How To Prevent Arcing And Sample Damage In Electroporation Cells
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electroporation Challenges
Electroporation, a widely used technique in molecular biology and biotechnology, faces several significant challenges that can hinder its effectiveness and reliability. One of the primary concerns is the occurrence of arcing, an electrical discharge that can severely damage both the sample and the electroporation apparatus. Arcing typically occurs when the electrical field strength exceeds the dielectric strength of the medium, leading to a sudden, uncontrolled current flow.
The risk of arcing is particularly high when working with high-conductivity buffers or samples with high salt concentrations. These conditions can create localized areas of increased conductivity, facilitating the formation of electrical arcs. Moreover, the presence of air bubbles in the electroporation cuvette can act as nucleation points for arc formation, exacerbating the problem.
Sample damage is another critical challenge in electroporation. The high-voltage electrical pulses used to create temporary pores in cell membranes can also cause irreversible damage to cellular structures and biomolecules. This damage can manifest as reduced cell viability, compromised membrane integrity, and degradation of nucleic acids or proteins. The delicate balance between achieving efficient transfection and minimizing cell damage is a constant concern for researchers and practitioners.
The heterogeneity of biological samples further complicates the electroporation process. Different cell types and tissues exhibit varying sensitivities to electrical fields, making it challenging to establish universal protocols. This variability can lead to inconsistent results and reduced reproducibility across experiments, hindering the widespread adoption of electroporation in certain applications.
Another significant challenge is the optimization of electroporation parameters. Factors such as pulse duration, voltage, and the number of pulses must be carefully tuned to achieve optimal results for each specific application. This optimization process can be time-consuming and resource-intensive, often requiring extensive trial-and-error experimentation.
The scalability of electroporation presents additional hurdles, particularly when transitioning from laboratory-scale experiments to industrial applications. Maintaining consistent and uniform electric field distribution in larger volumes or across multiple samples simultaneously remains a technical challenge. This limitation can impact the efficiency and cost-effectiveness of electroporation in large-scale bioprocessing or clinical applications.
Furthermore, the potential for sample contamination during the electroporation process is a persistent concern. The introduction of foreign materials or microorganisms can compromise the integrity of the experiment and lead to false results. Ensuring sterile conditions and preventing cross-contamination between samples are critical aspects that require careful consideration and specialized equipment.
The risk of arcing is particularly high when working with high-conductivity buffers or samples with high salt concentrations. These conditions can create localized areas of increased conductivity, facilitating the formation of electrical arcs. Moreover, the presence of air bubbles in the electroporation cuvette can act as nucleation points for arc formation, exacerbating the problem.
Sample damage is another critical challenge in electroporation. The high-voltage electrical pulses used to create temporary pores in cell membranes can also cause irreversible damage to cellular structures and biomolecules. This damage can manifest as reduced cell viability, compromised membrane integrity, and degradation of nucleic acids or proteins. The delicate balance between achieving efficient transfection and minimizing cell damage is a constant concern for researchers and practitioners.
The heterogeneity of biological samples further complicates the electroporation process. Different cell types and tissues exhibit varying sensitivities to electrical fields, making it challenging to establish universal protocols. This variability can lead to inconsistent results and reduced reproducibility across experiments, hindering the widespread adoption of electroporation in certain applications.
Another significant challenge is the optimization of electroporation parameters. Factors such as pulse duration, voltage, and the number of pulses must be carefully tuned to achieve optimal results for each specific application. This optimization process can be time-consuming and resource-intensive, often requiring extensive trial-and-error experimentation.
The scalability of electroporation presents additional hurdles, particularly when transitioning from laboratory-scale experiments to industrial applications. Maintaining consistent and uniform electric field distribution in larger volumes or across multiple samples simultaneously remains a technical challenge. This limitation can impact the efficiency and cost-effectiveness of electroporation in large-scale bioprocessing or clinical applications.
Furthermore, the potential for sample contamination during the electroporation process is a persistent concern. The introduction of foreign materials or microorganisms can compromise the integrity of the experiment and lead to false results. Ensuring sterile conditions and preventing cross-contamination between samples are critical aspects that require careful consideration and specialized equipment.
