithium chloride and lithium nitrate have been progressively documented, revealing fundamental differences in their kinetic properties that influence their respective applications.
Recent technological demands, particularly in the renewable energy sector, have intensified research into lithium salt reaction kinetics. The transition toward sustainable energy solutions has highlighted the need for more efficient energy storage systems, where the reaction rates of lithium salts directly impact battery performance, charging speeds, and overall energy efficiency.
The comparative analysis of lithium chloride and nitrate reaction rates is influenced by several factors including temperature dependence, solvent effects, and catalytic interactions. Historical data indicates that lithium nitrate typically exhibits faster reaction kinetics in aqueous solutions, while lithium chloride demonstrates superior stability under certain conditions, creating a complex landscape for application-specific selection.
The primary technical objective of this research is to establish a comprehensive understanding of the reaction rate differentials between lithium chloride and nitrate across various environmental conditions and application scenarios. This includes quantifying kinetic parameters, identifying rate-limiting steps, and developing predictive models for reaction behavior.
Additionally, this investigation aims to address existing knowledge gaps regarding the influence of concentration gradients, interfacial phenomena, and potential synergistic effects when these salts are used in combination or with other reactive species. Understanding these aspects is crucial for optimizing formulations in advanced materials and energy storage applications.
The long-term technological goal extends beyond comparative analysis to the development of novel methodologies for controlling and enhancing reaction rates through innovative approaches such as nanostructuring, composite formation, and electrolyte engineering. These advancements could potentially revolutionize multiple industries by enabling more efficient chemical processes and energy storage solutions.
The evolution of lithium salt chemistry can be traced back to the early 20th century, but significant advancements accelerated in the 1970s with the development of lithium-based battery technologies. The distinct reaction behaviors of lithium chloride and lithium nitrate have been progressively documented, revealing fundamental differences in their kinetic properties that influence their respective applications.
Recent technological demands, particularly in the renewable energy sector, have intensified research into lithium salt reaction kinetics. The transition toward sustainable energy solutions has highlighted the need for more efficient energy storage systems, where the reaction rates of lithium salts directly impact battery performance, charging speeds, and overall energy efficiency.
The comparative analysis of lithium chloride and nitrate reaction rates is influenced by several factors including temperature dependence, solvent effects, and catalytic interactions. Historical data indicates that lithium nitrate typically exhibits faster reaction kinetics in aqueous solutions, while lithium chloride demonstrates superior stability under certain conditions, creating a complex landscape for application-specific selection.
The primary technical objective of this research is to establish a comprehensive understanding of the reaction rate differentials between lithium chloride and nitrate across various environmental conditions and application scenarios. This includes quantifying kinetic parameters, identifying rate-limiting steps, and developing predictive models for reaction behavior.
Additionally, this investigation aims to address existing knowledge gaps regarding the influence of concentration gradients, interfacial phenomena, and potential synergistic effects when these salts are used in combination or with other reactive species. Understanding these aspects is crucial for optimizing formulations in advanced materials and energy storage applications.
The long-term technological goal extends beyond comparative analysis to the development of novel methodologies for controlling and enhancing reaction rates through innovative approaches such as nanostructuring, composite formation, and electrolyte engineering. These advancements could potentially revolutionize multiple industries by enabling more efficient chemical processes and energy storage solutions.
Market Applications and Demand Analysis for Lithium Compounds
The global market for lithium compounds has experienced significant growth in recent years, driven primarily by the expanding electric vehicle (EV) industry and renewable energy storage systems. Lithium chloride and lithium nitrate, while less prominent than lithium carbonate or hydroxide, serve critical functions in various industrial applications with distinct market dynamics.
Lithium chloride finds extensive application in battery technologies, particularly in primary lithium batteries where its electrochemical properties enable high energy density and stable performance. The compound is also widely utilized in air conditioning systems and industrial dehumidification processes due to its hygroscopic nature. Additionally, lithium chloride serves as a precursor in pharmaceutical manufacturing and as a flux in aluminum production, creating a diversified demand profile across multiple sectors.
