Computational Modeling of Muscimol and GABA Receptor Binding
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol-GABA Binding Modeling Background
Computational modeling of muscimol and GABA receptor binding represents a critical area of research in neuroscience and pharmacology. This field has evolved significantly over the past few decades, driven by advancements in computational power, molecular biology, and structural biology techniques. The primary goal of these modeling efforts is to understand the intricate interactions between ligands like muscimol and the GABA receptors at a molecular level.
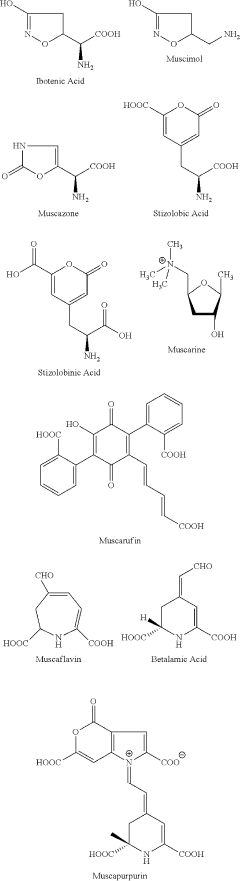
GABA (γ-aminobutyric acid) is the principal inhibitory neurotransmitter in the mammalian central nervous system, playing a crucial role in regulating neuronal excitability. GABA receptors, particularly the GABA-A receptors, are the primary targets for many clinically important drugs, including benzodiazepines, barbiturates, and certain anesthetics. Muscimol, a potent GABA-A receptor agonist derived from the mushroom Amanita muscaria, has been extensively used as a tool compound in neuropharmacological research.
The development of computational models for muscimol-GABA receptor binding has been driven by the need to understand the structural basis of ligand recognition, predict binding affinities, and design novel therapeutic agents. Early models were based on pharmacophore approaches, which identified key structural features necessary for ligand binding. As computational resources improved, more sophisticated methods emerged, including molecular docking, molecular dynamics simulations, and quantum mechanical calculations.
A significant milestone in this field was the determination of high-resolution crystal structures of GABA-A receptors, which provided crucial insights into the receptor architecture and binding sites. These structures have served as templates for homology modeling and have greatly enhanced the accuracy of computational predictions. The integration of experimental data from site-directed mutagenesis, electrophysiology, and ligand binding assays has further refined these models.
Recent advancements in artificial intelligence and machine learning have opened new avenues for modeling muscimol-GABA receptor interactions. These approaches can process vast amounts of experimental data to identify subtle patterns in binding behavior and predict the effects of structural modifications on ligand affinity and efficacy. Furthermore, the development of hybrid quantum mechanics/molecular mechanics (QM/MM) methods has allowed for more accurate modeling of the electronic interactions involved in ligand binding.
The ongoing research in this area aims to address several key challenges, including the accurate prediction of ligand binding modes, the incorporation of receptor dynamics and conformational changes, and the modeling of allosteric interactions. As computational methods continue to evolve, they promise to provide increasingly accurate and predictive models of muscimol-GABA receptor binding, facilitating drug discovery efforts and deepening our understanding of neural signaling mechanisms.
GABA (γ-aminobutyric acid) is the principal inhibitory neurotransmitter in the mammalian central nervous system, playing a crucial role in regulating neuronal excitability. GABA receptors, particularly the GABA-A receptors, are the primary targets for many clinically important drugs, including benzodiazepines, barbiturates, and certain anesthetics. Muscimol, a potent GABA-A receptor agonist derived from the mushroom Amanita muscaria, has been extensively used as a tool compound in neuropharmacological research.
The development of computational models for muscimol-GABA receptor binding has been driven by the need to understand the structural basis of ligand recognition, predict binding affinities, and design novel therapeutic agents. Early models were based on pharmacophore approaches, which identified key structural features necessary for ligand binding. As computational resources improved, more sophisticated methods emerged, including molecular docking, molecular dynamics simulations, and quantum mechanical calculations.
A significant milestone in this field was the determination of high-resolution crystal structures of GABA-A receptors, which provided crucial insights into the receptor architecture and binding sites. These structures have served as templates for homology modeling and have greatly enhanced the accuracy of computational predictions. The integration of experimental data from site-directed mutagenesis, electrophysiology, and ligand binding assays has further refined these models.
