Continuous Crystallization After Microreactor Synthesis: Best Practices
SEP 3, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microreactor-to-Crystallizer Integration Background and Objectives
Continuous crystallization following microreactor synthesis represents a significant advancement in process intensification for the pharmaceutical and fine chemical industries. This integration combines the precise control of microreactor technology with the efficiency of continuous crystallization, creating a seamless manufacturing pathway that addresses many limitations of traditional batch processes. The evolution of this technology can be traced back to the early 2000s when microreactor technology first gained prominence for its ability to handle highly exothermic and fast reactions with unprecedented control over reaction parameters.
The technological trajectory has since expanded to include downstream processing, with crystallization being a critical step in product isolation and purification. This integration aims to maintain the advantages of continuous processing throughout the entire production chain, eliminating the inefficiencies associated with batch-to-continuous transitions. Recent advancements have focused on real-time monitoring and control systems that enable adaptive process management across both synthesis and crystallization stages.
The primary objective of microreactor-to-crystallizer integration is to establish a robust continuous manufacturing platform that ensures consistent product quality while maximizing yield and minimizing waste. This involves developing strategies for managing the transition from the typically small-volume, high-concentration environment of microreactors to the controlled supersaturation conditions required for optimal crystallization outcomes.
Secondary objectives include reducing the overall process footprint, enhancing energy efficiency, and improving process safety profiles through the elimination of large solvent volumes and intermediate handling steps. The technology also aims to facilitate rapid scale-up from laboratory to production scales without significant redesign, addressing a long-standing challenge in pharmaceutical manufacturing.
Current research trends indicate growing interest in modular designs that allow for flexible configuration of integrated systems based on specific product requirements. This adaptability is particularly valuable for contract manufacturing organizations and pharmaceutical companies with diverse product portfolios. Additionally, there is increasing focus on developing universal interfaces between microreactors and crystallizers that can accommodate varying flow rates, solvent systems, and solid loading capacities.
The global regulatory landscape is also evolving to support continuous manufacturing initiatives, with the FDA and other agencies actively encouraging adoption through various guidance documents and initiatives. This regulatory support has accelerated industry investment in continuous crystallization technologies, particularly those that integrate seamlessly with upstream synthesis processes.
As we look toward future developments, the convergence of advanced process analytical technologies, artificial intelligence for process optimization, and novel materials for reactor and crystallizer construction promises to further enhance the capabilities and applicability of integrated microreactor-crystallizer systems across a broader range of chemical processes and products.
The technological trajectory has since expanded to include downstream processing, with crystallization being a critical step in product isolation and purification. This integration aims to maintain the advantages of continuous processing throughout the entire production chain, eliminating the inefficiencies associated with batch-to-continuous transitions. Recent advancements have focused on real-time monitoring and control systems that enable adaptive process management across both synthesis and crystallization stages.
The primary objective of microreactor-to-crystallizer integration is to establish a robust continuous manufacturing platform that ensures consistent product quality while maximizing yield and minimizing waste. This involves developing strategies for managing the transition from the typically small-volume, high-concentration environment of microreactors to the controlled supersaturation conditions required for optimal crystallization outcomes.
Secondary objectives include reducing the overall process footprint, enhancing energy efficiency, and improving process safety profiles through the elimination of large solvent volumes and intermediate handling steps. The technology also aims to facilitate rapid scale-up from laboratory to production scales without significant redesign, addressing a long-standing challenge in pharmaceutical manufacturing.
Current research trends indicate growing interest in modular designs that allow for flexible configuration of integrated systems based on specific product requirements. This adaptability is particularly valuable for contract manufacturing organizations and pharmaceutical companies with diverse product portfolios. Additionally, there is increasing focus on developing universal interfaces between microreactors and crystallizers that can accommodate varying flow rates, solvent systems, and solid loading capacities.
The global regulatory landscape is also evolving to support continuous manufacturing initiatives, with the FDA and other agencies actively encouraging adoption through various guidance documents and initiatives. This regulatory support has accelerated industry investment in continuous crystallization technologies, particularly those that integrate seamlessly with upstream synthesis processes.
As we look toward future developments, the convergence of advanced process analytical technologies, artificial intelligence for process optimization, and novel materials for reactor and crystallizer construction promises to further enhance the capabilities and applicability of integrated microreactor-crystallizer systems across a broader range of chemical processes and products.
