Electroporation For CRISPR Delivery: Targeting Efficiency Metrics
AUG 21, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CRISPR Electroporation Background and Objectives
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology has revolutionized gene editing since its discovery in the early 2000s. This powerful tool allows for precise modifications of genetic material in various organisms, including humans. As CRISPR applications continue to expand, the need for efficient delivery methods has become increasingly critical.
Electroporation has emerged as a promising technique for CRISPR delivery, offering several advantages over viral vector-based methods. This physical method uses electrical pulses to create temporary pores in cell membranes, allowing the entry of CRISPR components into target cells. The combination of CRISPR and electroporation has shown potential in both research and therapeutic applications.
The primary objective of exploring electroporation for CRISPR delivery is to enhance targeting efficiency metrics. These metrics are crucial for evaluating the effectiveness of gene editing procedures and include factors such as delivery efficiency, editing efficiency, and specificity. Improving these metrics is essential for advancing CRISPR technology towards clinical applications and expanding its use in basic research.
Recent developments in electroporation techniques have focused on optimizing parameters such as pulse duration, voltage, and electrode configuration to maximize CRISPR delivery while minimizing cellular damage. Researchers are also exploring the use of nanoparticles and other carriers in conjunction with electroporation to further improve targeting efficiency.
The evolution of CRISPR technology has been marked by significant milestones, including the discovery of Cas9 endonuclease, the development of various CRISPR systems (e.g., Cas12a, Cas13), and the creation of base editors and prime editors. Each advancement has brought new challenges and opportunities for delivery methods, with electroporation adapting to meet these changing requirements.
As the field progresses, the goals for CRISPR electroporation include achieving higher editing efficiencies in a broader range of cell types and tissues, reducing off-target effects, and developing in vivo electroporation methods for targeted gene editing in living organisms. These objectives align with the broader aims of CRISPR technology to enable precise genetic modifications for therapeutic purposes and to advance our understanding of gene function and regulation.
Electroporation has emerged as a promising technique for CRISPR delivery, offering several advantages over viral vector-based methods. This physical method uses electrical pulses to create temporary pores in cell membranes, allowing the entry of CRISPR components into target cells. The combination of CRISPR and electroporation has shown potential in both research and therapeutic applications.
The primary objective of exploring electroporation for CRISPR delivery is to enhance targeting efficiency metrics. These metrics are crucial for evaluating the effectiveness of gene editing procedures and include factors such as delivery efficiency, editing efficiency, and specificity. Improving these metrics is essential for advancing CRISPR technology towards clinical applications and expanding its use in basic research.
Recent developments in electroporation techniques have focused on optimizing parameters such as pulse duration, voltage, and electrode configuration to maximize CRISPR delivery while minimizing cellular damage. Researchers are also exploring the use of nanoparticles and other carriers in conjunction with electroporation to further improve targeting efficiency.
The evolution of CRISPR technology has been marked by significant milestones, including the discovery of Cas9 endonuclease, the development of various CRISPR systems (e.g., Cas12a, Cas13), and the creation of base editors and prime editors. Each advancement has brought new challenges and opportunities for delivery methods, with electroporation adapting to meet these changing requirements.
As the field progresses, the goals for CRISPR electroporation include achieving higher editing efficiencies in a broader range of cell types and tissues, reducing off-target effects, and developing in vivo electroporation methods for targeted gene editing in living organisms. These objectives align with the broader aims of CRISPR technology to enable precise genetic modifications for therapeutic purposes and to advance our understanding of gene function and regulation.
Market Analysis for CRISPR Delivery Methods
The CRISPR delivery methods market is experiencing significant growth, driven by the increasing adoption of gene editing technologies in various applications. Electroporation, as a key delivery method for CRISPR systems, plays a crucial role in this expanding market. The global CRISPR market size was valued at $3.7 billion in 2021 and is projected to reach $6.3 billion by 2028, with a compound annual growth rate (CAGR) of 21.7% during the forecast period.
Electroporation for CRISPR delivery has gained traction due to its versatility and efficiency in introducing genetic material into cells. This method has found applications across multiple sectors, including biotechnology, pharmaceuticals, agriculture, and academic research. The demand for electroporation-based CRISPR delivery is particularly strong in the development of gene therapies and the creation of genetically modified organisms for various purposes.
