Electroporation QC: Controls, Benchmarks And Reporting Formats
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electroporation QC Overview and Objectives
Electroporation, a pivotal technique in molecular biology and biotechnology, has revolutionized the field of gene transfer and cell manipulation. This method, which utilizes electrical pulses to create temporary pores in cell membranes, facilitates the introduction of foreign molecules into cells. As the applications of electroporation continue to expand, the need for robust quality control (QC) measures has become increasingly apparent.
The primary objective of Electroporation QC is to ensure consistent and reliable results across various experimental settings and applications. This encompasses the development and implementation of standardized controls, benchmarks, and reporting formats. By establishing these parameters, researchers and industry professionals can more effectively compare results, troubleshoot issues, and optimize protocols.
Controls in electroporation QC serve as reference points to validate the efficacy of the procedure. These may include positive controls to confirm successful gene transfer, negative controls to assess background levels, and internal controls to normalize for variations in cell number or transfection efficiency. The selection and implementation of appropriate controls are crucial for interpreting results accurately and identifying potential sources of error.
Benchmarks in electroporation QC provide standardized metrics against which experimental outcomes can be measured. These may include transfection efficiency rates, cell viability percentages, and gene expression levels. Establishing industry-wide benchmarks allows for the comparison of results across different laboratories and experimental setups, facilitating collaboration and advancing the field as a whole.
Reporting formats play a critical role in ensuring the reproducibility and transparency of electroporation experiments. Standardized reporting guidelines should encompass detailed descriptions of experimental conditions, including electrical parameters, cell types, buffer compositions, and post-electroporation handling procedures. By adopting uniform reporting practices, the scientific community can more easily validate findings and build upon existing knowledge.
The development of Electroporation QC standards is an ongoing process that requires collaboration between academic researchers, industry professionals, and regulatory bodies. As new applications and technologies emerge, these standards must evolve to address novel challenges and opportunities. The ultimate goal is to establish a comprehensive framework that enhances the reliability, reproducibility, and comparability of electroporation-based experiments across diverse fields of study.
The primary objective of Electroporation QC is to ensure consistent and reliable results across various experimental settings and applications. This encompasses the development and implementation of standardized controls, benchmarks, and reporting formats. By establishing these parameters, researchers and industry professionals can more effectively compare results, troubleshoot issues, and optimize protocols.
Controls in electroporation QC serve as reference points to validate the efficacy of the procedure. These may include positive controls to confirm successful gene transfer, negative controls to assess background levels, and internal controls to normalize for variations in cell number or transfection efficiency. The selection and implementation of appropriate controls are crucial for interpreting results accurately and identifying potential sources of error.
Benchmarks in electroporation QC provide standardized metrics against which experimental outcomes can be measured. These may include transfection efficiency rates, cell viability percentages, and gene expression levels. Establishing industry-wide benchmarks allows for the comparison of results across different laboratories and experimental setups, facilitating collaboration and advancing the field as a whole.
Reporting formats play a critical role in ensuring the reproducibility and transparency of electroporation experiments. Standardized reporting guidelines should encompass detailed descriptions of experimental conditions, including electrical parameters, cell types, buffer compositions, and post-electroporation handling procedures. By adopting uniform reporting practices, the scientific community can more easily validate findings and build upon existing knowledge.
The development of Electroporation QC standards is an ongoing process that requires collaboration between academic researchers, industry professionals, and regulatory bodies. As new applications and technologies emerge, these standards must evolve to address novel challenges and opportunities. The ultimate goal is to establish a comprehensive framework that enhances the reliability, reproducibility, and comparability of electroporation-based experiments across diverse fields of study.
Market Analysis for Electroporation QC Solutions
The market for electroporation quality control (QC) solutions is experiencing significant growth, driven by the increasing adoption of electroporation techniques in various fields, including gene therapy, cell-based therapies, and genetic engineering. As these applications become more prevalent in both research and clinical settings, the demand for reliable and standardized QC methods has surged.
The global electroporation market, which encompasses QC solutions, is projected to expand at a compound annual growth rate (CAGR) of over 6% from 2021 to 2026. This growth is primarily fueled by the rising investments in biotechnology and pharmaceutical research, as well as the growing focus on personalized medicine and advanced therapies.