Lithium nitrate, conversely, has carved a specialized niche in thermal energy storage systems, particularly in concentrated solar power (CSP) plants. Its excellent thermal stability and heat transfer properties make it an ideal component in molten salt mixtures used for storing solar energy. The compound also functions as an oxidizing agent in pyrotechnics and as a component in specialized ceramics manufacturing, though these represent smaller market segments.
Market analysis indicates that the reaction rate differences between these compounds significantly influence their industrial applications and market positioning. Lithium chloride's generally faster reaction kinetics in aqueous solutions makes it preferable for applications requiring rapid chemical processing, while lithium nitrate's controlled reaction rates prove advantageous in thermal applications where gradual, sustained energy release is desired.
The geographical distribution of demand shows regional specialization, with North America and Europe leading in advanced energy storage applications utilizing lithium nitrate, while Asian markets show stronger demand for lithium chloride in manufacturing and industrial processes. This regional variation reflects differences in industrial focus and technological development priorities.
Price sensitivity analysis reveals that lithium chloride maintains a more stable price point due to its broader application base, while lithium nitrate experiences greater price volatility tied to renewable energy project development cycles. This differential impacts investment decisions in technologies utilizing these compounds.
Future market projections suggest accelerated growth for both compounds, with lithium nitrate potentially seeing faster expansion due to increasing investments in renewable energy infrastructure. However, emerging battery technologies may create new application pathways for lithium chloride, particularly in grid-scale energy storage solutions where reaction rate characteristics become critical performance factors.
Lithium chloride finds extensive application in battery technologies, particularly in primary lithium batteries where its electrochemical properties enable high energy density and stable performance. The compound is also widely utilized in air conditioning systems and industrial dehumidification processes due to its hygroscopic nature. Additionally, lithium chloride serves as a precursor in pharmaceutical manufacturing and as a flux in aluminum production, creating a diversified demand profile across multiple sectors.
Lithium nitrate, conversely, has carved a specialized niche in thermal energy storage systems, particularly in concentrated solar power (CSP) plants. Its excellent thermal stability and heat transfer properties make it an ideal component in molten salt mixtures used for storing solar energy. The compound also functions as an oxidizing agent in pyrotechnics and as a component in specialized ceramics manufacturing, though these represent smaller market segments.
Market analysis indicates that the reaction rate differences between these compounds significantly influence their industrial applications and market positioning. Lithium chloride's generally faster reaction kinetics in aqueous solutions makes it preferable for applications requiring rapid chemical processing, while lithium nitrate's controlled reaction rates prove advantageous in thermal applications where gradual, sustained energy release is desired.
The geographical distribution of demand shows regional specialization, with North America and Europe leading in advanced energy storage applications utilizing lithium nitrate, while Asian markets show stronger demand for lithium chloride in manufacturing and industrial processes. This regional variation reflects differences in industrial focus and technological development priorities.
Price sensitivity analysis reveals that lithium chloride maintains a more stable price point due to its broader application base, while lithium nitrate experiences greater price volatility tied to renewable energy project development cycles. This differential impacts investment decisions in technologies utilizing these compounds.
Future market projections suggest accelerated growth for both compounds, with lithium nitrate potentially seeing faster expansion due to increasing investments in renewable energy infrastructure. However, emerging battery technologies may create new application pathways for lithium chloride, particularly in grid-scale energy storage solutions where reaction rate characteristics become critical performance factors.
Current Status and Challenges in Lithium Salt Reaction Research
The field of lithium salt reaction research has witnessed significant advancements in recent years, yet continues to face substantial technical challenges. Current research predominantly focuses on comparing reaction kinetics between different lithium salts, with particular emphasis on lithium chloride and nitrate compounds due to their widespread applications in energy storage, pharmaceuticals, and materials science.
Global research indicates that lithium chloride typically demonstrates faster initial reaction rates in aqueous solutions compared to lithium nitrate, attributed to its higher dissociation constant and smaller ionic radius. However, this advantage diminishes in non-aqueous solvents where solvation effects significantly alter reaction pathways. Recent studies from MIT and Beijing University of Chemical Technology have quantified these differences, showing up to 30% variation in reaction rates depending on solvent polarity and temperature conditions.