In the pharmaceutical and biotechnology sectors, electroporation is widely used for CRISPR delivery in drug discovery and development processes. The method's ability to achieve high transfection efficiency in a wide range of cell types makes it valuable for creating cell lines and animal models for disease research and drug screening. This application segment is expected to witness substantial growth in the coming years.
The agricultural sector is another key market for electroporation-based CRISPR delivery. The technology is being utilized to develop crop varieties with improved traits such as disease resistance, drought tolerance, and enhanced nutritional content. As global food security concerns intensify, the demand for precision breeding techniques using CRISPR is likely to drive market growth in this sector.
Academic and research institutions represent a significant portion of the market for electroporation-based CRISPR delivery methods. These institutions are at the forefront of developing new CRISPR applications and improving delivery techniques. The continuous influx of research funding and collaborations between academia and industry are expected to fuel market growth in this segment.
Geographically, North America dominates the market for CRISPR delivery methods, including electroporation, due to the presence of major biotechnology and pharmaceutical companies, well-established research infrastructure, and favorable regulatory environment. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by increasing investments in life sciences research and development of gene therapy technologies.
The market for electroporation-based CRISPR delivery is characterized by intense competition and rapid technological advancements. Key players in this space are focusing on developing innovative electroporation devices and protocols to enhance targeting efficiency and reduce off-target effects. Additionally, there is a growing trend towards the development of integrated systems that combine electroporation with other delivery methods to improve overall CRISPR delivery efficiency.
Electroporation for CRISPR delivery has gained traction due to its versatility and efficiency in introducing genetic material into cells. This method has found applications across multiple sectors, including biotechnology, pharmaceuticals, agriculture, and academic research. The demand for electroporation-based CRISPR delivery is particularly strong in the development of gene therapies and the creation of genetically modified organisms for various purposes.
In the pharmaceutical and biotechnology sectors, electroporation is widely used for CRISPR delivery in drug discovery and development processes. The method's ability to achieve high transfection efficiency in a wide range of cell types makes it valuable for creating cell lines and animal models for disease research and drug screening. This application segment is expected to witness substantial growth in the coming years.
The agricultural sector is another key market for electroporation-based CRISPR delivery. The technology is being utilized to develop crop varieties with improved traits such as disease resistance, drought tolerance, and enhanced nutritional content. As global food security concerns intensify, the demand for precision breeding techniques using CRISPR is likely to drive market growth in this sector.
Academic and research institutions represent a significant portion of the market for electroporation-based CRISPR delivery methods. These institutions are at the forefront of developing new CRISPR applications and improving delivery techniques. The continuous influx of research funding and collaborations between academia and industry are expected to fuel market growth in this segment.
Geographically, North America dominates the market for CRISPR delivery methods, including electroporation, due to the presence of major biotechnology and pharmaceutical companies, well-established research infrastructure, and favorable regulatory environment. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by increasing investments in life sciences research and development of gene therapy technologies.
The market for electroporation-based CRISPR delivery is characterized by intense competition and rapid technological advancements. Key players in this space are focusing on developing innovative electroporation devices and protocols to enhance targeting efficiency and reduce off-target effects. Additionally, there is a growing trend towards the development of integrated systems that combine electroporation with other delivery methods to improve overall CRISPR delivery efficiency.
Electroporation Challenges in CRISPR Delivery
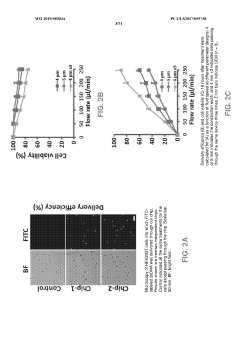
Electroporation, a widely used method for CRISPR delivery, faces several significant challenges that impact its efficiency and applicability in gene editing. One of the primary obstacles is the difficulty in achieving consistent and uniform transfection across a population of cells. The electrical pulses applied during electroporation can cause variability in membrane permeabilization, leading to inconsistent uptake of CRISPR components.
Another major challenge is the potential for cell damage or death due to the electrical shock. The balance between effective membrane disruption and cell viability is delicate, and finding the optimal parameters for different cell types and tissues remains a complex task. This issue is particularly pronounced when dealing with sensitive or hard-to-transfect cell types, limiting the technique's versatility.
The size of the CRISPR components, especially when using larger Cas9 proteins or complex ribonucleoprotein (RNP) assemblies, can also pose difficulties. Larger molecules are inherently more challenging to deliver through the transient pores created by electroporation, potentially reducing the overall efficiency of the gene-editing process.