Key market segments for electroporation QC solutions include academic and research institutions, biotechnology and pharmaceutical companies, and contract research organizations (CROs). Among these, biotechnology and pharmaceutical companies represent the largest market share due to their extensive use of electroporation in drug discovery and development processes.
Geographically, North America dominates the electroporation QC market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure and substantial investments in biomedical research. However, emerging markets in Asia-Pacific, especially China and India, are expected to witness rapid growth in the coming years.
The market for electroporation QC solutions is characterized by a high degree of fragmentation, with numerous small to medium-sized players competing alongside a few large, established companies. This competitive landscape has led to increased innovation and product development, particularly in the areas of automated QC systems and standardized reporting formats.
One of the key trends shaping the market is the growing demand for integrated QC solutions that offer comprehensive control, benchmarking, and reporting capabilities. End-users are increasingly seeking turnkey systems that can streamline their QC processes and ensure consistent, reproducible results across different experiments and laboratories.
Another significant market driver is the increasing regulatory scrutiny on cell and gene therapies, which has heightened the need for robust QC measures in electroporation processes. This has led to a growing emphasis on developing standardized controls and benchmarks that can meet regulatory requirements and facilitate the translation of research findings into clinical applications.
The market for electroporation QC solutions also faces certain challenges, including the high cost of advanced QC systems and the lack of standardized protocols across different research groups and industries. However, these challenges present opportunities for companies to develop cost-effective, user-friendly QC solutions that can address the diverse needs of the market.
The global electroporation market, which encompasses QC solutions, is projected to expand at a compound annual growth rate (CAGR) of over 6% from 2021 to 2026. This growth is primarily fueled by the rising investments in biotechnology and pharmaceutical research, as well as the growing focus on personalized medicine and advanced therapies.
Key market segments for electroporation QC solutions include academic and research institutions, biotechnology and pharmaceutical companies, and contract research organizations (CROs). Among these, biotechnology and pharmaceutical companies represent the largest market share due to their extensive use of electroporation in drug discovery and development processes.
Geographically, North America dominates the electroporation QC market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure and substantial investments in biomedical research. However, emerging markets in Asia-Pacific, especially China and India, are expected to witness rapid growth in the coming years.
The market for electroporation QC solutions is characterized by a high degree of fragmentation, with numerous small to medium-sized players competing alongside a few large, established companies. This competitive landscape has led to increased innovation and product development, particularly in the areas of automated QC systems and standardized reporting formats.
One of the key trends shaping the market is the growing demand for integrated QC solutions that offer comprehensive control, benchmarking, and reporting capabilities. End-users are increasingly seeking turnkey systems that can streamline their QC processes and ensure consistent, reproducible results across different experiments and laboratories.
Another significant market driver is the increasing regulatory scrutiny on cell and gene therapies, which has heightened the need for robust QC measures in electroporation processes. This has led to a growing emphasis on developing standardized controls and benchmarks that can meet regulatory requirements and facilitate the translation of research findings into clinical applications.
The market for electroporation QC solutions also faces certain challenges, including the high cost of advanced QC systems and the lack of standardized protocols across different research groups and industries. However, these challenges present opportunities for companies to develop cost-effective, user-friendly QC solutions that can address the diverse needs of the market.
Current Challenges in Electroporation QC
Electroporation quality control (QC) faces several significant challenges that hinder its widespread adoption and standardization in various applications. One of the primary issues is the lack of universally accepted controls and benchmarks. Different research groups and industries often use diverse cell types, electroporation conditions, and assessment methods, making it difficult to compare results across studies or establish industry-wide standards.
The variability in electroporation equipment and protocols further complicates the QC process. Factors such as electrode design, pulse parameters, and buffer composition can significantly impact electroporation efficiency and cell viability. This variability makes it challenging to develop consistent QC metrics that can be applied across different systems and experimental setups.