Recent advancements in artificial intelligence and machine learning have opened new avenues for modeling muscimol-GABA receptor interactions. These approaches can process vast amounts of experimental data to identify subtle patterns in binding behavior and predict the effects of structural modifications on ligand affinity and efficacy. Furthermore, the development of hybrid quantum mechanics/molecular mechanics (QM/MM) methods has allowed for more accurate modeling of the electronic interactions involved in ligand binding.
The ongoing research in this area aims to address several key challenges, including the accurate prediction of ligand binding modes, the incorporation of receptor dynamics and conformational changes, and the modeling of allosteric interactions. As computational methods continue to evolve, they promise to provide increasingly accurate and predictive models of muscimol-GABA receptor binding, facilitating drug discovery efforts and deepening our understanding of neural signaling mechanisms.
Neuropharmacology Market Analysis
The neuropharmacology market, particularly in relation to GABA receptor-targeted therapies, has shown significant growth and potential in recent years. This market segment is driven by the increasing prevalence of neurological disorders, such as anxiety, epilepsy, and insomnia, which are often treated with GABA receptor modulators. The global neuropharmacology market size was valued at over $30 billion in 2020, with GABA-related therapies accounting for a substantial portion of this value.
The demand for novel GABA receptor-targeted drugs has been steadily rising, fueled by the limitations of current treatments and the need for more effective, safer alternatives. This has led to increased research and development efforts in computational modeling of muscimol and GABA receptor binding, as pharmaceutical companies seek to develop more selective and potent compounds.
Market trends indicate a shift towards personalized medicine in neuropharmacology, with a focus on developing drugs that can be tailored to individual patient profiles. This trend aligns well with the advancements in computational modeling techniques, which allow for more precise predictions of drug-receptor interactions and potential side effects.
The competitive landscape in this market segment is characterized by a mix of established pharmaceutical giants and emerging biotech companies. Key players include Pfizer, Novartis, and Roche, who have significant investments in neuropharmacology research. Additionally, several smaller companies specializing in computational drug design have entered the market, offering innovative approaches to GABA receptor targeting.
Geographically, North America dominates the neuropharmacology market, followed by Europe and Asia-Pacific. However, emerging markets in Asia and Latin America are expected to show rapid growth in the coming years, driven by improving healthcare infrastructure and increasing awareness of neurological disorders.
The market for GABA receptor modulators is expected to grow at a CAGR of 6-8% over the next five years, with a particular emphasis on drugs targeting specific GABA receptor subtypes. This growth is supported by ongoing clinical trials and the potential for new drug approvals in the near future.
Challenges in the market include stringent regulatory requirements, high costs associated with drug development, and the complexity of neurological disorders. However, the integration of artificial intelligence and machine learning in computational modeling is expected to accelerate drug discovery processes and potentially reduce development costs.
In conclusion, the neuropharmacology market, especially in relation to GABA receptor-targeted therapies, presents significant opportunities for growth and innovation. The advancement of computational modeling techniques for muscimol and GABA receptor binding is likely to play a crucial role in shaping the future of this market segment.
The demand for novel GABA receptor-targeted drugs has been steadily rising, fueled by the limitations of current treatments and the need for more effective, safer alternatives. This has led to increased research and development efforts in computational modeling of muscimol and GABA receptor binding, as pharmaceutical companies seek to develop more selective and potent compounds.
Market trends indicate a shift towards personalized medicine in neuropharmacology, with a focus on developing drugs that can be tailored to individual patient profiles. This trend aligns well with the advancements in computational modeling techniques, which allow for more precise predictions of drug-receptor interactions and potential side effects.
The competitive landscape in this market segment is characterized by a mix of established pharmaceutical giants and emerging biotech companies. Key players include Pfizer, Novartis, and Roche, who have significant investments in neuropharmacology research. Additionally, several smaller companies specializing in computational drug design have entered the market, offering innovative approaches to GABA receptor targeting.
Geographically, North America dominates the neuropharmacology market, followed by Europe and Asia-Pacific. However, emerging markets in Asia and Latin America are expected to show rapid growth in the coming years, driven by improving healthcare infrastructure and increasing awareness of neurological disorders.
The market for GABA receptor modulators is expected to grow at a CAGR of 6-8% over the next five years, with a particular emphasis on drugs targeting specific GABA receptor subtypes. This growth is supported by ongoing clinical trials and the potential for new drug approvals in the near future.
Challenges in the market include stringent regulatory requirements, high costs associated with drug development, and the complexity of neurological disorders. However, the integration of artificial intelligence and machine learning in computational modeling is expected to accelerate drug discovery processes and potentially reduce development costs.