Market Demand Analysis
The market demand for effective electroporation solutions that prevent arcing and sample damage is experiencing significant growth across various sectors. This demand is primarily driven by the expanding applications of electroporation in biotechnology, pharmaceutical research, and medical treatments.
In the biotechnology sector, there is a growing need for reliable electroporation techniques to facilitate gene editing, cell transfection, and the development of genetically modified organisms. As CRISPR and other gene-editing technologies advance, the demand for precise and safe electroporation methods becomes increasingly critical. Research institutions and biotech companies are actively seeking solutions that can minimize sample damage and improve transfection efficiency.
The pharmaceutical industry is another major driver of market demand for improved electroporation technologies. Drug discovery and development processes heavily rely on efficient cell manipulation techniques. Electroporation plays a crucial role in introducing drug candidates into cells for testing and evaluation. The ability to prevent arcing and sample damage during these processes can significantly enhance the accuracy of drug screening and reduce the time and cost associated with pharmaceutical research.
In the medical field, electroporation is gaining traction as a promising method for various treatments, including cancer therapy and gene therapy. The demand for safe and effective electroporation devices in clinical settings is expected to rise as these treatments become more widespread. Hospitals and medical research centers are increasingly investing in advanced electroporation equipment that can deliver precise electrical pulses while minimizing the risk of tissue damage.
The global market for electroporation equipment and reagents is projected to grow substantially in the coming years. This growth is fueled by the increasing adoption of electroporation techniques in both research and clinical applications. North America currently holds the largest market share, followed by Europe and Asia-Pacific. However, emerging markets in developing countries are expected to show rapid growth as their biotechnology and pharmaceutical sectors expand.
Key factors driving market demand include the need for higher transfection efficiency, reduced cell mortality, and improved reproducibility of results. Researchers and clinicians are particularly interested in solutions that can address the challenges of arcing and sample damage, as these issues can significantly impact the success and reliability of electroporation procedures.
As the applications of electroporation continue to diversify, there is a growing demand for specialized equipment tailored to specific cell types and experimental conditions. This trend is creating opportunities for innovation in electrode design, pulse generation technology, and sample handling systems. Companies that can develop and commercialize solutions addressing these specific needs are likely to gain a competitive edge in the market.
In the biotechnology sector, there is a growing need for reliable electroporation techniques to facilitate gene editing, cell transfection, and the development of genetically modified organisms. As CRISPR and other gene-editing technologies advance, the demand for precise and safe electroporation methods becomes increasingly critical. Research institutions and biotech companies are actively seeking solutions that can minimize sample damage and improve transfection efficiency.
The pharmaceutical industry is another major driver of market demand for improved electroporation technologies. Drug discovery and development processes heavily rely on efficient cell manipulation techniques. Electroporation plays a crucial role in introducing drug candidates into cells for testing and evaluation. The ability to prevent arcing and sample damage during these processes can significantly enhance the accuracy of drug screening and reduce the time and cost associated with pharmaceutical research.
In the medical field, electroporation is gaining traction as a promising method for various treatments, including cancer therapy and gene therapy. The demand for safe and effective electroporation devices in clinical settings is expected to rise as these treatments become more widespread. Hospitals and medical research centers are increasingly investing in advanced electroporation equipment that can deliver precise electrical pulses while minimizing the risk of tissue damage.
The global market for electroporation equipment and reagents is projected to grow substantially in the coming years. This growth is fueled by the increasing adoption of electroporation techniques in both research and clinical applications. North America currently holds the largest market share, followed by Europe and Asia-Pacific. However, emerging markets in developing countries are expected to show rapid growth as their biotechnology and pharmaceutical sectors expand.
Key factors driving market demand include the need for higher transfection efficiency, reduced cell mortality, and improved reproducibility of results. Researchers and clinicians are particularly interested in solutions that can address the challenges of arcing and sample damage, as these issues can significantly impact the success and reliability of electroporation procedures.