Furthermore, the intracellular trafficking of CRISPR components post-electroporation presents another hurdle. Once inside the cell, the CRISPR machinery must navigate the cytoplasm and enter the nucleus to perform its gene-editing function. The efficiency of this process can vary significantly, affecting the overall success rate of the gene-editing attempt.
Off-target effects remain a concern in CRISPR applications, and electroporation's non-specific delivery mechanism can exacerbate this issue. The lack of targeted delivery means that CRISPR components may enter cells or cellular compartments where they are not intended to act, potentially leading to unintended genetic modifications.
Scalability is another challenge, particularly when considering clinical applications. While electroporation can be effective on a small scale, maintaining efficiency and consistency in large-scale operations or in vivo applications is problematic. This limitation hinders the translation of CRISPR therapies from laboratory settings to clinical practice.
Lastly, the optimization of electroporation protocols for CRISPR delivery is often empirical and cell-type specific. This necessitates extensive trial-and-error experimentation to determine the ideal conditions for each application, making the process time-consuming and resource-intensive. The lack of a universal, predictable protocol adds complexity to research and development efforts in this field.
Another major challenge is the potential for cell damage or death due to the electrical shock. The balance between effective membrane disruption and cell viability is delicate, and finding the optimal parameters for different cell types and tissues remains a complex task. This issue is particularly pronounced when dealing with sensitive or hard-to-transfect cell types, limiting the technique's versatility.
The size of the CRISPR components, especially when using larger Cas9 proteins or complex ribonucleoprotein (RNP) assemblies, can also pose difficulties. Larger molecules are inherently more challenging to deliver through the transient pores created by electroporation, potentially reducing the overall efficiency of the gene-editing process.
Furthermore, the intracellular trafficking of CRISPR components post-electroporation presents another hurdle. Once inside the cell, the CRISPR machinery must navigate the cytoplasm and enter the nucleus to perform its gene-editing function. The efficiency of this process can vary significantly, affecting the overall success rate of the gene-editing attempt.
Off-target effects remain a concern in CRISPR applications, and electroporation's non-specific delivery mechanism can exacerbate this issue. The lack of targeted delivery means that CRISPR components may enter cells or cellular compartments where they are not intended to act, potentially leading to unintended genetic modifications.
Scalability is another challenge, particularly when considering clinical applications. While electroporation can be effective on a small scale, maintaining efficiency and consistency in large-scale operations or in vivo applications is problematic. This limitation hinders the translation of CRISPR therapies from laboratory settings to clinical practice.
Lastly, the optimization of electroporation protocols for CRISPR delivery is often empirical and cell-type specific. This necessitates extensive trial-and-error experimentation to determine the ideal conditions for each application, making the process time-consuming and resource-intensive. The lack of a universal, predictable protocol adds complexity to research and development efforts in this field.
Current Electroporation Protocols for CRISPR
01 Optimization of electroporation parameters
Adjusting parameters such as voltage, pulse duration, and frequency can significantly improve the targeting efficiency of electroporation. Optimizing these factors enhances the permeability of cell membranes, allowing for more effective delivery of molecules into target cells while minimizing damage to surrounding tissues.- Optimization of electroporation parameters: Improving targeting efficiency in electroporation involves optimizing various parameters such as voltage, pulse duration, and frequency. These adjustments can enhance the permeability of cell membranes, allowing for more effective delivery of molecules into target cells while minimizing damage to surrounding tissues.
- Cell-specific targeting strategies: Developing cell-specific targeting strategies for electroporation can significantly increase efficiency. This may involve using specific electrode designs, modifying the composition of the electroporation medium, or employing cell-specific markers to enhance selectivity and reduce off-target effects.
- Nanoparticle-assisted electroporation: Incorporating nanoparticles into the electroporation process can enhance targeting efficiency. These nanoparticles can act as carriers for the molecules being delivered, improving their stability and increasing their uptake by target cells during electroporation.
- Combination with other delivery techniques: Combining electroporation with other delivery techniques, such as sonoporation or magnetofection, can synergistically improve targeting efficiency. This multi-modal approach can overcome limitations of individual methods and provide more precise and effective delivery to target cells or tissues.
- Real-time monitoring and feedback systems: Implementing real-time monitoring and feedback systems during electroporation can help optimize targeting efficiency. These systems can adjust electroporation parameters in response to cellular changes, ensuring optimal delivery conditions throughout the process and improving overall targeting efficiency.