In conclusion, the neuropharmacology market, especially in relation to GABA receptor-targeted therapies, presents significant opportunities for growth and innovation. The advancement of computational modeling techniques for muscimol and GABA receptor binding is likely to play a crucial role in shaping the future of this market segment.
Current Challenges in Receptor Binding Simulations
Computational modeling of receptor binding, particularly for muscimol and GABA receptors, faces several significant challenges that hinder accurate simulations and predictions. One of the primary obstacles is the complexity of receptor structures and their dynamic nature. GABA receptors, being ligand-gated ion channels, undergo conformational changes upon binding, which are difficult to capture in static models.
The heterogeneity of receptor subtypes further complicates modeling efforts. GABA receptors exist in various subunit compositions, each with distinct binding properties and functional characteristics. Accurately representing this diversity in computational models requires extensive parameterization and validation, which can be both time-consuming and computationally expensive.
Another challenge lies in the accurate representation of the binding site microenvironment. The local electrostatic fields, hydration patterns, and flexibility of the binding pocket significantly influence ligand-receptor interactions. Current force fields and scoring functions often struggle to capture these subtle effects, leading to discrepancies between computational predictions and experimental observations.
The treatment of water molecules in binding simulations presents an ongoing challenge. Water plays a crucial role in mediating ligand-receptor interactions, but its explicit inclusion in simulations dramatically increases computational costs. Balancing accuracy and efficiency in water modeling remains a key area of research.
Accounting for the flexibility of both the receptor and ligand during binding events is another significant hurdle. Traditional docking approaches often treat the receptor as rigid or only partially flexible, which may not accurately reflect the induced-fit mechanisms observed in muscimol and GABA binding. Advanced techniques like molecular dynamics simulations can address this issue but at the cost of increased computational demands.
The integration of experimental data into computational models poses its own set of challenges. While experimental structures provide valuable starting points, they often represent single snapshots of dynamic processes. Reconciling computational models with diverse experimental data, including binding affinities, kinetics, and structural information from various sources, requires sophisticated data integration and validation strategies.
Lastly, the development of accurate scoring functions for predicting binding affinities remains a critical challenge. Current scoring functions often struggle to account for entropic contributions, desolvation effects, and subtle electronic interactions that can significantly impact binding energetics. Improving these scoring functions is essential for enhancing the predictive power of computational models in muscimol and GABA receptor binding studies.
The heterogeneity of receptor subtypes further complicates modeling efforts. GABA receptors exist in various subunit compositions, each with distinct binding properties and functional characteristics. Accurately representing this diversity in computational models requires extensive parameterization and validation, which can be both time-consuming and computationally expensive.
Another challenge lies in the accurate representation of the binding site microenvironment. The local electrostatic fields, hydration patterns, and flexibility of the binding pocket significantly influence ligand-receptor interactions. Current force fields and scoring functions often struggle to capture these subtle effects, leading to discrepancies between computational predictions and experimental observations.
The treatment of water molecules in binding simulations presents an ongoing challenge. Water plays a crucial role in mediating ligand-receptor interactions, but its explicit inclusion in simulations dramatically increases computational costs. Balancing accuracy and efficiency in water modeling remains a key area of research.
Accounting for the flexibility of both the receptor and ligand during binding events is another significant hurdle. Traditional docking approaches often treat the receptor as rigid or only partially flexible, which may not accurately reflect the induced-fit mechanisms observed in muscimol and GABA binding. Advanced techniques like molecular dynamics simulations can address this issue but at the cost of increased computational demands.
The integration of experimental data into computational models poses its own set of challenges. While experimental structures provide valuable starting points, they often represent single snapshots of dynamic processes. Reconciling computational models with diverse experimental data, including binding affinities, kinetics, and structural information from various sources, requires sophisticated data integration and validation strategies.
Lastly, the development of accurate scoring functions for predicting binding affinities remains a critical challenge. Current scoring functions often struggle to account for entropic contributions, desolvation effects, and subtle electronic interactions that can significantly impact binding energetics. Improving these scoring functions is essential for enhancing the predictive power of computational models in muscimol and GABA receptor binding studies.