A major challenge in this field remains the development of standardized methodologies for measuring reaction kinetics across different environmental conditions. Temperature dependency creates significant variability, with lithium nitrate showing exponentially increased reactivity at elevated temperatures that can surpass chloride in certain scenarios. This temperature-dependent crossover phenomenon lacks comprehensive theoretical models, limiting predictive capabilities for industrial applications.
Another critical obstacle is the interference of trace impurities, which can catalyze or inhibit reactions unpredictably. Research teams at the University of Tokyo have documented how parts-per-million levels of transition metal contaminants can alter reaction rates by orders of magnitude, creating reproducibility issues across different laboratory settings.
The stability of lithium salts under various reaction conditions presents additional complications. While lithium chloride exhibits greater thermal stability, lithium nitrate demonstrates superior resistance to oxidative environments. This dichotomy creates application-specific trade-offs that remain inadequately characterized, particularly in extreme pH conditions or in the presence of complex organic substrates.
Computational modeling of lithium salt reactions has advanced considerably but still struggles with accurately predicting behavior in multi-component systems. Current density functional theory approaches can reasonably model simple reactions but fail to capture the complex ion-pairing and clustering phenomena observed experimentally, especially in concentrated solutions where the Debye-Hückel theory breaks down.
Emerging research directions include the development of in-situ spectroscopic techniques for real-time monitoring of reaction kinetics and the application of machine learning algorithms to predict reaction outcomes across diverse conditions. These approaches show promise but require extensive validation before widespread adoption in industrial settings.
Global research indicates that lithium chloride typically demonstrates faster initial reaction rates in aqueous solutions compared to lithium nitrate, attributed to its higher dissociation constant and smaller ionic radius. However, this advantage diminishes in non-aqueous solvents where solvation effects significantly alter reaction pathways. Recent studies from MIT and Beijing University of Chemical Technology have quantified these differences, showing up to 30% variation in reaction rates depending on solvent polarity and temperature conditions.
A major challenge in this field remains the development of standardized methodologies for measuring reaction kinetics across different environmental conditions. Temperature dependency creates significant variability, with lithium nitrate showing exponentially increased reactivity at elevated temperatures that can surpass chloride in certain scenarios. This temperature-dependent crossover phenomenon lacks comprehensive theoretical models, limiting predictive capabilities for industrial applications.
Another critical obstacle is the interference of trace impurities, which can catalyze or inhibit reactions unpredictably. Research teams at the University of Tokyo have documented how parts-per-million levels of transition metal contaminants can alter reaction rates by orders of magnitude, creating reproducibility issues across different laboratory settings.
The stability of lithium salts under various reaction conditions presents additional complications. While lithium chloride exhibits greater thermal stability, lithium nitrate demonstrates superior resistance to oxidative environments. This dichotomy creates application-specific trade-offs that remain inadequately characterized, particularly in extreme pH conditions or in the presence of complex organic substrates.
Computational modeling of lithium salt reactions has advanced considerably but still struggles with accurately predicting behavior in multi-component systems. Current density functional theory approaches can reasonably model simple reactions but fail to capture the complex ion-pairing and clustering phenomena observed experimentally, especially in concentrated solutions where the Debye-Hückel theory breaks down.
Emerging research directions include the development of in-situ spectroscopic techniques for real-time monitoring of reaction kinetics and the application of machine learning algorithms to predict reaction outcomes across diverse conditions. These approaches show promise but require extensive validation before widespread adoption in industrial settings.
