Electroporation For Plant Transformation: Optimized Pulse Schedules
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electroporation Background
Electroporation is a powerful technique that has revolutionized plant transformation processes. This method involves the application of electrical pulses to create temporary pores in cell membranes, allowing the introduction of foreign DNA into plant cells. The technique was first developed in the 1980s and has since become a widely adopted method for genetic modification in various plant species.
The fundamental principle behind electroporation is based on the concept of increasing cell membrane permeability through the application of controlled electric fields. When subjected to these fields, the lipid bilayer of the cell membrane undergoes a temporary rearrangement, forming transient pores. These pores allow for the passage of macromolecules, such as DNA, RNA, or proteins, into the cell.
In the context of plant transformation, electroporation offers several advantages over other methods. It is relatively simple to perform, requires minimal specialized equipment, and can be applied to a wide range of plant species and cell types. Additionally, electroporation can be used for both transient and stable transformation, making it a versatile tool in plant biotechnology.
The efficiency of electroporation for plant transformation is influenced by various factors, including the strength and duration of the electric field, the composition of the electroporation buffer, and the physiological state of the target cells. One of the critical aspects of optimizing electroporation protocols is the design of pulse schedules, which refers to the specific pattern and parameters of the electric pulses applied to the cells.
Over the years, researchers have explored different pulse schedules to enhance transformation efficiency and minimize cell damage. These schedules can vary in terms of voltage, pulse duration, number of pulses, and intervals between pulses. The optimal parameters often depend on the specific plant species and tissue type being transformed.
Recent advancements in electroporation technology have focused on developing more sophisticated pulse generators and electrodes, as well as refining the composition of electroporation buffers. These improvements aim to increase transformation efficiency while reducing cell mortality and tissue damage.
As the field of plant biotechnology continues to evolve, electroporation remains a crucial tool for genetic engineering and functional genomics studies. Ongoing research in this area is directed towards further optimizing pulse schedules and developing species-specific protocols to expand the range of plants amenable to this transformation method.
The fundamental principle behind electroporation is based on the concept of increasing cell membrane permeability through the application of controlled electric fields. When subjected to these fields, the lipid bilayer of the cell membrane undergoes a temporary rearrangement, forming transient pores. These pores allow for the passage of macromolecules, such as DNA, RNA, or proteins, into the cell.
In the context of plant transformation, electroporation offers several advantages over other methods. It is relatively simple to perform, requires minimal specialized equipment, and can be applied to a wide range of plant species and cell types. Additionally, electroporation can be used for both transient and stable transformation, making it a versatile tool in plant biotechnology.
The efficiency of electroporation for plant transformation is influenced by various factors, including the strength and duration of the electric field, the composition of the electroporation buffer, and the physiological state of the target cells. One of the critical aspects of optimizing electroporation protocols is the design of pulse schedules, which refers to the specific pattern and parameters of the electric pulses applied to the cells.
Over the years, researchers have explored different pulse schedules to enhance transformation efficiency and minimize cell damage. These schedules can vary in terms of voltage, pulse duration, number of pulses, and intervals between pulses. The optimal parameters often depend on the specific plant species and tissue type being transformed.
Recent advancements in electroporation technology have focused on developing more sophisticated pulse generators and electrodes, as well as refining the composition of electroporation buffers. These improvements aim to increase transformation efficiency while reducing cell mortality and tissue damage.
As the field of plant biotechnology continues to evolve, electroporation remains a crucial tool for genetic engineering and functional genomics studies. Ongoing research in this area is directed towards further optimizing pulse schedules and developing species-specific protocols to expand the range of plants amenable to this transformation method.
Market Demand Analysis
The market demand for electroporation technology in plant transformation has been steadily growing, driven by the increasing need for genetically modified crops to address global food security challenges and environmental concerns. This technology offers a non-viral method for introducing foreign DNA into plant cells, making it an attractive alternative to traditional Agrobacterium-mediated transformation.
The agricultural biotechnology sector, valued at over $30 billion globally, is a key driver of demand for electroporation technology. As countries seek to improve crop yields, enhance nutritional content, and develop resistance to pests and diseases, the market for efficient plant transformation methods continues to expand. Electroporation, with its potential for optimized pulse schedules, addresses the growing demand for precise and controllable genetic modification techniques.
Research institutions and biotechnology companies are increasingly investing in electroporation technology for plant transformation. The ability to fine-tune pulse parameters allows for improved transformation efficiency across a wide range of plant species, including those traditionally considered recalcitrant to genetic modification. This versatility has led to a surge in demand from both public and private sector researchers working on crop improvement programs.