Market Analysis for Continuous Crystallization Technologies
The continuous crystallization technology market is experiencing robust growth, driven by increasing demand for more efficient and sustainable pharmaceutical and fine chemical manufacturing processes. Current market valuations indicate that the global continuous manufacturing market in pharmaceuticals alone reached approximately $650 million in 2022, with continuous crystallization technologies representing a significant segment within this space. Industry analysts project a compound annual growth rate of 12.8% for continuous pharmaceutical manufacturing technologies through 2028, with crystallization processes showing particularly strong momentum.
Demand for continuous crystallization technologies following microreactor synthesis is primarily concentrated in pharmaceutical manufacturing, where regulatory bodies like the FDA have actively encouraged adoption of continuous manufacturing approaches. This regulatory support has created a favorable market environment, particularly in North America and Europe where pharmaceutical manufacturing hubs are concentrated. The market is also seeing increased penetration in specialty chemicals, agrochemicals, and advanced materials sectors.
Key market drivers include significant cost reduction potential, with studies demonstrating 15-30% operational cost savings compared to batch processes. Quality improvements represent another critical factor, as continuous crystallization enables more precise control over crystal size distribution, morphology, and purity—parameters essential for pharmaceutical applications. Environmental considerations further bolster market growth, with continuous processes typically reducing solvent usage by 20-40% and energy consumption by 15-25% compared to traditional batch crystallization.
Regional analysis reveals North America currently holds the largest market share at approximately 38%, followed by Europe at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by rapid expansion of pharmaceutical manufacturing capabilities in China and India, coupled with increasing adoption of advanced manufacturing technologies.
Market segmentation by industry application shows pharmaceuticals leading with approximately 45% market share, followed by fine chemicals (28%), agrochemicals (15%), and other applications including food processing and advanced materials (12%). Within pharmaceuticals, small molecule API crystallization represents the dominant application, though continuous crystallization for biologics and peptide-based therapeutics is emerging as a high-growth segment.
Customer demand patterns indicate increasing preference for integrated continuous manufacturing solutions that seamlessly connect microreactor synthesis with downstream crystallization processes. End users particularly value technologies offering flexibility in handling different compounds, rapid changeover capabilities, and robust process analytical technology (PAT) integration for real-time monitoring and control.
Demand for continuous crystallization technologies following microreactor synthesis is primarily concentrated in pharmaceutical manufacturing, where regulatory bodies like the FDA have actively encouraged adoption of continuous manufacturing approaches. This regulatory support has created a favorable market environment, particularly in North America and Europe where pharmaceutical manufacturing hubs are concentrated. The market is also seeing increased penetration in specialty chemicals, agrochemicals, and advanced materials sectors.
Key market drivers include significant cost reduction potential, with studies demonstrating 15-30% operational cost savings compared to batch processes. Quality improvements represent another critical factor, as continuous crystallization enables more precise control over crystal size distribution, morphology, and purity—parameters essential for pharmaceutical applications. Environmental considerations further bolster market growth, with continuous processes typically reducing solvent usage by 20-40% and energy consumption by 15-25% compared to traditional batch crystallization.
Regional analysis reveals North America currently holds the largest market share at approximately 38%, followed by Europe at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by rapid expansion of pharmaceutical manufacturing capabilities in China and India, coupled with increasing adoption of advanced manufacturing technologies.
Market segmentation by industry application shows pharmaceuticals leading with approximately 45% market share, followed by fine chemicals (28%), agrochemicals (15%), and other applications including food processing and advanced materials (12%). Within pharmaceuticals, small molecule API crystallization represents the dominant application, though continuous crystallization for biologics and peptide-based therapeutics is emerging as a high-growth segment.
Customer demand patterns indicate increasing preference for integrated continuous manufacturing solutions that seamlessly connect microreactor synthesis with downstream crystallization processes. End users particularly value technologies offering flexibility in handling different compounds, rapid changeover capabilities, and robust process analytical technology (PAT) integration for real-time monitoring and control.
Current Challenges in Post-Microreactor Crystallization
Despite significant advancements in microreactor technology for continuous synthesis, the integration with downstream crystallization processes remains problematic. The transition from microreactor-based synthesis to crystallization presents several critical challenges that impede the full realization of end-to-end continuous manufacturing in pharmaceutical and fine chemical industries.