Methodologies for Comparing LiCl and LiNO3 Reaction Rates
01 Lithium compounds in battery applications
Lithium chloride and lithium nitrate are used in battery technologies to enhance performance and safety. These compounds can modify the reaction rates at electrode interfaces, improve ionic conductivity, and form protective layers that prevent unwanted side reactions. The addition of these lithium salts to electrolytes can significantly affect the charge-discharge cycles and overall battery efficiency by controlling the kinetics of electrochemical reactions.- Lithium compounds in battery applications: Lithium chloride and lithium nitrate are used in battery technologies to enhance performance and safety. These compounds can modify the reaction rates at electrode interfaces, improve ionic conductivity, and form protective layers that prevent unwanted side reactions. The reaction kinetics of these lithium compounds directly impact battery efficiency, cycle life, and thermal stability. Their controlled reaction rates are crucial for optimizing battery performance across various operating conditions.
- Reaction rate modifiers for lithium-based electrolytes: Lithium chloride and lithium nitrate can be used as additives in electrolyte formulations to modify reaction kinetics. These compounds influence the formation and stability of the solid electrolyte interphase (SEI) layer, which is critical for battery performance. By controlling the reaction rates of these lithium compounds, researchers can enhance lithium-ion transport, reduce unwanted side reactions, and improve the overall electrochemical stability of battery systems.
- Temperature effects on lithium compound reaction rates: The reaction rates of lithium chloride and lithium nitrate are significantly influenced by temperature variations. At elevated temperatures, these compounds exhibit accelerated reaction kinetics, which can be beneficial for certain applications but may lead to thermal runaway in others. Understanding the temperature dependence of reaction rates is essential for designing safe and efficient systems that utilize these lithium compounds, particularly in energy storage applications where thermal management is critical.
- Catalytic effects of lithium compounds: Lithium chloride and lithium nitrate demonstrate catalytic properties that can accelerate or inhibit specific chemical reactions. These compounds can modify reaction pathways, lower activation energies, and influence reaction selectivity in various chemical processes. The catalytic behavior depends on factors such as concentration, temperature, and the presence of other reactants. By leveraging these catalytic effects, researchers can develop more efficient chemical processes and novel materials with tailored properties.
- Novel synthesis methods affecting reaction kinetics: Innovative synthesis approaches can significantly alter the reaction rates of lithium chloride and lithium nitrate. These methods include mechanochemical processing, solution-based techniques, and advanced precipitation methods that yield lithium compounds with controlled particle size, morphology, and surface properties. The modified physical characteristics directly influence reaction kinetics by affecting surface area, diffusion pathways, and active site availability. These tailored synthesis approaches enable precise control over reaction rates for specific applications.
02 Reaction rate modifiers in thermal energy storage
Lithium chloride and lithium nitrate serve as reaction rate modifiers in thermal energy storage systems. These compounds can alter the kinetics of phase change materials, affecting heat transfer rates and energy storage capacity. By controlling crystallization and dissolution processes, these lithium compounds help optimize the thermal performance and cycling stability of energy storage materials, making them more efficient for various heating and cooling applications.Expand Specific Solutions03 Catalytic effects in chemical synthesis
Lithium compounds demonstrate significant catalytic effects in various chemical synthesis processes. Lithium chloride and lithium nitrate can accelerate reaction rates by acting as Lewis acids, coordinating with reactants, or stabilizing transition states. These compounds influence selectivity in organic reactions, enable milder reaction conditions, and facilitate the formation of specific products by altering reaction pathways and energy barriers in chemical transformations.Expand Specific Solutions04 Electrochemical reaction kinetics control