The market for electroporation equipment and consumables is also experiencing growth. Manufacturers of electroporation devices are developing more sophisticated systems with programmable pulse schedules, catering to the specific needs of plant scientists. This has created a niche market within the broader laboratory equipment industry, with annual growth rates exceeding the industry average.
Emerging trends in precision agriculture and sustainable farming practices are further fueling the demand for electroporation technology. As farmers and agribusinesses seek crops with enhanced traits such as drought tolerance, improved nutrient uptake, and resistance to climate change-induced stresses, the need for efficient plant transformation methods becomes more critical.
The pharmaceutical and nutraceutical industries are also contributing to the market demand for plant transformation technologies. The production of plant-based vaccines, therapeutic proteins, and bioactive compounds requires efficient methods for introducing foreign genes into plant cells. Electroporation, with its potential for high-throughput applications, is well-positioned to meet this growing demand.
Geographically, North America and Europe lead in terms of market demand for electroporation technology in plant transformation. However, rapidly developing economies in Asia-Pacific, particularly China and India, are showing increased interest as they invest heavily in agricultural biotechnology to ensure food security for their growing populations.
The agricultural biotechnology sector, valued at over $30 billion globally, is a key driver of demand for electroporation technology. As countries seek to improve crop yields, enhance nutritional content, and develop resistance to pests and diseases, the market for efficient plant transformation methods continues to expand. Electroporation, with its potential for optimized pulse schedules, addresses the growing demand for precise and controllable genetic modification techniques.
Research institutions and biotechnology companies are increasingly investing in electroporation technology for plant transformation. The ability to fine-tune pulse parameters allows for improved transformation efficiency across a wide range of plant species, including those traditionally considered recalcitrant to genetic modification. This versatility has led to a surge in demand from both public and private sector researchers working on crop improvement programs.
The market for electroporation equipment and consumables is also experiencing growth. Manufacturers of electroporation devices are developing more sophisticated systems with programmable pulse schedules, catering to the specific needs of plant scientists. This has created a niche market within the broader laboratory equipment industry, with annual growth rates exceeding the industry average.
Emerging trends in precision agriculture and sustainable farming practices are further fueling the demand for electroporation technology. As farmers and agribusinesses seek crops with enhanced traits such as drought tolerance, improved nutrient uptake, and resistance to climate change-induced stresses, the need for efficient plant transformation methods becomes more critical.
The pharmaceutical and nutraceutical industries are also contributing to the market demand for plant transformation technologies. The production of plant-based vaccines, therapeutic proteins, and bioactive compounds requires efficient methods for introducing foreign genes into plant cells. Electroporation, with its potential for high-throughput applications, is well-positioned to meet this growing demand.
Geographically, North America and Europe lead in terms of market demand for electroporation technology in plant transformation. However, rapidly developing economies in Asia-Pacific, particularly China and India, are showing increased interest as they invest heavily in agricultural biotechnology to ensure food security for their growing populations.
Technical Challenges
Electroporation for plant transformation faces several significant technical challenges that hinder its widespread adoption and efficiency. One of the primary obstacles is the optimization of pulse schedules, which is crucial for successful gene delivery without causing excessive damage to plant cells.
The delicate balance between effective cell membrane permeabilization and cell viability remains a major hurdle. High voltage pulses are necessary to create temporary pores in the cell membrane, allowing DNA to enter. However, excessive electrical stress can lead to irreversible damage and cell death. Researchers struggle to determine the ideal voltage, pulse duration, and number of pulses for different plant species and tissue types.
Another challenge lies in the variability of results across different plant species and even within the same species. The cell wall composition and thickness, which vary significantly among plants, affect the efficiency of electroporation. This variability necessitates the development of species-specific protocols, making the technique labor-intensive and time-consuming to optimize for each new plant variety.
The size and conformation of the DNA to be introduced also present technical difficulties. Larger plasmids or DNA constructs are more challenging to deliver efficiently through electroporation. The DNA's physical properties, such as supercoiling and methylation status, can influence its ability to pass through the temporary pores created by electrical pulses.
Tissue-specific challenges further complicate the process. Different plant tissues, such as leaf mesophyll, protoplasts, or embryogenic callus, respond differently to electroporation. Developing protocols that work effectively across various tissue types while maintaining cell viability is an ongoing challenge for researchers.