State-of-the-Art Binding Simulation Techniques
01 Muscimol binding to GABA receptors
Muscimol, a psychoactive compound found in certain mushrooms, acts as a potent agonist of GABA receptors. It binds to these receptors with high affinity, mimicking the effects of the neurotransmitter GABA. This binding leads to increased inhibitory neurotransmission in the central nervous system, resulting in various physiological and behavioral effects.- Muscimol binding to GABA receptors: Muscimol, a psychoactive compound found in certain mushrooms, acts as a potent GABA receptor agonist. It binds to GABA-A receptors with high affinity, mimicking the effects of the neurotransmitter GABA. This binding leads to increased inhibitory neurotransmission in the central nervous system, resulting in various physiological and behavioral effects.
- GABA receptor binding assays and screening methods: Various assays and screening methods have been developed to study GABA receptor binding, including muscimol binding. These techniques involve the use of radiolabeled ligands, fluorescent probes, or other molecular tools to measure binding affinity and characterize receptor-ligand interactions. Such methods are crucial for drug discovery and understanding the pharmacology of GABA receptor modulators.
- Therapeutic applications of muscimol and GABA receptor modulators: Research into muscimol and other GABA receptor modulators has led to potential therapeutic applications for various neurological and psychiatric disorders. These compounds may be useful in treating conditions such as anxiety, epilepsy, insomnia, and neurodegenerative diseases. The development of novel GABA receptor agonists and antagonists aims to improve efficacy and reduce side effects compared to existing treatments.
- Structure-activity relationships of muscimol analogs: Studies on the structure-activity relationships of muscimol and its analogs have provided insights into the molecular determinants of GABA receptor binding and activation. Modifications to the muscimol structure have led to the development of compounds with altered potency, selectivity, and pharmacokinetic properties. This research contributes to the design of novel GABA receptor ligands with improved therapeutic potential.
- GABA receptor subtype selectivity: Research has focused on developing compounds with selectivity for specific GABA receptor subtypes. This approach aims to target particular receptor populations to achieve desired therapeutic effects while minimizing unwanted side effects. Understanding the binding properties of muscimol and related compounds at different GABA receptor subtypes contributes to the development of more selective and effective drugs.
02 GABA receptor subtypes and muscimol selectivity
Research has focused on the selectivity of muscimol for different GABA receptor subtypes. Studies have shown that muscimol has varying affinities for GABA-A and GABA-C receptors, with potential implications for drug development targeting specific receptor subtypes. Understanding these binding preferences is crucial for developing more targeted therapeutic approaches.Expand Specific Solutions03 Muscimol analogs and GABA receptor binding
Researchers have developed and studied various muscimol analogs to investigate their binding properties to GABA receptors. These analogs may exhibit different binding affinities, selectivities, or pharmacological profiles compared to muscimol. Such studies contribute to the understanding of structure-activity relationships and aid in the design of novel GABA receptor ligands.Expand Specific Solutions04 Imaging and detection of muscimol-GABA receptor interactions
Advanced imaging techniques and detection methods have been developed to study the interactions between muscimol and GABA receptors. These methods include radioligand binding assays, fluorescence-based techniques, and electrophysiological measurements. Such tools enable researchers to visualize and quantify the binding of muscimol to GABA receptors in various experimental settings.Expand Specific Solutions05 Therapeutic applications of muscimol-GABA receptor binding
The understanding of muscimol's binding to GABA receptors has led to investigations into potential therapeutic applications. These include the development of treatments for neurological disorders, anxiety, sleep disorders, and other conditions associated with GABAergic dysfunction. Research in this area aims to harness the properties of muscimol or its analogs for clinical benefit.Expand Specific Solutions
Key Players in Molecular Modeling Software
The computational modeling of Muscimol and GABA receptor binding is an emerging field at the intersection of neuroscience and computational chemistry. The market is in its early growth stage, with increasing interest from pharmaceutical companies due to its potential in drug discovery for neurological disorders. Key players like F. Hoffmann-La Roche, Pfizer, and Janssen Pharmaceutica are investing in this technology, leveraging their expertise in neuropharmacology. The market size is expanding, driven by the growing demand for novel CNS therapeutics. While the technology is still evolving, recent advancements in computational power and machine learning algorithms are accelerating its maturity, promising more accurate predictions of drug-receptor interactions and potentially revolutionizing the development of GABA-targeted therapies.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed advanced computational models for GABA receptor binding, utilizing machine learning algorithms and molecular dynamics simulations. Their approach combines high-throughput virtual screening with structure-based drug design to identify novel GABA receptor modulators. The company employs quantum mechanical calculations to accurately predict binding affinities and optimize lead compounds[1]. Roche's platform integrates experimental data from electrophysiology and X-ray crystallography to refine their models, enabling more precise predictions of muscimol and other GABA ligand interactions[3].