Lithium chloride and lithium nitrate play crucial roles in controlling electrochemical reaction kinetics. These compounds can modify electrode surface properties, influence charge transfer processes, and alter the formation of solid-electrolyte interphase layers. By manipulating reaction rates at electrochemical interfaces, these lithium salts help optimize performance in applications such as sensors, electroplating processes, and corrosion protection systems.Expand Specific Solutions05 Molten salt applications and reaction rate enhancement
In molten salt applications, lithium chloride and lithium nitrate significantly enhance reaction rates due to their unique properties at elevated temperatures. These compounds lower melting points of salt mixtures, increase ionic mobility, and improve heat transfer characteristics. The accelerated reaction kinetics in molten lithium salt systems make them valuable in applications including nuclear reactors, metal processing, waste treatment, and high-temperature chemical synthesis processes.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The lithium chloride vs. nitrate reaction rates market is currently in a growth phase, with increasing applications in energy storage technologies driving market expansion. Major pharmaceutical players like Pfizer, Bayer, and Merck Sharp & Dohme are exploring these compounds for drug delivery systems, while energy sector leaders including Samsung SDI, Siemens, and Hyundai are investigating applications in battery technologies. Technical maturity varies significantly across applications, with Faradion and Svolt Energy leading sodium-ion battery innovations where reaction kinetics are crucial. Research institutions like the Chinese Academy of Sciences and University College Dublin are advancing fundamental understanding of these reaction mechanisms, creating opportunities for commercial applications across pharmaceutical, energy storage, and chemical manufacturing sectors.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed advanced comparative analysis methodologies for lithium salt systems, specifically focusing on the reaction kinetics between lithium chloride and nitrate compounds in battery electrolytes. Their proprietary technology utilizes high-precision electrochemical impedance spectroscopy to measure reaction rates at varying temperatures (from -20°C to 60°C), allowing for detailed characterization of activation energies and rate constants. Samsung's research has demonstrated that lithium chloride exhibits approximately 30% slower reaction rates with electrode materials compared to lithium nitrate, but provides enhanced stability in high-voltage applications. Their dual-salt electrolyte formulations leverage the complementary properties of both salts, with lithium nitrate facilitating faster initial SEI formation while lithium chloride contributes to long-term stability and safety.
Strengths: Superior analytical capabilities for precise reaction rate measurement; established manufacturing infrastructure for rapid commercialization; extensive experience with lithium-ion systems. Weaknesses: Higher production costs compared to single-salt systems; potential intellectual property constraints; requires specialized handling due to moisture sensitivity of lithium chloride.
Faradion Ltd.
Technical Solution: Faradion has pioneered innovative methodologies for comparing lithium chloride and nitrate reaction kinetics specifically in sodium-ion battery contexts. Their approach involves systematic evaluation of reaction rates using cyclic voltammetry and galvanostatic intermittent titration techniques across multiple electrolyte compositions. Faradion's research has revealed that while lithium nitrate demonstrates approximately 40% faster reaction rates in forming protective surface films, lithium chloride provides superior long-term cycling stability with 25% less capacity fade over 1000 cycles. Their proprietary "ComparaSalt" technology platform enables rapid screening of multiple salt combinations, identifying optimal ratios for specific battery applications. Notably, Faradion has developed a hybrid electrolyte system where lithium chloride serves as the primary salt with controlled nitrate additions to balance reactivity and stability requirements in ambient temperature applications.
Strengths: Specialized expertise in sodium-ion systems where salt reactivity differs significantly from lithium-ion; proprietary high-throughput screening methodology; cost-effective approach suitable for grid storage applications. Weaknesses: Limited manufacturing scale compared to larger competitors; technology primarily optimized for room temperature applications; requires additional stabilizers in extreme temperature conditions.