The scalability of electroporation for large-scale plant transformation projects remains a significant hurdle. Current methods often yield low transformation efficiencies, making it difficult to generate large numbers of transgenic plants efficiently. This limitation is particularly problematic for crop improvement programs that require high-throughput transformation capabilities.
Additionally, the reproducibility of electroporation results poses a challenge. Slight variations in experimental conditions, such as buffer composition, temperature, or even the physiological state of the plant tissue, can lead to inconsistent outcomes. This variability makes it difficult to establish standardized protocols across different laboratories and research groups.
Lastly, the integration of foreign DNA into the plant genome following electroporation is not always predictable or stable. Ensuring consistent gene expression and inheritance in subsequent generations remains a technical challenge that researchers continue to address through various molecular and breeding strategies.
The delicate balance between effective cell membrane permeabilization and cell viability remains a major hurdle. High voltage pulses are necessary to create temporary pores in the cell membrane, allowing DNA to enter. However, excessive electrical stress can lead to irreversible damage and cell death. Researchers struggle to determine the ideal voltage, pulse duration, and number of pulses for different plant species and tissue types.
Another challenge lies in the variability of results across different plant species and even within the same species. The cell wall composition and thickness, which vary significantly among plants, affect the efficiency of electroporation. This variability necessitates the development of species-specific protocols, making the technique labor-intensive and time-consuming to optimize for each new plant variety.
The size and conformation of the DNA to be introduced also present technical difficulties. Larger plasmids or DNA constructs are more challenging to deliver efficiently through electroporation. The DNA's physical properties, such as supercoiling and methylation status, can influence its ability to pass through the temporary pores created by electrical pulses.
Tissue-specific challenges further complicate the process. Different plant tissues, such as leaf mesophyll, protoplasts, or embryogenic callus, respond differently to electroporation. Developing protocols that work effectively across various tissue types while maintaining cell viability is an ongoing challenge for researchers.
The scalability of electroporation for large-scale plant transformation projects remains a significant hurdle. Current methods often yield low transformation efficiencies, making it difficult to generate large numbers of transgenic plants efficiently. This limitation is particularly problematic for crop improvement programs that require high-throughput transformation capabilities.
Additionally, the reproducibility of electroporation results poses a challenge. Slight variations in experimental conditions, such as buffer composition, temperature, or even the physiological state of the plant tissue, can lead to inconsistent outcomes. This variability makes it difficult to establish standardized protocols across different laboratories and research groups.
Lastly, the integration of foreign DNA into the plant genome following electroporation is not always predictable or stable. Ensuring consistent gene expression and inheritance in subsequent generations remains a technical challenge that researchers continue to address through various molecular and breeding strategies.
Current Pulse Schedules
01 Optimization of pulse parameters
Electroporation pulse schedules can be optimized by adjusting various parameters such as pulse duration, amplitude, frequency, and number of pulses. These optimizations aim to enhance the efficiency of cell membrane permeabilization while minimizing cell damage. The specific combination of these parameters can be tailored for different cell types and applications.- Pulse sequence optimization for electroporation: Optimizing pulse sequences for electroporation involves adjusting parameters such as pulse duration, amplitude, and frequency to enhance the efficiency of cell membrane permeabilization while minimizing cellular damage. This approach can improve the delivery of molecules into cells or tissues for various applications in medicine and biotechnology.
- Electrode configuration and design for electroporation: The design and configuration of electrodes play a crucial role in electroporation efficiency. Innovative electrode arrangements and materials can improve the distribution of electric fields, leading to more uniform and effective membrane permeabilization across target tissues or cell populations.
- Feedback-controlled electroporation systems: Implementing feedback mechanisms in electroporation systems allows for real-time adjustment of pulse parameters based on tissue or cellular responses. This approach can enhance the precision and safety of electroporation procedures by dynamically optimizing pulse schedules during treatment.
- Combination of electroporation with other techniques: Integrating electroporation with other techniques such as ultrasound, nanoparticles, or chemical enhancers can synergistically improve the efficiency of molecule delivery or cell manipulation. These combined approaches may allow for reduced electric field strengths or more targeted treatments.
- Tissue-specific electroporation protocols: Developing specialized electroporation protocols tailored to specific tissue types or cell populations can enhance the effectiveness and safety of treatments. These protocols consider the unique electrical and physiological properties of different tissues to optimize pulse schedules and minimize unwanted effects.