Another critical challenge is the absence of standardized reporting formats for electroporation QC data. The lack of a unified approach to data presentation and analysis makes it difficult for researchers and practitioners to interpret and compare results from different sources. This inconsistency hampers the development of best practices and slows down the progress in optimizing electroporation protocols for various applications.
The complexity of biological systems adds another layer of difficulty to electroporation QC. Cell-to-cell variability, differences in membrane composition, and varying susceptibility to electric fields can lead to inconsistent results even within the same experimental setup. This inherent biological variability makes it challenging to establish reliable QC parameters that can account for these differences.
Furthermore, the multifactorial nature of electroporation outcomes poses a significant challenge in developing comprehensive QC measures. Factors such as cell viability, transfection efficiency, and long-term cell function need to be considered simultaneously, but their relative importance may vary depending on the specific application. Balancing these factors in a meaningful QC framework remains a complex task.
The time-sensitive nature of electroporation QC also presents practical challenges. Many applications require rapid assessment of electroporation success, but current methods often involve time-consuming processes such as cell culture and gene expression analysis. Developing quick and reliable QC methods that can provide real-time feedback on electroporation efficiency is an ongoing challenge in the field.
Lastly, the integration of electroporation QC into automated and high-throughput systems remains a significant hurdle. As electroporation finds increasing use in large-scale applications such as cell therapy manufacturing, there is a growing need for robust QC methods that can be seamlessly incorporated into automated workflows. Developing such systems while maintaining accuracy and reliability is a complex engineering challenge that requires interdisciplinary approaches.
The variability in electroporation equipment and protocols further complicates the QC process. Factors such as electrode design, pulse parameters, and buffer composition can significantly impact electroporation efficiency and cell viability. This variability makes it challenging to develop consistent QC metrics that can be applied across different systems and experimental setups.
Another critical challenge is the absence of standardized reporting formats for electroporation QC data. The lack of a unified approach to data presentation and analysis makes it difficult for researchers and practitioners to interpret and compare results from different sources. This inconsistency hampers the development of best practices and slows down the progress in optimizing electroporation protocols for various applications.
The complexity of biological systems adds another layer of difficulty to electroporation QC. Cell-to-cell variability, differences in membrane composition, and varying susceptibility to electric fields can lead to inconsistent results even within the same experimental setup. This inherent biological variability makes it challenging to establish reliable QC parameters that can account for these differences.
Furthermore, the multifactorial nature of electroporation outcomes poses a significant challenge in developing comprehensive QC measures. Factors such as cell viability, transfection efficiency, and long-term cell function need to be considered simultaneously, but their relative importance may vary depending on the specific application. Balancing these factors in a meaningful QC framework remains a complex task.
The time-sensitive nature of electroporation QC also presents practical challenges. Many applications require rapid assessment of electroporation success, but current methods often involve time-consuming processes such as cell culture and gene expression analysis. Developing quick and reliable QC methods that can provide real-time feedback on electroporation efficiency is an ongoing challenge in the field.
Lastly, the integration of electroporation QC into automated and high-throughput systems remains a significant hurdle. As electroporation finds increasing use in large-scale applications such as cell therapy manufacturing, there is a growing need for robust QC methods that can be seamlessly incorporated into automated workflows. Developing such systems while maintaining accuracy and reliability is a complex engineering challenge that requires interdisciplinary approaches.
Existing Electroporation QC Methodologies
01 Monitoring and control systems for electroporation
Advanced monitoring and control systems are implemented to ensure the quality and efficiency of electroporation processes. These systems may include real-time feedback mechanisms, automated parameter adjustments, and data logging capabilities to maintain optimal conditions throughout the procedure.- Monitoring and control systems for electroporation: Advanced monitoring and control systems are implemented to ensure the quality and efficiency of electroporation processes. These systems may include real-time feedback mechanisms, automated parameter adjustments, and data logging capabilities to maintain optimal conditions throughout the procedure.
- Electrode design and configuration for improved electroporation: Innovative electrode designs and configurations are developed to enhance the uniformity and effectiveness of electric field distribution during electroporation. This includes optimizing electrode geometry, spacing, and materials to achieve consistent and high-quality results across various applications.