02 Cell-specific targeting strategies
Developing cell-specific targeting strategies involves designing electrodes and electric field distributions to focus on particular cell types or tissues. This approach increases the precision of electroporation, improving efficiency by reducing off-target effects and enhancing the delivery of molecules to desired cellular locations.Expand Specific Solutions03 Combination with nanoparticles or carriers
Incorporating nanoparticles or molecular carriers with electroporation can enhance targeting efficiency. These carriers can be designed to interact specifically with target cells, improving the localization and uptake of therapeutic molecules when combined with electroporation techniques.Expand Specific Solutions04 Real-time monitoring and feedback systems
Implementing real-time monitoring and feedback systems during electroporation allows for dynamic adjustment of parameters. This approach enables continuous optimization of the process, improving targeting efficiency by adapting to variations in tissue properties and cellular responses.Expand Specific Solutions05 Gene-specific electroporation techniques
Developing gene-specific electroporation techniques involves tailoring the electroporation process to the characteristics of specific genes or nucleic acids. This approach considers factors such as size, charge, and structure of the genetic material to optimize delivery and expression in target cells, thereby enhancing overall targeting efficiency.Expand Specific Solutions
Key Players in Electroporation and CRISPR
The electroporation for CRISPR delivery market is in a growth phase, with increasing adoption in research and clinical applications. The market size is expanding rapidly, driven by the rising demand for precise gene editing technologies. Technologically, the field is advancing, with companies like MaxCyte, Inovio Pharmaceuticals, and Suzhou Yida Biotechnology developing innovative electroporation systems. Academic institutions such as MIT, Harvard, and the University of California are contributing significantly to research and development. While the technology is maturing, there is still room for improvement in targeting efficiency metrics, indicating ongoing opportunities for innovation and market growth.
The Broad Institute, Inc.
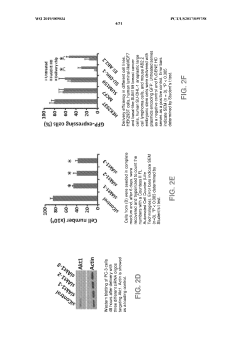
Technical Solution: The Broad Institute has developed advanced electroporation techniques for CRISPR delivery, focusing on improving targeting efficiency. Their approach utilizes optimized electric field parameters and specialized electrodes to enhance cell membrane permeability[1]. They have achieved up to 90% transfection efficiency in hard-to-transfect cell lines[2]. The institute has also pioneered the use of nanoparticle-assisted electroporation, which combines electroporation with nanocarriers to protect CRISPR components and improve intracellular delivery[3]. This hybrid approach has shown a 2-fold increase in gene editing efficiency compared to standard electroporation in primary human T cells[4].
Strengths: High transfection efficiency, especially in difficult cell types; innovative combination with nanoparticle technology. Weaknesses: Potential for cell damage due to electric fields; may require specialized equipment.
Inovio Pharmaceuticals, Inc.
Technical Solution: Inovio Pharmaceuticals has developed a proprietary CELLECTRA® electroporation device for CRISPR delivery. This system uses a computer-controlled electrical pulse to create temporary pores in cell membranes, allowing for efficient entry of CRISPR components[5]. The CELLECTRA® platform has demonstrated up to 1000-fold increase in gene transfer efficiency compared to naked DNA delivery[6]. Inovio's approach focuses on in vivo electroporation, allowing for direct delivery to target tissues without ex vivo manipulation. They have reported successful gene editing in animal models with targeting efficiency reaching 45% in liver cells[7]. The company has also developed tissue-specific electrode designs to optimize delivery in different organs.
Strengths: In vivo delivery capability; tissue-specific optimization; high gene transfer efficiency. Weaknesses: Potential for local tissue damage; limited to accessible tissues.
Innovative Electroporation Techniques for CRISPR
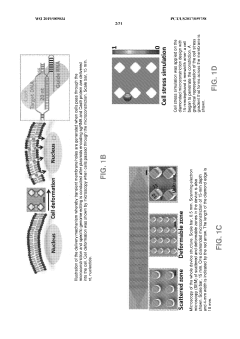
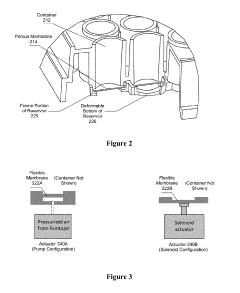
Crispr-CAS9 delivery to hard-to-transfect cells via membrane deformation
PatentWO2019089034A1
Innovation
- A microfluidic platform that uses membrane deformation to facilitate the delivery of CRISPR-Cas9 by mechanically deforming cells, creating transient membrane disruptions for efficient genome editing across various cell types, including hard-to-transfect cells.