Temperature gradient management constitutes a primary obstacle, as the thermal conditions optimal for reaction completion in microreactors often differ substantially from those required for controlled nucleation and crystal growth. This mismatch frequently results in premature crystallization within transfer lines or uncontrolled nucleation events that compromise product quality and process reliability.
Residence time distribution represents another significant challenge. While microreactors typically operate with residence times of seconds to minutes, crystallization processes generally require extended timeframes ranging from minutes to hours. This temporal disparity necessitates sophisticated engineering solutions to bridge these fundamentally different process dynamics without compromising the continuous nature of the overall process.
Scale-up considerations further complicate implementation efforts. Laboratory-scale continuous crystallization systems that perform adequately often encounter unforeseen complications during industrial scale-up, including fouling, clogging, and inconsistent crystal size distribution. These issues are particularly pronounced at the interface between microreactor outputs and crystallization inputs.
Solvent compatibility issues frequently arise when the reaction medium optimal for synthesis differs from that required for efficient crystallization. This necessitates either in-line solvent exchange capabilities or compromise solutions that may not be optimal for either process stage, potentially affecting yield and product quality.
Process analytical technology (PAT) integration presents technical difficulties in monitoring crystallization parameters in real-time after microreactor synthesis. Current sensor technologies often struggle with the high solid loadings, potential fouling, and rapid process dynamics characteristic of continuous crystallization environments.
Regulatory compliance adds another layer of complexity, as continuous crystallization processes must demonstrate consistent product quality attributes to meet stringent pharmaceutical manufacturing standards. The variable nature of post-microreactor crystallization makes validation particularly challenging under current regulatory frameworks.
Material of construction compatibility issues arise when considering the entire process train. Materials suitable for handling corrosive reaction conditions in microreactors may not be optimal for crystallization equipment, creating design constraints that limit process flexibility and performance.
Temperature gradient management constitutes a primary obstacle, as the thermal conditions optimal for reaction completion in microreactors often differ substantially from those required for controlled nucleation and crystal growth. This mismatch frequently results in premature crystallization within transfer lines or uncontrolled nucleation events that compromise product quality and process reliability.
Residence time distribution represents another significant challenge. While microreactors typically operate with residence times of seconds to minutes, crystallization processes generally require extended timeframes ranging from minutes to hours. This temporal disparity necessitates sophisticated engineering solutions to bridge these fundamentally different process dynamics without compromising the continuous nature of the overall process.
Scale-up considerations further complicate implementation efforts. Laboratory-scale continuous crystallization systems that perform adequately often encounter unforeseen complications during industrial scale-up, including fouling, clogging, and inconsistent crystal size distribution. These issues are particularly pronounced at the interface between microreactor outputs and crystallization inputs.
Solvent compatibility issues frequently arise when the reaction medium optimal for synthesis differs from that required for efficient crystallization. This necessitates either in-line solvent exchange capabilities or compromise solutions that may not be optimal for either process stage, potentially affecting yield and product quality.
Process analytical technology (PAT) integration presents technical difficulties in monitoring crystallization parameters in real-time after microreactor synthesis. Current sensor technologies often struggle with the high solid loadings, potential fouling, and rapid process dynamics characteristic of continuous crystallization environments.
Regulatory compliance adds another layer of complexity, as continuous crystallization processes must demonstrate consistent product quality attributes to meet stringent pharmaceutical manufacturing standards. The variable nature of post-microreactor crystallization makes validation particularly challenging under current regulatory frameworks.
Material of construction compatibility issues arise when considering the entire process train. Materials suitable for handling corrosive reaction conditions in microreactors may not be optimal for crystallization equipment, creating design constraints that limit process flexibility and performance.
State-of-the-Art Continuous Crystallization Solutions
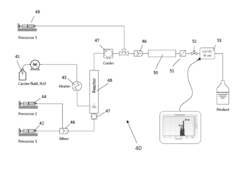
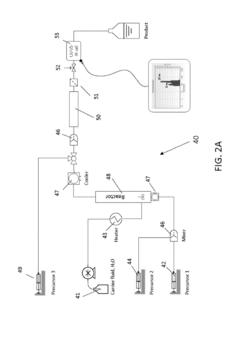
01 Microreactor-based continuous crystallization systems
Microreactor technology enables continuous crystallization processes with precise control over reaction conditions. These systems integrate synthesis and crystallization in a continuous flow, allowing for improved heat and mass transfer, consistent crystal size distribution, and enhanced product quality. The compact design of microreactors facilitates efficient scale-up from laboratory to industrial production while maintaining consistent product characteristics.- Microreactor-based continuous crystallization systems: Microreactor technology enables precise control over reaction conditions for continuous crystallization processes. These systems integrate synthesis and crystallization in a continuous flow, allowing for better control of crystal size, morphology, and purity. The small dimensions of microreactors provide enhanced heat and mass transfer, resulting in more uniform crystallization conditions and improved product quality compared to batch processes.