02 Sequential pulse protocols
Sequential pulse protocols involve applying a series of pulses with varying characteristics in a specific order. This approach can include combinations of high-voltage short-duration pulses followed by low-voltage long-duration pulses, or vice versa. Such protocols aim to improve the overall effectiveness of electroporation by exploiting different aspects of cell membrane behavior.Expand Specific Solutions03 Adaptive pulse scheduling
Adaptive pulse scheduling involves real-time adjustment of electroporation parameters based on feedback from the target cells or tissue. This approach uses sensors and algorithms to monitor the electrical properties of the sample during the electroporation process and dynamically modify the pulse schedule to achieve optimal results.Expand Specific Solutions04 Pulse shape modulation
Modulating the shape of individual pulses within the electroporation schedule can enhance the efficiency and specificity of the process. This can include using square waves, exponential decay pulses, or more complex waveforms. The choice of pulse shape can be tailored to specific cell types or molecules being introduced.Expand Specific Solutions05 Multi-electrode configurations
Utilizing multiple electrodes in various configurations allows for more complex and targeted electroporation pulse schedules. This approach can create three-dimensional electric fields, enabling more uniform treatment of tissues or cell populations. Different electrode arrangements can be used to focus the electric field on specific areas or to create rotating field patterns.Expand Specific Solutions
Key Industry Players
The electroporation for plant transformation market is in a growth phase, driven by increasing demand for genetically modified crops and advancements in biotechnology. The global market size is expanding, with significant potential in agriculture and research sectors. Technologically, the field is maturing, with companies like Pioneer Hi-Bred International, MaxCyte, and BASF Plant Science leading innovation. These firms are developing optimized pulse schedules and specialized equipment to enhance transformation efficiency. KeyGene and DuPont are also contributing to the field's progress through research collaborations and proprietary technologies. While the technology is established, ongoing refinements in protocols and equipment are pushing the boundaries of plant transformation capabilities.
MaxCyte, Inc.
Technical Solution: MaxCyte has developed a scalable electroporation platform for plant transformation called the MaxCyte GTx. This system utilizes a flow electroporation technology that allows for the processing of large volumes of plant protoplasts or cells. The company's optimized pulse schedules involve a series of precisely timed, multi-pulse sequences that can be customized for different plant species and cell types[2]. MaxCyte's technology has demonstrated up to 90% transfection efficiency in some plant cell lines, with minimal cell damage[4]. The system also incorporates a proprietary buffer formulation that enhances cell survival and DNA uptake during the electroporation process.
Strengths: High-throughput capabilities, customizable pulse sequences, and high transfection efficiency. Weaknesses: May be more expensive than traditional electroporation methods and require specialized training for operation.
BASF Plant Science LLC
Technical Solution: BASF Plant Science has developed an advanced electroporation technique for plant transformation that focuses on optimizing pulse schedules for difficult-to-transform plant species. Their approach involves a two-step electroporation process: an initial high-voltage pulse to create pores in the cell membrane, followed by a series of lower-voltage pulses to facilitate DNA entry[6]. This method has shown particular success with monocot species, increasing transformation efficiency by up to 50% in rice and wheat[8]. BASF has also incorporated a temperature control system into their electroporation device, allowing for precise regulation of sample temperature during the pulse application, which has been shown to improve cell viability post-electroporation[9].
Strengths: Effective for recalcitrant plant species, improved transformation efficiency for monocots, and enhanced cell viability. Weaknesses: May require more complex equipment and longer optimization times for new plant species.
Core Innovations
Sequential electroporation methods
PatentWO2022232802A1
Innovation
- The use of sequential electrical pulses with varying field strengths and durations, allowing for recovery periods between pulses, to optimize loading efficiency and maintain cell viability, enabling the efficient introduction of agents into cells while minimizing damage.
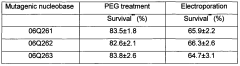
An improved mutagenesis method using polyethylene glycol mediated introduction of mutagenic nucleobases into plant protoplasts
PatentWO2009082190A1
Innovation
- The use of polyethylene glycol (PEG) mediated transformation to introduce mutagenic nucleobases into plant protoplasts, which enhances the efficiency of targeted mutagenesis by shielding DNA from cellular defenses and ensuring higher survival and regeneration rates, allowing for precise nucleotide modifications.