As the applications of electroporation continue to diversify, there is a growing demand for specialized equipment tailored to specific cell types and experimental conditions. This trend is creating opportunities for innovation in electrode design, pulse generation technology, and sample handling systems. Companies that can develop and commercialize solutions addressing these specific needs are likely to gain a competitive edge in the market.
Current Arcing Issues
Electroporation is a widely used technique in molecular biology and biotechnology for introducing foreign molecules into cells. However, one of the major challenges in this process is the occurrence of arcing, which can lead to significant sample damage and reduced transfection efficiency. Arcing is an electrical discharge that occurs when the applied voltage exceeds the dielectric strength of the sample buffer or when there are air bubbles present in the electroporation cuvette.
The current issues with arcing in electroporation cells are multifaceted and can have severe consequences on experimental outcomes. One of the primary concerns is the generation of excessive heat during the arcing event. This sudden temperature increase can cause localized cell death, compromising the integrity of the sample and potentially leading to the loss of valuable biological material. Moreover, the high-energy discharge associated with arcing can create shockwaves within the sample, causing mechanical stress on the cells and further contributing to cellular damage.
Another significant issue is the alteration of the electric field distribution within the electroporation cuvette. Arcing events can create non-uniform electric fields, resulting in inconsistent pore formation across the cell population. This variability in membrane permeabilization can lead to unpredictable transfection efficiencies and potentially skew experimental results. Additionally, the occurrence of arcing can cause rapid changes in the conductivity of the sample buffer, further exacerbating the non-uniformity of the electric field and potentially damaging sensitive electronic components of the electroporation device.
The presence of metal ions or impurities in the sample buffer can also contribute to arcing issues. These contaminants can lower the dielectric strength of the buffer, making it more susceptible to electrical breakdown at lower voltages. Furthermore, the formation of metal complexes during arcing can generate reactive oxygen species, which may cause oxidative stress and damage to cellular components, including DNA, proteins, and lipids.
Arcing events can also lead to the degradation of the electrodes in the electroporation cuvette. The high current densities associated with arcing can cause electrode erosion, releasing metal particles into the sample. These particles not only contaminate the biological material but can also act as nucleation sites for further arcing events in subsequent experiments, creating a cycle of ongoing issues.
To address these challenges, researchers and equipment manufacturers have been exploring various strategies. These include optimizing buffer compositions to increase dielectric strength, developing advanced electrode materials resistant to erosion, and implementing sophisticated pulse-shaping techniques to minimize the likelihood of arcing. Additionally, the integration of real-time monitoring systems to detect and mitigate arcing events during electroporation is an area of active research and development in the field.
The current issues with arcing in electroporation cells are multifaceted and can have severe consequences on experimental outcomes. One of the primary concerns is the generation of excessive heat during the arcing event. This sudden temperature increase can cause localized cell death, compromising the integrity of the sample and potentially leading to the loss of valuable biological material. Moreover, the high-energy discharge associated with arcing can create shockwaves within the sample, causing mechanical stress on the cells and further contributing to cellular damage.
Another significant issue is the alteration of the electric field distribution within the electroporation cuvette. Arcing events can create non-uniform electric fields, resulting in inconsistent pore formation across the cell population. This variability in membrane permeabilization can lead to unpredictable transfection efficiencies and potentially skew experimental results. Additionally, the occurrence of arcing can cause rapid changes in the conductivity of the sample buffer, further exacerbating the non-uniformity of the electric field and potentially damaging sensitive electronic components of the electroporation device.
The presence of metal ions or impurities in the sample buffer can also contribute to arcing issues. These contaminants can lower the dielectric strength of the buffer, making it more susceptible to electrical breakdown at lower voltages. Furthermore, the formation of metal complexes during arcing can generate reactive oxygen species, which may cause oxidative stress and damage to cellular components, including DNA, proteins, and lipids.
Arcing events can also lead to the degradation of the electrodes in the electroporation cuvette. The high current densities associated with arcing can cause electrode erosion, releasing metal particles into the sample. These particles not only contaminate the biological material but can also act as nucleation sites for further arcing events in subsequent experiments, creating a cycle of ongoing issues.