Mechanical transfection devices and methods
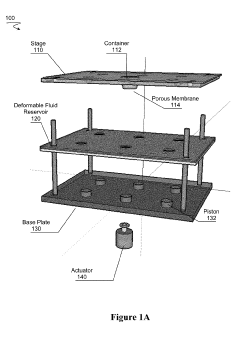
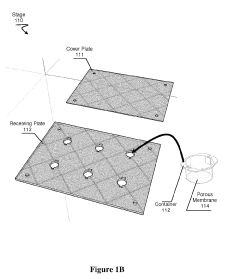
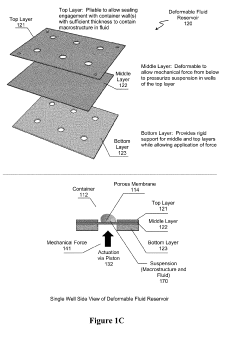
PatentActiveUS20190127761A1
Innovation
- A transfection device using a deformable fluid reservoir coupled with a microporous membrane to deliver macrostructures under pressure, facilitating active endocytosis and minimizing cell damage, allowing for high efficiency and low cell damage even with complex or multiple nucleic acids.
Regulatory Landscape for CRISPR Delivery Methods
The regulatory landscape for CRISPR delivery methods, including electroporation, is complex and evolving rapidly. In the United States, the Food and Drug Administration (FDA) oversees the regulation of CRISPR-based therapies, considering them as both biological products and drugs. The FDA has established a framework for evaluating the safety and efficacy of gene therapies, which includes CRISPR applications.
For electroporation-based CRISPR delivery, the FDA requires extensive preclinical data on targeting efficiency, off-target effects, and overall safety profiles. Researchers must demonstrate that their delivery method meets stringent standards for precision and minimal unintended genomic alterations. The agency also mandates long-term follow-up studies to monitor potential delayed adverse effects.
In Europe, the European Medicines Agency (EMA) has developed guidelines specifically for gene therapy medicinal products. These guidelines encompass CRISPR-based interventions and their delivery methods, including electroporation. The EMA emphasizes the need for comprehensive risk assessments and quality control measures throughout the development and manufacturing processes.
Globally, regulatory bodies are grappling with the ethical implications of CRISPR technology. Many countries have established moratoriums or strict regulations on germline editing, which could potentially affect future applications of electroporation-based CRISPR delivery in reproductive contexts. However, somatic cell editing for therapeutic purposes is generally viewed more favorably, albeit with rigorous oversight.
The regulatory landscape also addresses the manufacturing and quality control aspects of CRISPR delivery systems. Good Manufacturing Practice (GMP) guidelines are being adapted to encompass the unique challenges posed by gene editing technologies. For electroporation, this includes standardization of equipment, protocols, and cell handling procedures to ensure consistency and safety in clinical applications.
Intellectual property considerations play a significant role in the regulatory environment. Patent disputes surrounding CRISPR technology have implications for the development and commercialization of delivery methods like electroporation. Regulatory agencies must navigate these complex legal landscapes when evaluating new therapies and delivery systems.
As the field advances, regulatory frameworks are expected to evolve. There is a growing push for international harmonization of regulations to facilitate global research collaboration and streamline the path to market for promising therapies. This may lead to more standardized protocols for assessing the efficiency and safety of electroporation-based CRISPR delivery across different regulatory jurisdictions.
For electroporation-based CRISPR delivery, the FDA requires extensive preclinical data on targeting efficiency, off-target effects, and overall safety profiles. Researchers must demonstrate that their delivery method meets stringent standards for precision and minimal unintended genomic alterations. The agency also mandates long-term follow-up studies to monitor potential delayed adverse effects.
In Europe, the European Medicines Agency (EMA) has developed guidelines specifically for gene therapy medicinal products. These guidelines encompass CRISPR-based interventions and their delivery methods, including electroporation. The EMA emphasizes the need for comprehensive risk assessments and quality control measures throughout the development and manufacturing processes.