Strengths: Comprehensive integration of experimental and computational methods, access to vast proprietary databases. Weaknesses: High computational costs, potential overreliance on in silico predictions.
Janssen Pharmaceutica NV
Technical Solution: Janssen has developed a sophisticated computational platform for modeling GABA receptor binding, focusing on allosteric modulation. Their approach utilizes machine learning algorithms trained on extensive proprietary datasets to predict binding modes and efficacy of novel compounds. The company employs molecular dynamics simulations to study the conformational changes induced by muscimol and other GABA receptor ligands[2]. Janssen's models incorporate pharmacophore mapping and fragment-based drug design to guide the development of new GABA receptor modulators with improved selectivity and potency[4].
Strengths: Strong focus on allosteric modulation, extensive proprietary data for model training. Weaknesses: May be limited by the availability of experimental structural data for certain GABA receptor subtypes.
Innovations in GABA Receptor Modeling
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
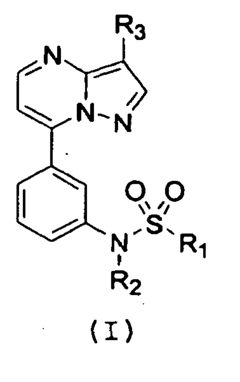
N- [3-(3-substituted-pyrazolo[1,5-a] pyrimidin-7-yl) phenyl]-sulfonamides, and compositions, and methods related thereto
PatentInactiveEP1648897B1
Innovation
- Development of novel N-[3-(3-substituted-pyrazolo[1,5-a]pyrimidin-7-yl)-phenyl]-sulfonamides with high affinity for GABA A receptor α1 and α2 subunits, which are used to treat insomnia, anxiety, and epilepsy, offering rapid sleep induction with reduced hangover effects and dependence risk.
Regulatory Aspects of In Silico Drug Discovery
The regulatory landscape for in silico drug discovery is rapidly evolving as computational methods gain prominence in pharmaceutical research and development. Regulatory agencies, including the FDA and EMA, are increasingly recognizing the value of computational modeling in drug discovery and development processes. However, they also emphasize the need for rigorous validation and standardization of these methods.
For computational modeling of muscimol and GABA receptor binding, regulatory considerations primarily focus on the reliability and reproducibility of the models used. Agencies require thorough documentation of the computational methods, including the algorithms, software packages, and data sources employed. Validation of these models against experimental data is crucial, with regulatory bodies expecting clear demonstrations of the model's predictive power and limitations.
The use of in silico methods in regulatory submissions is governed by various guidelines. For instance, the FDA's guidance on "Physiologically Based Pharmacokinetic Analyses — Format and Content" provides a framework for submitting computational modeling results. While this guidance is not specific to receptor binding models, its principles can be applied to the computational modeling of muscimol and GABA receptor interactions.
Regulatory agencies also emphasize the importance of transparency in computational modeling. Researchers are expected to provide detailed information on model parameters, assumptions, and any adjustments made during the modeling process. This transparency allows regulators to assess the validity of the models and their applicability to drug development decisions.
Data quality and integrity are paramount in regulatory considerations. Agencies require that the data used to build and validate computational models be of high quality, well-documented, and traceable. For muscimol and GABA receptor binding models, this includes ensuring the accuracy and reliability of structural data, binding affinity measurements, and other relevant experimental inputs.
As computational methods advance, regulatory frameworks are adapting to incorporate these new approaches. There is a growing trend towards the acceptance of in silico evidence in regulatory submissions, particularly when used in conjunction with traditional experimental data. However, the extent to which computational modeling can replace in vitro or in vivo studies varies depending on the specific application and regulatory context.
For computational modeling of muscimol and GABA receptor binding, regulatory considerations primarily focus on the reliability and reproducibility of the models used. Agencies require thorough documentation of the computational methods, including the algorithms, software packages, and data sources employed. Validation of these models against experimental data is crucial, with regulatory bodies expecting clear demonstrations of the model's predictive power and limitations.
The use of in silico methods in regulatory submissions is governed by various guidelines. For instance, the FDA's guidance on "Physiologically Based Pharmacokinetic Analyses — Format and Content" provides a framework for submitting computational modeling results. While this guidance is not specific to receptor binding models, its principles can be applied to the computational modeling of muscimol and GABA receptor interactions.