- Integrated synthesis-crystallization continuous processes: Integrated continuous processes combine chemical synthesis in microreactors with downstream crystallization units. This approach eliminates intermediate handling steps, reduces impurities, and improves overall process efficiency. The seamless transition from synthesis to crystallization allows for real-time adjustments of process parameters, resulting in consistent crystal properties and higher yields of the desired product form.
- Advanced control strategies for continuous crystallization: Advanced control strategies employ sensors, monitoring systems, and automation to maintain optimal crystallization conditions after microreactor synthesis. These strategies include real-time monitoring of supersaturation, temperature profiles, and crystal growth rates. Feedback control systems adjust process parameters to ensure consistent crystal quality despite variations in the synthesis output, enabling robust continuous manufacturing of crystalline products.
- Novel crystallizer designs for continuous processing: Specialized crystallizer designs have been developed to handle the continuous output from microreactors. These include tubular crystallizers, oscillatory flow crystallizers, and multi-stage crystallization units that can accommodate varying residence times and cooling profiles. The designs focus on preventing clogging, controlling nucleation rates, and facilitating crystal growth while maintaining continuous operation for extended periods.
- Solvent selection and supersaturation control techniques: Solvent selection and supersaturation control are critical for successful continuous crystallization following microreactor synthesis. Techniques include anti-solvent addition, temperature cycling, and controlled cooling profiles to achieve desired crystal properties. These approaches manage the delicate balance between nucleation and growth rates, preventing undesired phenomena such as oiling out, agglomeration, or formation of undesired polymorphs in continuous crystallization processes.
02 Continuous crystallization process control and monitoring
Advanced control systems for continuous crystallization processes after microreactor synthesis enable real-time monitoring and adjustment of critical parameters. These systems utilize sensors to track temperature, concentration, supersaturation, and crystal growth rates, allowing for automated adjustments to maintain optimal crystallization conditions. Process analytical technologies (PAT) provide feedback for maintaining consistent crystal quality and size distribution throughout extended production runs.Expand Specific Solutions03 Integrated synthesis-crystallization flow systems
Integrated continuous flow systems combine microreactor synthesis with downstream crystallization units in a single process train. These systems eliminate intermediate handling steps, reducing contamination risks and improving efficiency. The seamless transition from synthesis to crystallization allows for immediate product recovery and purification, with specialized interfaces designed to manage the transition from homogeneous reaction mixtures to heterogeneous crystallizing suspensions.Expand Specific Solutions04 Novel crystallizer designs for continuous processing
Specialized crystallizer designs have been developed for continuous processing following microreactor synthesis. These include oscillatory flow crystallizers, tubular crystallizers with controlled cooling zones, and mixed-suspension mixed-product-removal (MSMPR) crystallizers optimized for continuous operation. Novel designs incorporate features for managing crystal growth, preventing fouling, and facilitating continuous solid-liquid separation while maintaining narrow particle size distribution.Expand Specific Solutions05 Solvent selection and supersaturation control strategies
Effective solvent selection and supersaturation control strategies are critical for successful continuous crystallization after microreactor synthesis. These approaches involve careful selection of solvent systems compatible with both reaction and crystallization steps, along with methods to precisely control supersaturation through temperature profiles, anti-solvent addition, or evaporation. Advanced strategies include the use of seed crystals, ultrasound, or controlled cooling rates to initiate and maintain controlled crystallization.Expand Specific Solutions
Industry Leaders in Continuous Manufacturing Equipment
Continuous crystallization after microreactor synthesis is currently in the growth phase, with increasing market adoption driven by pharmaceutical and chemical industries seeking more efficient manufacturing processes. The global market is expanding rapidly, estimated at several billion dollars, as companies pursue continuous manufacturing solutions. Technologically, the field is advancing from early-stage development to commercial implementation, with academic institutions (Tianjin University, MIT, Illinois Institute of Technology) establishing fundamental research while industrial players (FUJIFILM Manufacturing Europe, Sumitomo Pharmaceuticals, Ricoh) focus on practical applications. Research organizations like CNRS and Chinese Academy of Sciences are bridging fundamental science with industrial needs, creating a competitive landscape where collaboration between academia and industry is accelerating technology maturation and adoption.