Globally, regulatory bodies are grappling with the ethical implications of CRISPR technology. Many countries have established moratoriums or strict regulations on germline editing, which could potentially affect future applications of electroporation-based CRISPR delivery in reproductive contexts. However, somatic cell editing for therapeutic purposes is generally viewed more favorably, albeit with rigorous oversight.
The regulatory landscape also addresses the manufacturing and quality control aspects of CRISPR delivery systems. Good Manufacturing Practice (GMP) guidelines are being adapted to encompass the unique challenges posed by gene editing technologies. For electroporation, this includes standardization of equipment, protocols, and cell handling procedures to ensure consistency and safety in clinical applications.
Intellectual property considerations play a significant role in the regulatory environment. Patent disputes surrounding CRISPR technology have implications for the development and commercialization of delivery methods like electroporation. Regulatory agencies must navigate these complex legal landscapes when evaluating new therapies and delivery systems.
As the field advances, regulatory frameworks are expected to evolve. There is a growing push for international harmonization of regulations to facilitate global research collaboration and streamline the path to market for promising therapies. This may lead to more standardized protocols for assessing the efficiency and safety of electroporation-based CRISPR delivery across different regulatory jurisdictions.
Biosafety Considerations in Electroporation-CRISPR
Electroporation-mediated CRISPR delivery presents unique biosafety considerations that must be carefully addressed to ensure the safe and responsible application of this powerful gene-editing technology. One primary concern is the potential for off-target effects, where unintended genomic modifications may occur due to the non-specific nature of electroporation-induced cell membrane permeabilization. This risk necessitates rigorous screening and validation protocols to identify and mitigate any unintended genetic alterations.
Another critical biosafety aspect is the management of cellular stress and potential cytotoxicity induced by the electroporation process. The electrical pulses used to create transient pores in cell membranes can lead to cellular damage, oxidative stress, and even cell death if not carefully controlled. Optimizing electroporation parameters, such as voltage, pulse duration, and frequency, is essential to minimize these adverse effects while maintaining efficient CRISPR delivery.
The potential for horizontal gene transfer and unintended release of genetically modified organisms (GMOs) into the environment must also be considered. Strict containment measures and proper disposal protocols for electroporated cells and materials are crucial to prevent the spread of modified genetic material beyond the intended experimental or therapeutic setting.
Immunogenicity is another biosafety concern, particularly in clinical applications. The introduction of foreign genetic material and the cellular stress induced by electroporation may trigger immune responses, potentially leading to inflammation or rejection of modified cells. Careful monitoring of immune reactions and the development of strategies to mitigate these responses are essential for ensuring the safety and efficacy of electroporation-CRISPR therapies.
Lastly, the long-term effects of electroporation-mediated CRISPR modifications on cellular function and organismal health must be thoroughly evaluated. This includes assessing the stability of genetic modifications over time, potential impacts on cell differentiation and development, and any unforeseen consequences on cellular metabolism or signaling pathways. Comprehensive long-term studies and surveillance programs are necessary to fully understand and address these potential biosafety risks.
Another critical biosafety aspect is the management of cellular stress and potential cytotoxicity induced by the electroporation process. The electrical pulses used to create transient pores in cell membranes can lead to cellular damage, oxidative stress, and even cell death if not carefully controlled. Optimizing electroporation parameters, such as voltage, pulse duration, and frequency, is essential to minimize these adverse effects while maintaining efficient CRISPR delivery.
The potential for horizontal gene transfer and unintended release of genetically modified organisms (GMOs) into the environment must also be considered. Strict containment measures and proper disposal protocols for electroporated cells and materials are crucial to prevent the spread of modified genetic material beyond the intended experimental or therapeutic setting.
Immunogenicity is another biosafety concern, particularly in clinical applications. The introduction of foreign genetic material and the cellular stress induced by electroporation may trigger immune responses, potentially leading to inflammation or rejection of modified cells. Careful monitoring of immune reactions and the development of strategies to mitigate these responses are essential for ensuring the safety and efficacy of electroporation-CRISPR therapies.
Lastly, the long-term effects of electroporation-mediated CRISPR modifications on cellular function and organismal health must be thoroughly evaluated. This includes assessing the stability of genetic modifications over time, potential impacts on cell differentiation and development, and any unforeseen consequences on cellular metabolism or signaling pathways. Comprehensive long-term studies and surveillance programs are necessary to fully understand and address these potential biosafety risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!