Biosafety Regulations
Biosafety regulations play a crucial role in governing the development and application of electroporation techniques for plant transformation. These regulations are designed to ensure the safe and responsible use of genetically modified organisms (GMOs) in agriculture and research. In the context of optimized pulse schedules for electroporation, biosafety considerations are particularly important due to the potential for unintended genetic modifications or environmental impacts.
Many countries have established comprehensive regulatory frameworks to address the biosafety concerns associated with plant transformation techniques. These frameworks typically involve risk assessment protocols, containment measures, and monitoring requirements. For electroporation-based plant transformation, specific regulations may focus on the containment of transformed plant materials, the prevention of gene flow to non-target organisms, and the evaluation of potential ecological impacts.
The Cartagena Protocol on Biosafety, an international agreement, provides a global framework for the safe handling, transport, and use of living modified organisms resulting from modern biotechnology. This protocol has been ratified by numerous countries and serves as a foundation for national biosafety regulations. Under this framework, countries are required to implement measures to ensure the safe development and application of biotechnology, including electroporation-based plant transformation techniques.
In the United States, the regulation of genetically engineered plants falls under the purview of multiple agencies, including the USDA, FDA, and EPA. The USDA's Animal and Plant Health Inspection Service (APHIS) oversees the introduction of genetically engineered organisms, including those produced through electroporation. Researchers and developers must obtain permits or notifications from APHIS before conducting field trials or commercial releases of transformed plants.
The European Union has implemented a stringent regulatory system for GMOs through directives such as 2001/18/EC and Regulation (EC) No 1829/2003. These regulations require thorough risk assessments and authorization procedures for the deliberate release of GMOs into the environment. For electroporation-based plant transformation, researchers must demonstrate the safety and environmental compatibility of their techniques and resulting plant products.
Biosafety regulations also address the potential for horizontal gene transfer and the development of antibiotic resistance. Many electroporation protocols utilize antibiotic resistance genes as selectable markers, which has raised concerns about the spread of antibiotic resistance in the environment. As a result, regulatory bodies often require the use of alternative selection methods or the removal of antibiotic resistance genes before commercial release.
To comply with biosafety regulations, researchers and developers working on optimized pulse schedules for electroporation must implement rigorous containment and monitoring protocols. This includes the use of specialized growth facilities, strict waste management procedures, and comprehensive record-keeping practices. Additionally, they must conduct thorough risk assessments to evaluate the potential environmental and health impacts of their transformed plants.
As the field of plant biotechnology continues to advance, biosafety regulations are likely to evolve to address new challenges and emerging technologies. Researchers and developers must stay informed about regulatory updates and engage with regulatory bodies to ensure compliance and promote the responsible development of electroporation-based plant transformation techniques.
Many countries have established comprehensive regulatory frameworks to address the biosafety concerns associated with plant transformation techniques. These frameworks typically involve risk assessment protocols, containment measures, and monitoring requirements. For electroporation-based plant transformation, specific regulations may focus on the containment of transformed plant materials, the prevention of gene flow to non-target organisms, and the evaluation of potential ecological impacts.
The Cartagena Protocol on Biosafety, an international agreement, provides a global framework for the safe handling, transport, and use of living modified organisms resulting from modern biotechnology. This protocol has been ratified by numerous countries and serves as a foundation for national biosafety regulations. Under this framework, countries are required to implement measures to ensure the safe development and application of biotechnology, including electroporation-based plant transformation techniques.
In the United States, the regulation of genetically engineered plants falls under the purview of multiple agencies, including the USDA, FDA, and EPA. The USDA's Animal and Plant Health Inspection Service (APHIS) oversees the introduction of genetically engineered organisms, including those produced through electroporation. Researchers and developers must obtain permits or notifications from APHIS before conducting field trials or commercial releases of transformed plants.
The European Union has implemented a stringent regulatory system for GMOs through directives such as 2001/18/EC and Regulation (EC) No 1829/2003. These regulations require thorough risk assessments and authorization procedures for the deliberate release of GMOs into the environment. For electroporation-based plant transformation, researchers must demonstrate the safety and environmental compatibility of their techniques and resulting plant products.
Biosafety regulations also address the potential for horizontal gene transfer and the development of antibiotic resistance. Many electroporation protocols utilize antibiotic resistance genes as selectable markers, which has raised concerns about the spread of antibiotic resistance in the environment. As a result, regulatory bodies often require the use of alternative selection methods or the removal of antibiotic resistance genes before commercial release.