Nederlandse Organisatie voor Toegepast-Natuurwetenschappelijk
Technical Solution: The Nederlandse Organisatie voor Toegepast-Natuurwetenschappelijk (TNO) has developed an innovative continuous crystallization platform called CrystalLine that seamlessly integrates with microreactor synthesis. Their system employs a series of tubular crystallizers with precisely controlled temperature zones and residence time distribution. TNO's technology features proprietary static mixers designed to promote uniform supersaturation while minimizing crystal damage. The platform incorporates advanced process analytical technology including in-line Raman spectroscopy, focused beam reflectance measurement, and particle vision microscopy for real-time crystal characterization. TNO has implemented a sophisticated control system that uses machine learning algorithms to predict and adjust crystallization parameters based on upstream microreactor conditions. Their technology has been successfully demonstrated for pharmaceutical intermediates, specialty chemicals, and food ingredients, showing consistent improvements in crystal purity, size distribution, and polymorphic control compared to batch processes.
Strengths: Highly versatile platform applicable across multiple industries; excellent scalability from laboratory to production scale; sophisticated control systems enabling robust operation. Weaknesses: Requires significant expertise in both microreactor technology and crystallization science; higher initial capital investment compared to conventional batch crystallization equipment.
Massachusetts Institute of Technology
Technical Solution: MIT has developed an integrated microreactor-crystallizer system that combines continuous-flow synthesis with controlled crystallization. Their approach utilizes segmented flow crystallization where product solution from the microreactor is directly fed into a crystallization module with precise temperature control zones. MIT researchers have implemented Process Analytical Technology (PAT) tools including in-line Raman spectroscopy and focused beam reflectance measurement (FBRM) to monitor crystal formation in real-time, allowing for feedback control of supersaturation levels. Their system incorporates ultrasonic irradiation at specific points to control nucleation events and achieve narrow crystal size distribution. MIT has also pioneered the use of anti-solvent addition strategies within their continuous crystallization platform to enhance yield and purity of pharmaceutical compounds.
Strengths: Superior integration of PAT tools enabling real-time process monitoring and control; advanced temperature profile management for precise supersaturation control. Weaknesses: Complex system architecture requires specialized expertise; higher implementation costs compared to conventional batch crystallization approaches.
Critical Patents and Literature in Crystallization Control
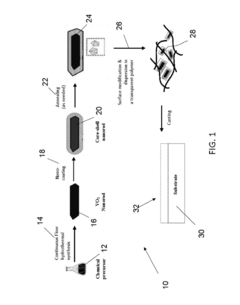
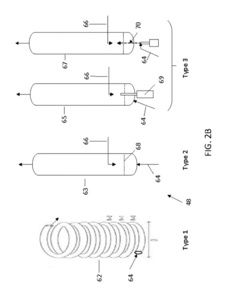
Continuous flow synthesis of VO2 nanoparticles or nanorods by using a microreactor
PatentActiveUS9975804B2
Innovation
- A method for producing anisotropic VO2 nanoparticles using a continuous flow hydrothermal synthesis process, encapsulating them in a core-shell construct, and applying them to transparent substrates, allowing for enhanced light transmission and heat control without moving parts or electrical power, using relatively inexpensive materials and milder reaction conditions.
Continuous crystallisation method
PatentWO2009047408A1
Innovation
- A continuous crystallization process using an enantiomerically pure resolving agent and solvent in a continuously stirred system, where the mixture and resolving agent are injected at constant rates, maintaining a non-zero reactor volume and concentration, and recovering the diastereoisomer salt continuously, without seeding, to achieve high-purity crystallization.
Scale-up Considerations for Industrial Implementation
Scaling up continuous crystallization processes from laboratory microreactor synthesis to industrial implementation requires careful consideration of multiple engineering and operational factors. The transition from small-scale to large-scale production introduces challenges related to heat and mass transfer, which can significantly impact crystal quality, size distribution, and process efficiency. Industrial implementation necessitates maintaining consistent supersaturation levels across larger volumes, which becomes increasingly difficult as equipment dimensions increase.