To address these challenges, researchers and equipment manufacturers have been exploring various strategies. These include optimizing buffer compositions to increase dielectric strength, developing advanced electrode materials resistant to erosion, and implementing sophisticated pulse-shaping techniques to minimize the likelihood of arcing. Additionally, the integration of real-time monitoring systems to detect and mitigate arcing events during electroporation is an area of active research and development in the field.
Existing Anti-Arcing Solutions
01 Prevention of arcing during electroporation
Various methods are employed to prevent arcing during electroporation, which can damage cells and samples. These include optimizing electrode design, controlling pulse parameters, and using specialized buffer solutions. By minimizing the risk of arcing, the efficiency and safety of electroporation procedures can be significantly improved.- Prevention of arcing during electroporation: Various methods are employed to prevent arcing during electroporation, which can damage cells and samples. These include optimizing electrode design, controlling pulse parameters, and using specialized buffer solutions. Implementing these techniques helps maintain sample integrity and improves the efficiency of the electroporation process.
- Electrode design for minimizing sample damage: Advanced electrode designs are developed to minimize sample damage during electroporation. These designs focus on reducing localized electric field concentrations, improving heat dissipation, and ensuring uniform field distribution across the sample. Such improvements help protect cells from excessive stress and maintain their viability.
- Pulse optimization for cell protection: Optimizing pulse parameters is crucial for protecting cells during electroporation. This includes adjusting pulse duration, amplitude, and frequency to achieve effective membrane permeabilization while minimizing cellular stress. Advanced pulse protocols are developed to balance transfection efficiency with cell viability.
- Buffer composition for sample preservation: Specialized buffer solutions are formulated to preserve sample integrity during electroporation. These buffers often contain components that stabilize cell membranes, reduce oxidative stress, and maintain optimal conductivity. The right buffer composition can significantly reduce the risk of sample damage and improve overall electroporation outcomes.
- Post-electroporation cell recovery techniques: Various techniques are developed to enhance cell recovery after electroporation. These include the use of growth factors, antioxidants, and specialized recovery media. Implementing effective post-electroporation protocols helps mitigate cellular stress and damage, improving overall cell survival and transfection efficiency.
02 Sample protection strategies
To protect samples from damage during electroporation, researchers have developed various strategies. These include the use of protective agents, temperature control systems, and specialized sample holders. These approaches help maintain sample integrity and viability throughout the electroporation process.Expand Specific Solutions03 Pulse optimization techniques
Optimizing pulse parameters is crucial for reducing the risk of arcing and sample damage. This involves adjusting pulse duration, amplitude, and frequency to achieve efficient electroporation while minimizing harmful effects. Advanced pulse generators and control systems are used to fine-tune these parameters for different cell types and applications.Expand Specific Solutions04 Electrode design and materials
Innovative electrode designs and materials play a significant role in preventing arcing and sample damage. This includes the development of specialized electrode geometries, surface treatments, and novel conductive materials. These advancements help distribute electric fields more uniformly and reduce the likelihood of localized high-voltage areas that can cause arcing.Expand Specific Solutions05 Monitoring and feedback systems
Advanced monitoring and feedback systems are implemented to detect and prevent arcing in real-time during electroporation. These systems can rapidly adjust pulse parameters or terminate the process if arcing is detected, thereby protecting cells and samples from damage. Additionally, they provide valuable data for optimizing electroporation protocols.Expand Specific Solutions
Key Industry Players
The electroporation cell technology market is in a growth phase, with increasing demand driven by applications in gene therapy, cell-based therapies, and biotechnology research. The global market size is projected to expand significantly in the coming years. Technologically, the field is advancing rapidly, with companies like MaxCyte, Bio-Rad Laboratories, and Suzhou Yida Biotechnology leading innovation. These firms are developing more sophisticated electroporation systems that address key challenges such as arcing and sample damage. The focus is on improving efficiency, scalability, and cell viability. While established players dominate, emerging companies are introducing novel approaches, indicating a dynamic and competitive landscape with potential for further technological breakthroughs.
MaxCyte, Inc.