To comply with biosafety regulations, researchers and developers working on optimized pulse schedules for electroporation must implement rigorous containment and monitoring protocols. This includes the use of specialized growth facilities, strict waste management procedures, and comprehensive record-keeping practices. Additionally, they must conduct thorough risk assessments to evaluate the potential environmental and health impacts of their transformed plants.
As the field of plant biotechnology continues to advance, biosafety regulations are likely to evolve to address new challenges and emerging technologies. Researchers and developers must stay informed about regulatory updates and engage with regulatory bodies to ensure compliance and promote the responsible development of electroporation-based plant transformation techniques.
Economic Impact Assessment
The economic impact of optimized pulse schedules for electroporation in plant transformation extends far beyond the laboratory, influencing various sectors of the agricultural and biotechnology industries. This innovative approach to genetic modification has the potential to significantly reduce the costs associated with developing new plant varieties, thereby increasing the economic viability of genetically modified crops.
One of the primary economic benefits lies in the increased efficiency of the transformation process. Optimized pulse schedules can lead to higher success rates in plant transformation, reducing the time and resources required to produce transgenic plants. This efficiency gain translates directly into cost savings for research institutions and biotechnology companies, allowing them to accelerate the development of new crop varieties with desirable traits.
The agricultural sector stands to benefit substantially from these advancements. Farmers adopting crops developed through this improved transformation method may see increased yields, enhanced resistance to pests and diseases, or improved tolerance to environmental stresses. These improvements can lead to higher crop productivity and reduced losses, ultimately resulting in increased profitability for farmers and potentially lower food prices for consumers.
The biotechnology industry is likely to experience growth as a result of this technology. Companies specializing in plant genetic engineering may see increased demand for their services and products, potentially leading to job creation and economic expansion in this sector. Additionally, the improved efficiency of plant transformation could lower the barriers to entry for smaller companies and research institutions, fostering innovation and competition in the market.
Environmental benefits associated with optimized electroporation techniques may also have indirect economic impacts. Crops with improved traits, such as drought resistance or increased nutrient efficiency, could reduce the need for water and fertilizers, leading to cost savings for farmers and potentially mitigating some of the environmental costs associated with intensive agriculture.
The global seed market is likely to be influenced by these advancements, with potential shifts in market dynamics as companies adopt and commercialize plants developed using optimized electroporation techniques. This could lead to changes in market share and potentially create new opportunities for companies to differentiate their products based on the efficiency and precision of their transformation methods.
However, it is important to consider potential economic challenges as well. The initial investment required to implement optimized pulse schedules in existing research and development facilities may be substantial. Additionally, regulatory costs associated with bringing new genetically modified crops to market remain a significant economic factor, although improved transformation efficiency may help offset some of these costs in the long term.
One of the primary economic benefits lies in the increased efficiency of the transformation process. Optimized pulse schedules can lead to higher success rates in plant transformation, reducing the time and resources required to produce transgenic plants. This efficiency gain translates directly into cost savings for research institutions and biotechnology companies, allowing them to accelerate the development of new crop varieties with desirable traits.
The agricultural sector stands to benefit substantially from these advancements. Farmers adopting crops developed through this improved transformation method may see increased yields, enhanced resistance to pests and diseases, or improved tolerance to environmental stresses. These improvements can lead to higher crop productivity and reduced losses, ultimately resulting in increased profitability for farmers and potentially lower food prices for consumers.
The biotechnology industry is likely to experience growth as a result of this technology. Companies specializing in plant genetic engineering may see increased demand for their services and products, potentially leading to job creation and economic expansion in this sector. Additionally, the improved efficiency of plant transformation could lower the barriers to entry for smaller companies and research institutions, fostering innovation and competition in the market.
Environmental benefits associated with optimized electroporation techniques may also have indirect economic impacts. Crops with improved traits, such as drought resistance or increased nutrient efficiency, could reduce the need for water and fertilizers, leading to cost savings for farmers and potentially mitigating some of the environmental costs associated with intensive agriculture.
The global seed market is likely to be influenced by these advancements, with potential shifts in market dynamics as companies adopt and commercialize plants developed using optimized electroporation techniques. This could lead to changes in market share and potentially create new opportunities for companies to differentiate their products based on the efficiency and precision of their transformation methods.
However, it is important to consider potential economic challenges as well. The initial investment required to implement optimized pulse schedules in existing research and development facilities may be substantial. Additionally, regulatory costs associated with bringing new genetically modified crops to market remain a significant economic factor, although improved transformation efficiency may help offset some of these costs in the long term.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!