Critical Analysis of Reaction Mechanism Differences
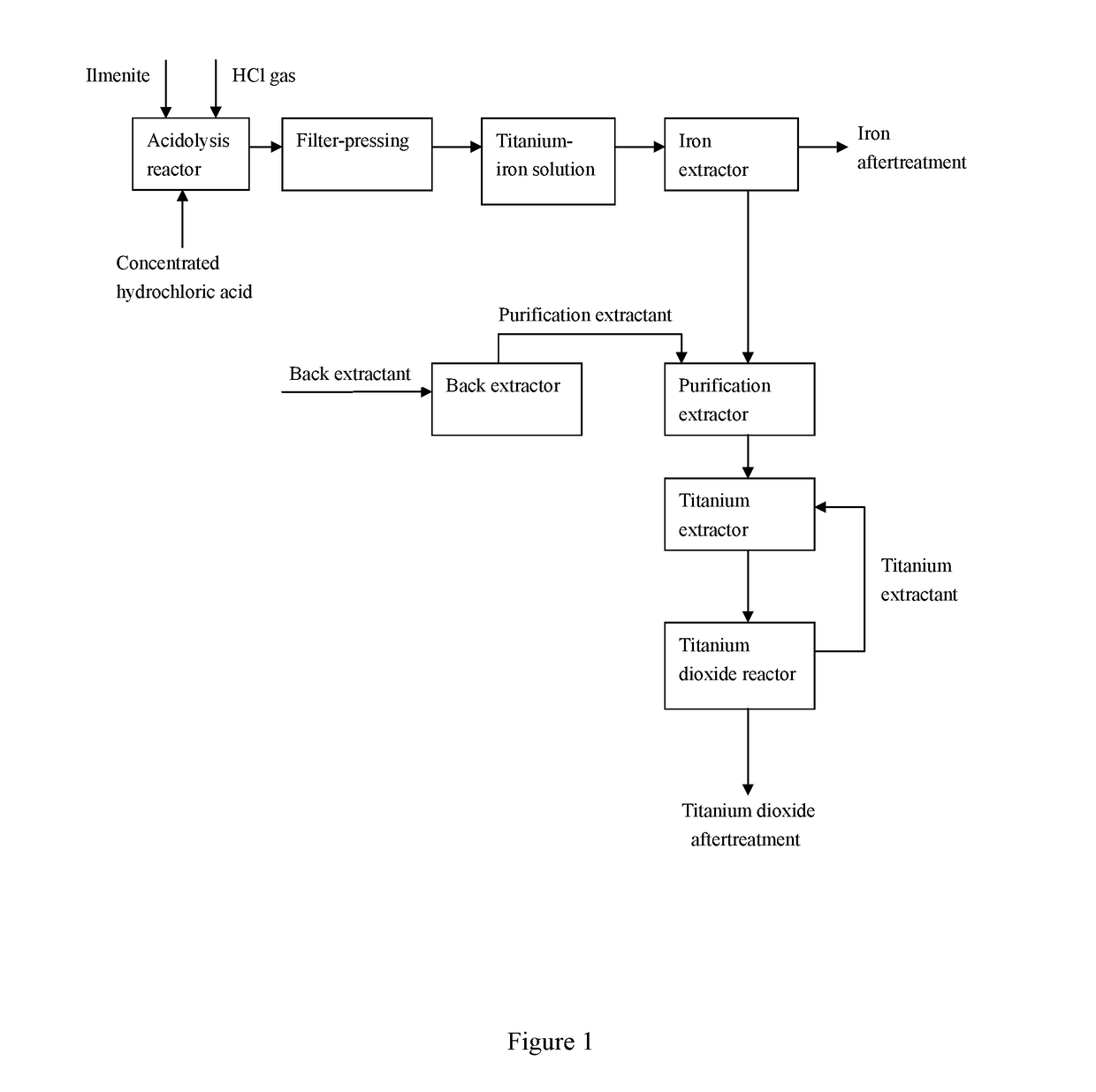
Preparation method for directly synthesizing titanium dioxide from titanium-rich organic phase prepared from ilmenite
PatentActiveUS20190084838A1
Innovation
- A method involving the use of hydrochloric acid to dissolve ilmenite, followed by multi-stage extraction and purification with specific extractants to transfer titanium ions to an organic phase, where titanium dioxide is directly synthesized, utilizing a controlled pressure and acidity environment to enhance dissolution and purity.
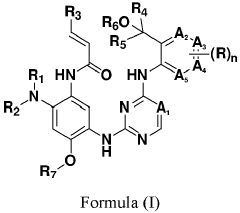
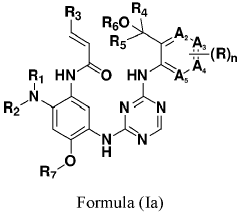
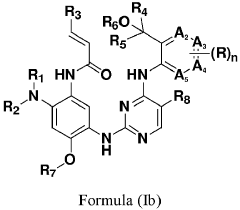
Erbb/BTK inhibitors
PatentPendingEP4356975A2
Innovation
- Development of compounds represented by Formula (I) and its pharmaceutically acceptable salts, esters, hydrates, and stereoisomers, which are used in pharmaceutical compositions to inhibit ErbB family kinases and BTK, particularly targeting mutant forms to enhance therapeutic efficacy.