Equipment selection represents a critical decision point in scale-up strategies. While laboratory microreactors typically utilize glass or small-diameter tubing, industrial-scale operations require more robust materials such as stainless steel or specialized alloys that can withstand higher pressures and provide better temperature control. Tubular crystallizers, continuous stirred tank crystallizers (CSTC), and mixed suspension mixed product removal (MSMPR) crystallizers each offer distinct advantages depending on the specific crystallization kinetics and desired product characteristics.
Process control systems must be significantly more sophisticated at industrial scale to maintain the precise conditions necessary for consistent crystal formation. Advanced monitoring technologies, including Process Analytical Technology (PAT) tools such as in-line particle size analyzers, Raman spectroscopy, and focused beam reflectance measurement (FBRM), become essential for real-time process adjustment. These systems allow operators to detect deviations from optimal crystallization conditions and implement corrective actions before product quality is compromised.
Hydrodynamic considerations become increasingly important during scale-up. Flow patterns, mixing efficiency, and residence time distributions can vary dramatically between small and large-scale equipment. Computational fluid dynamics (CFD) modeling has emerged as a valuable tool for predicting these behaviors and optimizing equipment design before physical implementation. Maintaining consistent shear rates throughout larger crystallizers helps prevent unwanted secondary nucleation or crystal breakage.
Economic feasibility must be thoroughly evaluated when scaling up continuous crystallization processes. Capital expenditure requirements for specialized equipment must be balanced against operational benefits such as reduced labor costs, improved energy efficiency, and enhanced product consistency. Modular approaches to scale-up have gained popularity, where multiple smaller units operate in parallel rather than designing a single large-scale system, offering flexibility in production capacity and reducing scale-up risks.
Regulatory considerations present additional challenges for pharmaceutical and fine chemical applications. Process validation protocols must demonstrate that the scaled-up continuous crystallization process consistently produces material meeting all quality specifications. This typically requires extensive documentation of process parameters, design space mapping, and demonstration of process control strategies that ensure consistent crystal properties across production batches.
Equipment selection represents a critical decision point in scale-up strategies. While laboratory microreactors typically utilize glass or small-diameter tubing, industrial-scale operations require more robust materials such as stainless steel or specialized alloys that can withstand higher pressures and provide better temperature control. Tubular crystallizers, continuous stirred tank crystallizers (CSTC), and mixed suspension mixed product removal (MSMPR) crystallizers each offer distinct advantages depending on the specific crystallization kinetics and desired product characteristics.
Process control systems must be significantly more sophisticated at industrial scale to maintain the precise conditions necessary for consistent crystal formation. Advanced monitoring technologies, including Process Analytical Technology (PAT) tools such as in-line particle size analyzers, Raman spectroscopy, and focused beam reflectance measurement (FBRM), become essential for real-time process adjustment. These systems allow operators to detect deviations from optimal crystallization conditions and implement corrective actions before product quality is compromised.
Hydrodynamic considerations become increasingly important during scale-up. Flow patterns, mixing efficiency, and residence time distributions can vary dramatically between small and large-scale equipment. Computational fluid dynamics (CFD) modeling has emerged as a valuable tool for predicting these behaviors and optimizing equipment design before physical implementation. Maintaining consistent shear rates throughout larger crystallizers helps prevent unwanted secondary nucleation or crystal breakage.
Economic feasibility must be thoroughly evaluated when scaling up continuous crystallization processes. Capital expenditure requirements for specialized equipment must be balanced against operational benefits such as reduced labor costs, improved energy efficiency, and enhanced product consistency. Modular approaches to scale-up have gained popularity, where multiple smaller units operate in parallel rather than designing a single large-scale system, offering flexibility in production capacity and reducing scale-up risks.
Regulatory considerations present additional challenges for pharmaceutical and fine chemical applications. Process validation protocols must demonstrate that the scaled-up continuous crystallization process consistently produces material meeting all quality specifications. This typically requires extensive documentation of process parameters, design space mapping, and demonstration of process control strategies that ensure consistent crystal properties across production batches.
Quality by Design Approaches for Regulatory Compliance
Quality by Design (QbD) approaches have become increasingly critical for regulatory compliance in continuous crystallization processes following microreactor synthesis. The pharmaceutical industry faces stringent regulatory requirements from agencies such as the FDA and EMA, which have embraced QbD principles as essential frameworks for ensuring product quality and process consistency.