- Quality control through cell viability and transfection efficiency assessment: Methods for assessing cell viability and transfection efficiency are crucial for electroporation quality control. These may involve fluorescence-based assays, flow cytometry, or other analytical techniques to quantify successful gene transfer and cell survival rates post-electroporation.
- Optimization of electroporation parameters: Systematic approaches to optimize electroporation parameters such as voltage, pulse duration, and frequency are developed. This involves the use of algorithms, machine learning techniques, or experimental design methodologies to identify the most effective combination of parameters for specific cell types or applications.
- Integration of quality control measures in electroporation devices: Electroporation devices are designed with built-in quality control features, including sensors for temperature monitoring, impedance measurement, and pulse delivery verification. These integrated measures ensure consistent performance and allow for immediate detection of potential issues during the electroporation process.
02 Electrode design and configuration for improved electroporation
Innovative electrode designs and configurations are developed to enhance the uniformity and effectiveness of electric field distribution during electroporation. This includes optimizing electrode geometry, spacing, and materials to achieve consistent and high-quality results across various applications.Expand Specific Solutions03 Quality control through cell viability and transfection efficiency assessment
Methods for assessing cell viability and transfection efficiency are crucial for electroporation quality control. These may involve fluorescence-based assays, flow cytometry, or other analytical techniques to quantify successful gene transfer and cell survival rates post-electroporation.Expand Specific Solutions04 Optimization of electroporation parameters
Systematic approaches to optimize electroporation parameters such as voltage, pulse duration, and frequency are developed. This may involve machine learning algorithms or statistical methods to determine the ideal conditions for specific cell types or applications, ensuring consistent and high-quality results.Expand Specific Solutions05 Integration of quality control measures in electroporation devices
Electroporation devices are designed with built-in quality control features, such as impedance measurement systems, temperature monitoring, and automated error detection. These integrated measures help maintain consistent performance and alert users to potential issues that may affect electroporation quality.Expand Specific Solutions
Key Players in Electroporation QC Industry
The electroporation QC technology market is in a growth phase, driven by increasing applications in gene therapy and cell-based research. The market size is expanding, with projections indicating significant growth in the coming years. Technologically, the field is advancing rapidly, with major players like St. Jude Medical and TDK Corp leading innovations. Academic institutions such as Tsinghua University and EPFL are contributing to research advancements. The involvement of diverse companies like MaxCyte and Renesas Electronics suggests a competitive landscape with opportunities for specialized solutions in controls, benchmarks, and reporting formats for electroporation QC.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has made significant contributions to electroporation QC through the development of novel physical and chemical controls. They have pioneered the use of nanosecond pulsed electric fields for more precise control over membrane permeabilization[11]. Their QC protocols include the use of fluorescent voltage-sensitive dyes to directly visualize and quantify membrane potential changes during electroporation[12]. CNRS researchers have also developed microfluidic devices for high-throughput electroporation with integrated optical sensors for real-time monitoring[13]. Their benchmarking approach involves systematic comparison of electroporation efficiency across a wide range of pulse parameters and buffer compositions. The CNRS reporting format emphasizes detailed characterization of the electric field distribution and its effects on cellular physiology.
Strengths: Innovative physical approaches to electroporation, high-precision monitoring techniques. Weaknesses: Some methods may be challenging to translate to standard laboratory or industrial settings, potentially requiring specialized equipment.
The Regents of the University of California
Technical Solution: The University of California system has contributed significantly to electroporation QC through multi-campus collaborative research. They have developed a standardized set of control experiments using well-characterized cell lines and plasmids to ensure consistency across different electroporation devices and protocols[8]. Their QC approach includes the use of multi-color flow cytometry for precise quantification of transfection efficiency and cell health markers[9]. UC researchers have also pioneered the use of machine learning algorithms to predict optimal electroporation parameters based on cell type and cargo characteristics[10]. Their reporting format includes detailed experimental conditions, raw data from flow cytometry and other analytical techniques, and statistical analyses of reproducibility across multiple trials.
Strengths: Comprehensive control experiments, advanced analytical techniques, and predictive modeling capabilities. Weaknesses: May be resource-intensive to implement fully, requires expertise in data science for optimal utilization.