Technical Solution: MaxCyte has developed a proprietary Flow Electroporation Technology to prevent arcing and sample damage in electroporation cells. Their system utilizes a unique flow-based approach, where cells and genetic material are continuously passed through a controlled electric field[1]. This method ensures uniform exposure to the electric field, reducing the risk of arcing and cell damage. The technology incorporates precise voltage control and pulse timing mechanisms, allowing for optimal membrane permeabilization without compromising cell viability[2]. Additionally, MaxCyte's system employs specialized electrodes and buffer solutions that minimize local field intensities, further reducing the likelihood of arcing events[3].
Strengths: Highly efficient for large-scale transfections, suitable for a wide range of cell types, and maintains high cell viability. Weaknesses: Requires specialized equipment and may have higher initial costs compared to traditional electroporation methods.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has introduced the Gene Pulser Xcell Electroporation System to address arcing and sample damage issues in electroporation. This system employs advanced pulse delivery technology that automatically optimizes electrical parameters based on sample characteristics[4]. It utilizes a combination of square wave and exponential decay waveforms to achieve efficient cell permeabilization while minimizing the risk of arcing. The system also incorporates real-time monitoring of voltage and current during pulse delivery, allowing for immediate adjustment to prevent potential arcing events[5]. Bio-Rad's technology includes specialized cuvettes with uniform electrode spacing and surface treatment to ensure consistent field distribution and reduce hotspots that could lead to arcing[6].
Strengths: Versatile system suitable for various cell types, user-friendly interface with pre-optimized protocols. Weaknesses: May require some optimization for specific cell lines or applications, and the system's complexity could present a learning curve for new users.
Core Anti-Arcing Innovations
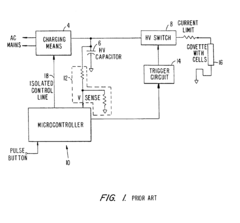
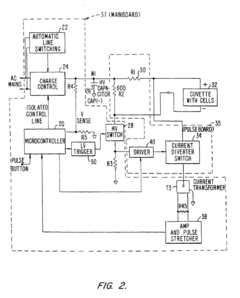
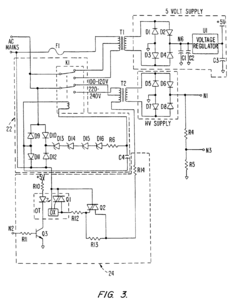
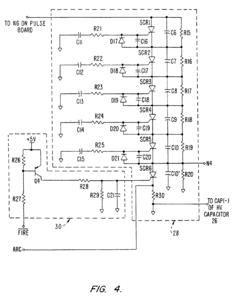
Electroporation cell with arc prevention/reduction
PatentInactiveEP1190075B1
Innovation
- A current diverting circuit is integrated into the electroporator system to divert current away from the sample during an arc-over event, preventing damage and reducing the duration of high voltage application.
In-situ discharge to avoid arcing during plasma etch processes
PatentInactiveUS6914007B2
Innovation
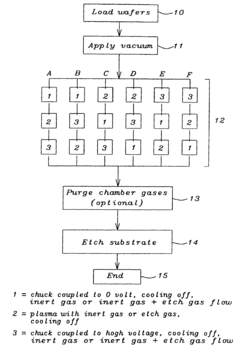
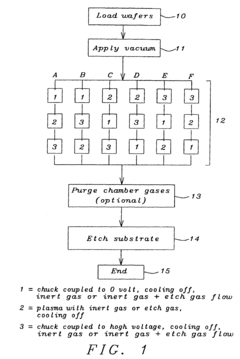
- A discharge sequence is implemented before the plasma etch process, involving three steps: coupling the wafer chuck to a 0 voltage source, generating a plasma for 0 to 30 seconds, and then coupling it to a high voltage source, with inert or fluorocarbon gases flowing through the chamber to stabilize and release charges, applicable at any point in a multiple-step etch process.
Safety Regulations
Electroporation is a powerful technique used in various biological applications, but it comes with inherent risks that necessitate strict safety regulations. These regulations are designed to protect both the operators and the integrity of the samples being processed. The primary safety concerns in electroporation revolve around electrical hazards, biological containment, and sample preservation.