Regulatory agencies also emphasize the importance of transparency in computational modeling. Researchers are expected to provide detailed information on model parameters, assumptions, and any adjustments made during the modeling process. This transparency allows regulators to assess the validity of the models and their applicability to drug development decisions.
Data quality and integrity are paramount in regulatory considerations. Agencies require that the data used to build and validate computational models be of high quality, well-documented, and traceable. For muscimol and GABA receptor binding models, this includes ensuring the accuracy and reliability of structural data, binding affinity measurements, and other relevant experimental inputs.
As computational methods advance, regulatory frameworks are adapting to incorporate these new approaches. There is a growing trend towards the acceptance of in silico evidence in regulatory submissions, particularly when used in conjunction with traditional experimental data. However, the extent to which computational modeling can replace in vitro or in vivo studies varies depending on the specific application and regulatory context.
Ethical Considerations in Neuropharmacology Research
The ethical considerations in neuropharmacology research, particularly in the context of computational modeling of muscimol and GABA receptor binding, are multifaceted and require careful attention. One primary concern is the potential for misuse or abuse of the knowledge gained from such research. As our understanding of receptor binding mechanisms improves, there is a risk that this information could be exploited to develop substances with harmful effects or to manipulate neural processes in unethical ways.
Another critical ethical issue is the use of animal models in neuropharmacology research. While computational modeling can reduce the need for animal testing, some level of in vivo experimentation is often necessary to validate findings. Researchers must balance the potential benefits of their work against the welfare of laboratory animals, ensuring that experiments are designed to minimize suffering and use the fewest number of animals possible.
Data privacy and security also present significant ethical challenges in this field. Computational models often rely on large datasets, which may include sensitive information about individuals' neurological profiles. Protecting this data from breaches and ensuring that it is not used for purposes beyond the scope of the original research is paramount.
The potential for unintended consequences in neuropharmacology research cannot be overlooked. Even with rigorous modeling and testing, the complex nature of the brain means that interventions targeting GABA receptors could have unforeseen effects on cognition, behavior, or other neurological functions. Researchers must carefully consider the long-term implications of their work and implement robust safety protocols.
There are also ethical considerations surrounding the equitable distribution of benefits from neuropharmacological advancements. As new treatments or interventions are developed based on improved understanding of receptor binding, ensuring access to these innovations across different socioeconomic groups becomes an important ethical imperative.
Informed consent is another crucial ethical aspect, particularly when research moves from computational models to human trials. Participants must be fully aware of the potential risks and benefits associated with experimental neuropharmacological interventions, and their autonomy must be respected throughout the research process.
Lastly, there is an ethical responsibility to communicate research findings accurately and transparently. This includes acknowledging the limitations of computational models and being clear about the uncertainties that remain in our understanding of GABA receptor binding mechanisms. Responsible dissemination of research outcomes helps prevent misinterpretation and ensures that policy decisions and further research are based on accurate information.
Another critical ethical issue is the use of animal models in neuropharmacology research. While computational modeling can reduce the need for animal testing, some level of in vivo experimentation is often necessary to validate findings. Researchers must balance the potential benefits of their work against the welfare of laboratory animals, ensuring that experiments are designed to minimize suffering and use the fewest number of animals possible.
Data privacy and security also present significant ethical challenges in this field. Computational models often rely on large datasets, which may include sensitive information about individuals' neurological profiles. Protecting this data from breaches and ensuring that it is not used for purposes beyond the scope of the original research is paramount.
The potential for unintended consequences in neuropharmacology research cannot be overlooked. Even with rigorous modeling and testing, the complex nature of the brain means that interventions targeting GABA receptors could have unforeseen effects on cognition, behavior, or other neurological functions. Researchers must carefully consider the long-term implications of their work and implement robust safety protocols.
There are also ethical considerations surrounding the equitable distribution of benefits from neuropharmacological advancements. As new treatments or interventions are developed based on improved understanding of receptor binding, ensuring access to these innovations across different socioeconomic groups becomes an important ethical imperative.
Informed consent is another crucial ethical aspect, particularly when research moves from computational models to human trials. Participants must be fully aware of the potential risks and benefits associated with experimental neuropharmacological interventions, and their autonomy must be respected throughout the research process.
Lastly, there is an ethical responsibility to communicate research findings accurately and transparently. This includes acknowledging the limitations of computational models and being clear about the uncertainties that remain in our understanding of GABA receptor binding mechanisms. Responsible dissemination of research outcomes helps prevent misinterpretation and ensures that policy decisions and further research are based on accurate information.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!