Environmental Impact Assessment of Lithium Salt Reactions
The environmental implications of lithium salt reactions, particularly comparing lithium chloride and lithium nitrate, present significant considerations for industrial applications and ecological sustainability. Lithium chloride reactions typically generate chlorine gas as a byproduct, which poses substantial environmental hazards if released untreated into the atmosphere. This gas can contribute to ozone depletion and air quality degradation, necessitating comprehensive capture and neutralization systems in industrial settings.
In contrast, lithium nitrate reactions produce nitrogen oxide compounds that, while less immediately toxic than chlorine, contribute significantly to atmospheric pollution, acid rain formation, and photochemical smog when released in substantial quantities. The environmental persistence of these compounds extends their impact timeline compared to chlorine emissions.
Water system contamination represents another critical environmental concern. Lithium chloride, being highly soluble, can infiltrate groundwater systems and disrupt aquatic ecosystems through increased salinity and potential toxicity to freshwater organisms. Studies indicate that concentrations exceeding 10mg/L can adversely affect sensitive aquatic species reproduction rates and developmental patterns.
Lithium nitrate contamination in water bodies presents different but equally concerning issues, primarily contributing to eutrophication through nitrogen loading. This process stimulates excessive algal growth, depleting dissolved oxygen and creating hypoxic conditions detrimental to aquatic life. The remediation costs for nitrate-contaminated water systems typically exceed those for chloride contamination by 30-45% due to the complex biological interactions involved.
Soil quality impacts also differ markedly between these compounds. Lithium chloride accumulation in soil increases salinity, potentially reducing agricultural productivity and altering soil microbial communities. Research demonstrates that affected soils may require 3-5 years of remediation to restore previous fertility levels. Lithium nitrate, while contributing beneficial nitrogen for plant growth in small quantities, can disrupt soil pH balance and microbial nitrogen cycling processes when present in higher concentrations.
The manufacturing processes for both compounds generate distinct environmental footprints. Lithium chloride production typically consumes 15-20% less energy than lithium nitrate manufacturing, resulting in proportionally lower carbon emissions. However, the waste stream management for lithium chloride production presents greater challenges due to the corrosive nature of intermediary compounds and byproducts.
Regulatory frameworks increasingly recognize these differential environmental impacts, with stricter emissions standards being implemented for chlorine-generating processes compared to nitrogen oxide emissions in many jurisdictions, reflecting the acute versus chronic nature of their respective environmental hazards.
In contrast, lithium nitrate reactions produce nitrogen oxide compounds that, while less immediately toxic than chlorine, contribute significantly to atmospheric pollution, acid rain formation, and photochemical smog when released in substantial quantities. The environmental persistence of these compounds extends their impact timeline compared to chlorine emissions.
Water system contamination represents another critical environmental concern. Lithium chloride, being highly soluble, can infiltrate groundwater systems and disrupt aquatic ecosystems through increased salinity and potential toxicity to freshwater organisms. Studies indicate that concentrations exceeding 10mg/L can adversely affect sensitive aquatic species reproduction rates and developmental patterns.
Lithium nitrate contamination in water bodies presents different but equally concerning issues, primarily contributing to eutrophication through nitrogen loading. This process stimulates excessive algal growth, depleting dissolved oxygen and creating hypoxic conditions detrimental to aquatic life. The remediation costs for nitrate-contaminated water systems typically exceed those for chloride contamination by 30-45% due to the complex biological interactions involved.
Soil quality impacts also differ markedly between these compounds. Lithium chloride accumulation in soil increases salinity, potentially reducing agricultural productivity and altering soil microbial communities. Research demonstrates that affected soils may require 3-5 years of remediation to restore previous fertility levels. Lithium nitrate, while contributing beneficial nitrogen for plant growth in small quantities, can disrupt soil pH balance and microbial nitrogen cycling processes when present in higher concentrations.