The implementation of QbD in continuous crystallization requires systematic identification of Critical Quality Attributes (CQAs) that directly impact product performance. These typically include crystal size distribution, polymorphic form, purity profile, and residual solvent content. By establishing clear relationships between these attributes and clinical performance, manufacturers can develop robust control strategies that satisfy regulatory expectations.
Risk assessment methodologies form the cornerstone of QbD implementation for continuous crystallization processes. Techniques such as Failure Mode and Effects Analysis (FMEA) and Hazard Analysis and Critical Control Points (HACCP) enable systematic evaluation of potential failure points in the crystallization process. This proactive approach to risk management aligns perfectly with regulatory agencies' expectations for comprehensive process understanding.
Design of Experiments (DoE) methodologies provide the statistical framework necessary for establishing design spaces that ensure consistent product quality. For continuous crystallization following microreactor synthesis, key process parameters typically include temperature profiles, residence time, antisolvent addition rates, and seed crystal characteristics. Regulatory bodies increasingly expect manufacturers to demonstrate how these parameters interact within a multidimensional design space.
Process Analytical Technology (PAT) implementation represents another crucial aspect of regulatory compliance through QbD. Real-time monitoring tools such as Focused Beam Reflectance Measurement (FBRM), Raman spectroscopy, and Near-Infrared (NIR) analysis enable continuous verification that the process remains within the established design space. Regulatory agencies increasingly expect manufacturers to implement such technologies for real-time release testing.
Documentation requirements for QbD-based regulatory submissions have evolved significantly. Manufacturers must provide comprehensive evidence of process understanding, including multivariate models that describe the relationship between Critical Process Parameters (CPPs) and CQAs. Case studies demonstrate that well-structured QbD documentation can significantly expedite regulatory approval processes and reduce post-approval change requirements.
Lifecycle management considerations represent the final component of QbD-based regulatory compliance. Continuous improvement mechanisms must be established to incorporate new knowledge gained during commercial manufacturing. This approach aligns with regulatory expectations for ongoing process verification and enables manufacturers to implement process improvements without extensive regulatory resubmissions.
The implementation of QbD in continuous crystallization requires systematic identification of Critical Quality Attributes (CQAs) that directly impact product performance. These typically include crystal size distribution, polymorphic form, purity profile, and residual solvent content. By establishing clear relationships between these attributes and clinical performance, manufacturers can develop robust control strategies that satisfy regulatory expectations.
Risk assessment methodologies form the cornerstone of QbD implementation for continuous crystallization processes. Techniques such as Failure Mode and Effects Analysis (FMEA) and Hazard Analysis and Critical Control Points (HACCP) enable systematic evaluation of potential failure points in the crystallization process. This proactive approach to risk management aligns perfectly with regulatory agencies' expectations for comprehensive process understanding.
Design of Experiments (DoE) methodologies provide the statistical framework necessary for establishing design spaces that ensure consistent product quality. For continuous crystallization following microreactor synthesis, key process parameters typically include temperature profiles, residence time, antisolvent addition rates, and seed crystal characteristics. Regulatory bodies increasingly expect manufacturers to demonstrate how these parameters interact within a multidimensional design space.
Process Analytical Technology (PAT) implementation represents another crucial aspect of regulatory compliance through QbD. Real-time monitoring tools such as Focused Beam Reflectance Measurement (FBRM), Raman spectroscopy, and Near-Infrared (NIR) analysis enable continuous verification that the process remains within the established design space. Regulatory agencies increasingly expect manufacturers to implement such technologies for real-time release testing.
Documentation requirements for QbD-based regulatory submissions have evolved significantly. Manufacturers must provide comprehensive evidence of process understanding, including multivariate models that describe the relationship between Critical Process Parameters (CPPs) and CQAs. Case studies demonstrate that well-structured QbD documentation can significantly expedite regulatory approval processes and reduce post-approval change requirements.
Lifecycle management considerations represent the final component of QbD-based regulatory compliance. Continuous improvement mechanisms must be established to incorporate new knowledge gained during commercial manufacturing. This approach aligns with regulatory expectations for ongoing process verification and enables manufacturers to implement process improvements without extensive regulatory resubmissions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!