Electrical safety is paramount when working with electroporation equipment. Regulations typically require that all devices be properly grounded and insulated to prevent electrical shock. Operators must be trained in the safe use of high-voltage equipment and should wear appropriate personal protective equipment (PPE), including insulated gloves and safety goggles. Emergency shut-off switches must be easily accessible, and regular maintenance checks of the equipment are mandatory to ensure all safety features are functioning correctly.
Biological safety is another critical aspect of electroporation regulations. As the technique often involves working with potentially hazardous biological materials, proper containment measures are essential. This includes the use of biosafety cabinets when handling samples, adherence to sterile techniques, and proper disposal of biological waste. Depending on the risk level of the materials being used, additional containment measures may be required, such as negative pressure rooms or specialized air filtration systems.
To prevent arcing and sample damage, regulations often specify the use of specialized electroporation cuvettes designed to minimize these risks. These cuvettes must meet specific standards for material composition and electrical resistance. Additionally, protocols for sample preparation and handling are typically outlined to reduce the likelihood of contamination or damage during the electroporation process.
Safety regulations also address the importance of proper training and documentation. All personnel involved in electroporation procedures must receive comprehensive training on both the technical aspects of the technique and the associated safety protocols. Detailed records of training, equipment maintenance, and experimental procedures must be maintained and regularly audited to ensure compliance with safety standards.
Environmental considerations are also incorporated into safety regulations for electroporation. This includes guidelines for the proper storage and handling of chemicals used in the process, as well as protocols for managing electromagnetic emissions from the equipment. In some cases, shielding may be required to protect nearby sensitive instruments or to comply with workplace electromagnetic field exposure limits.
Lastly, safety regulations often mandate the implementation of emergency response procedures specific to electroporation-related incidents. This includes protocols for addressing electrical fires, chemical spills, and potential biological exposures. Regular safety drills and updates to emergency procedures are typically required to ensure all personnel are prepared to respond effectively in case of an accident.
Electrical safety is paramount when working with electroporation equipment. Regulations typically require that all devices be properly grounded and insulated to prevent electrical shock. Operators must be trained in the safe use of high-voltage equipment and should wear appropriate personal protective equipment (PPE), including insulated gloves and safety goggles. Emergency shut-off switches must be easily accessible, and regular maintenance checks of the equipment are mandatory to ensure all safety features are functioning correctly.
Biological safety is another critical aspect of electroporation regulations. As the technique often involves working with potentially hazardous biological materials, proper containment measures are essential. This includes the use of biosafety cabinets when handling samples, adherence to sterile techniques, and proper disposal of biological waste. Depending on the risk level of the materials being used, additional containment measures may be required, such as negative pressure rooms or specialized air filtration systems.
To prevent arcing and sample damage, regulations often specify the use of specialized electroporation cuvettes designed to minimize these risks. These cuvettes must meet specific standards for material composition and electrical resistance. Additionally, protocols for sample preparation and handling are typically outlined to reduce the likelihood of contamination or damage during the electroporation process.
Safety regulations also address the importance of proper training and documentation. All personnel involved in electroporation procedures must receive comprehensive training on both the technical aspects of the technique and the associated safety protocols. Detailed records of training, equipment maintenance, and experimental procedures must be maintained and regularly audited to ensure compliance with safety standards.
Environmental considerations are also incorporated into safety regulations for electroporation. This includes guidelines for the proper storage and handling of chemicals used in the process, as well as protocols for managing electromagnetic emissions from the equipment. In some cases, shielding may be required to protect nearby sensitive instruments or to comply with workplace electromagnetic field exposure limits.
Lastly, safety regulations often mandate the implementation of emergency response procedures specific to electroporation-related incidents. This includes protocols for addressing electrical fires, chemical spills, and potential biological exposures. Regular safety drills and updates to emergency procedures are typically required to ensure all personnel are prepared to respond effectively in case of an accident.