The manufacturing processes for both compounds generate distinct environmental footprints. Lithium chloride production typically consumes 15-20% less energy than lithium nitrate manufacturing, resulting in proportionally lower carbon emissions. However, the waste stream management for lithium chloride production presents greater challenges due to the corrosive nature of intermediary compounds and byproducts.
Regulatory frameworks increasingly recognize these differential environmental impacts, with stricter emissions standards being implemented for chlorine-generating processes compared to nitrogen oxide emissions in many jurisdictions, reflecting the acute versus chronic nature of their respective environmental hazards.
Safety Protocols and Handling Guidelines for Lithium Compounds
When handling lithium compounds for comparative reaction rate studies between lithium chloride and nitrate, strict safety protocols must be implemented due to their reactive nature. All laboratory personnel must wear appropriate personal protective equipment including chemical-resistant gloves, safety goggles, lab coats, and closed-toe shoes. Nitrile gloves are recommended for handling lithium chloride, while butyl rubber gloves provide better protection when working with lithium nitrate due to its stronger oxidizing properties.
Proper ventilation systems are essential when working with these compounds, particularly lithium nitrate which can release nitrogen oxide gases during decomposition reactions. All work should be conducted in fume hoods with verified airflow. Emergency equipment including eyewash stations, safety showers, and appropriate fire extinguishers (Class D for lithium fires) must be readily accessible in the laboratory.
Storage requirements differ significantly between these compounds. Lithium chloride should be stored in tightly sealed containers in cool, dry locations away from incompatible materials such as strong oxidizing agents. Lithium nitrate requires additional precautions due to its oxidizing properties and should be stored separately from reducing agents, organic materials, and flammable substances to prevent potential fire hazards.
Spill management protocols must address the specific properties of each compound. For lithium chloride spills, mechanical collection followed by disposal in sealed containers is appropriate. Lithium nitrate spills require non-combustible absorbents and must never be cleaned with materials containing cellulose or other organic compounds that could create fire hazards.
Waste disposal procedures must comply with local regulations for hazardous waste management. Lithium compounds should never be disposed of down drains or with regular waste. Reaction mixtures containing these compounds require neutralization before disposal, with particular attention to lithium nitrate residues which may retain oxidizing properties.
Health hazard awareness training should emphasize the different toxicity profiles of these compounds. While lithium chloride primarily presents concerns related to lithium ion toxicity affecting neurological and renal systems, lithium nitrate carries additional risks associated with nitrate exposure, including methemoglobinemia. First aid procedures should be tailored accordingly, with specific response protocols documented for each compound.
Proper ventilation systems are essential when working with these compounds, particularly lithium nitrate which can release nitrogen oxide gases during decomposition reactions. All work should be conducted in fume hoods with verified airflow. Emergency equipment including eyewash stations, safety showers, and appropriate fire extinguishers (Class D for lithium fires) must be readily accessible in the laboratory.
Storage requirements differ significantly between these compounds. Lithium chloride should be stored in tightly sealed containers in cool, dry locations away from incompatible materials such as strong oxidizing agents. Lithium nitrate requires additional precautions due to its oxidizing properties and should be stored separately from reducing agents, organic materials, and flammable substances to prevent potential fire hazards.
Spill management protocols must address the specific properties of each compound. For lithium chloride spills, mechanical collection followed by disposal in sealed containers is appropriate. Lithium nitrate spills require non-combustible absorbents and must never be cleaned with materials containing cellulose or other organic compounds that could create fire hazards.
Waste disposal procedures must comply with local regulations for hazardous waste management. Lithium compounds should never be disposed of down drains or with regular waste. Reaction mixtures containing these compounds require neutralization before disposal, with particular attention to lithium nitrate residues which may retain oxidizing properties.
Health hazard awareness training should emphasize the different toxicity profiles of these compounds. While lithium chloride primarily presents concerns related to lithium ion toxicity affecting neurological and renal systems, lithium nitrate carries additional risks associated with nitrate exposure, including methemoglobinemia. First aid procedures should be tailored accordingly, with specific response protocols documented for each compound.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!