Cell Viability Optimization
Optimizing cell viability is crucial in electroporation to ensure successful transfection while minimizing cellular damage. One of the primary challenges in this process is preventing arcing and sample damage, which can significantly reduce cell viability and transfection efficiency. To address this issue, several strategies have been developed and implemented in recent years.
A key approach to preventing arcing is the careful control of buffer composition and conductivity. By using low-conductivity buffers, researchers can reduce the risk of electrical arcing during the electroporation process. These buffers typically contain lower concentrations of ions compared to standard cell culture media, which helps to minimize the flow of electric current through the sample. Additionally, the use of specialized electroporation buffers that contain osmoprotectants, such as sucrose or trehalose, can help maintain cell integrity during the procedure.
Another important factor in optimizing cell viability is the precise control of electrical parameters. This includes adjusting the voltage, pulse duration, and number of pulses applied to the sample. Recent advancements in electroporation technology have led to the development of systems that allow for fine-tuning of these parameters, enabling researchers to optimize conditions for specific cell types and applications. Some modern electroporators even incorporate real-time monitoring of electrical resistance, allowing for automatic adjustment of parameters to prevent arcing.
The design of electroporation cuvettes and chambers has also evolved to enhance cell viability. Newer cuvette designs feature improved electrode materials and configurations that promote more uniform electric field distribution across the sample. This helps to reduce localized areas of high field strength that can lead to cell damage or arcing. Some advanced systems now utilize microfluidic channels or plate-based formats, which allow for better control of sample volume and cell density, further reducing the risk of arcing and improving overall transfection efficiency.
Temperature management during electroporation has emerged as a critical factor in maintaining cell viability. Cooling the sample and electroporation chamber can help mitigate thermal damage caused by Joule heating during the procedure. Some systems now incorporate built-in cooling mechanisms or allow for the use of pre-chilled cuvettes to maintain optimal temperature conditions throughout the process.
Lastly, the development of gentler electroporation protocols has contributed significantly to improving cell viability. These protocols often involve the use of multiple low-voltage pulses rather than a single high-voltage pulse, which can be less damaging to cells while still achieving efficient transfection. Combined with advanced waveform shaping techniques, these approaches allow for better control of membrane permeabilization while minimizing cellular stress.
A key approach to preventing arcing is the careful control of buffer composition and conductivity. By using low-conductivity buffers, researchers can reduce the risk of electrical arcing during the electroporation process. These buffers typically contain lower concentrations of ions compared to standard cell culture media, which helps to minimize the flow of electric current through the sample. Additionally, the use of specialized electroporation buffers that contain osmoprotectants, such as sucrose or trehalose, can help maintain cell integrity during the procedure.
Another important factor in optimizing cell viability is the precise control of electrical parameters. This includes adjusting the voltage, pulse duration, and number of pulses applied to the sample. Recent advancements in electroporation technology have led to the development of systems that allow for fine-tuning of these parameters, enabling researchers to optimize conditions for specific cell types and applications. Some modern electroporators even incorporate real-time monitoring of electrical resistance, allowing for automatic adjustment of parameters to prevent arcing.
The design of electroporation cuvettes and chambers has also evolved to enhance cell viability. Newer cuvette designs feature improved electrode materials and configurations that promote more uniform electric field distribution across the sample. This helps to reduce localized areas of high field strength that can lead to cell damage or arcing. Some advanced systems now utilize microfluidic channels or plate-based formats, which allow for better control of sample volume and cell density, further reducing the risk of arcing and improving overall transfection efficiency.
Temperature management during electroporation has emerged as a critical factor in maintaining cell viability. Cooling the sample and electroporation chamber can help mitigate thermal damage caused by Joule heating during the procedure. Some systems now incorporate built-in cooling mechanisms or allow for the use of pre-chilled cuvettes to maintain optimal temperature conditions throughout the process.
Lastly, the development of gentler electroporation protocols has contributed significantly to improving cell viability. These protocols often involve the use of multiple low-voltage pulses rather than a single high-voltage pulse, which can be less damaging to cells while still achieving efficient transfection. Combined with advanced waveform shaping techniques, these approaches allow for better control of membrane permeabilization while minimizing cellular stress.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!