Innovative Approaches in Electroporation QC
Monitoring and control of an electroporation
PatentWO2006018528A1
Innovation
- An electroporation assembly with a monitoring system that estimates the effective electrical power delivery to cellular parts using bio-impedance data, allowing for real-time adjustment of the electric field to optimize electroporation, including the use of an estimator to calculate specific electrical quantities and adapt the pulse shape to the biological sample, regardless of its nature or location.
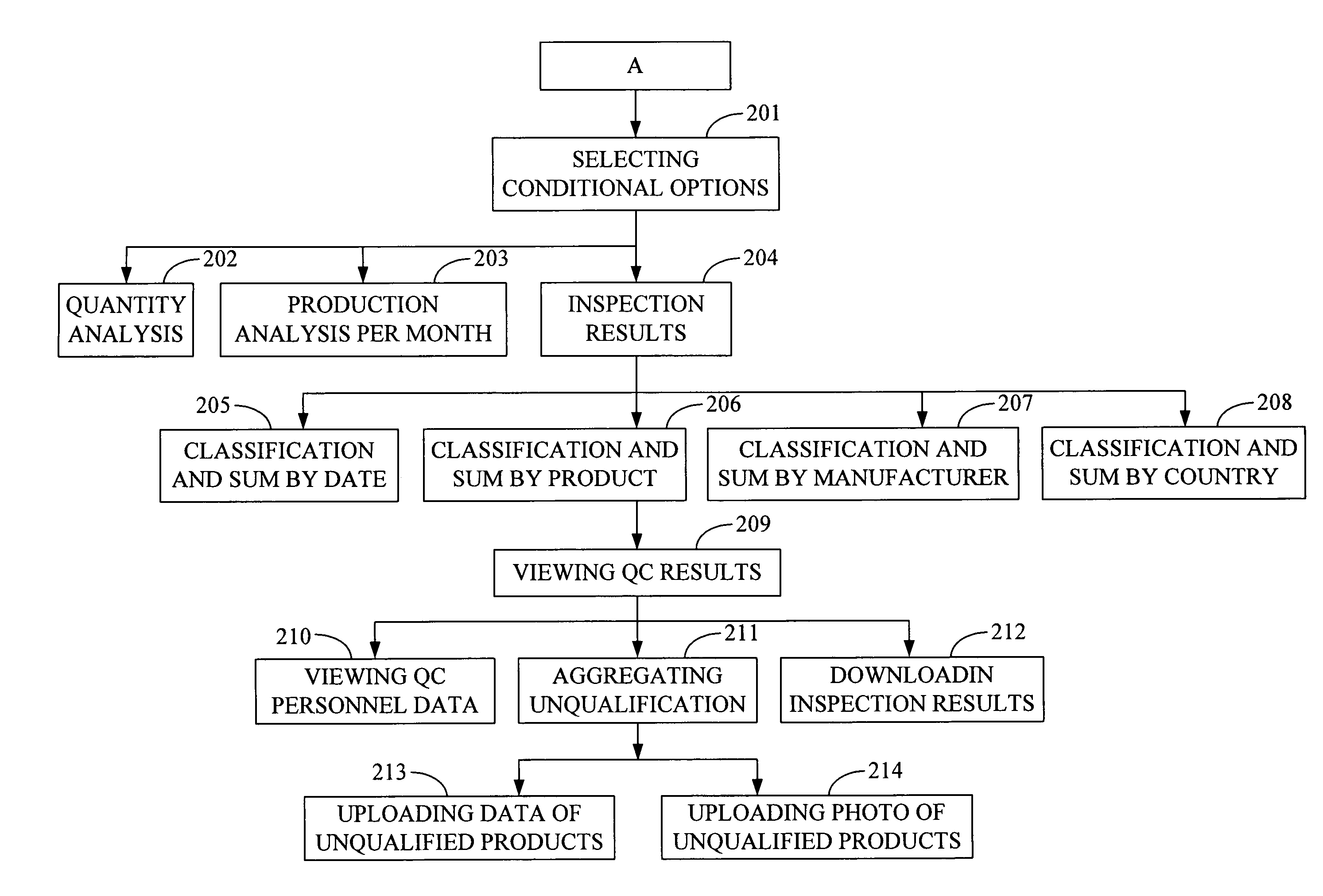
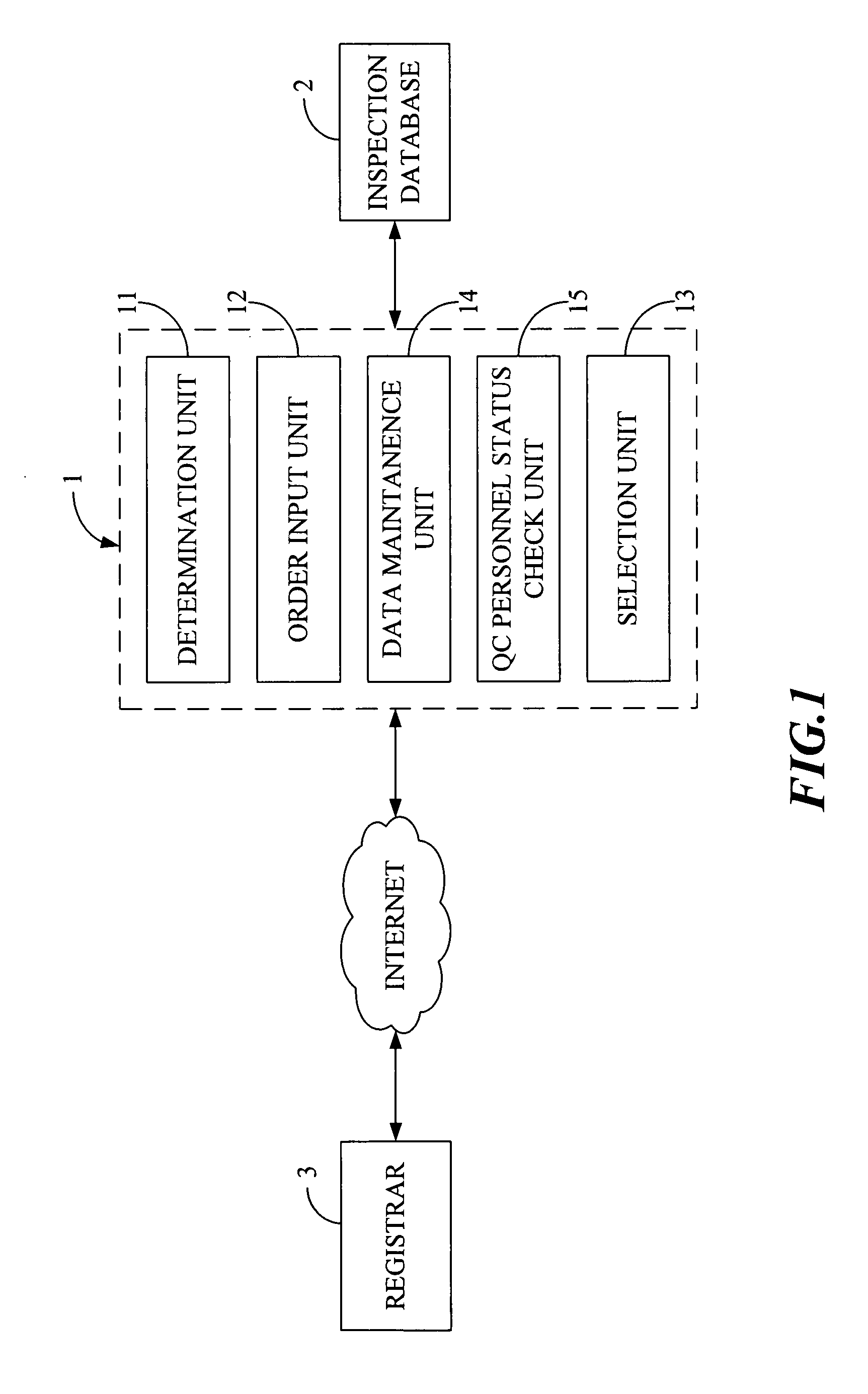
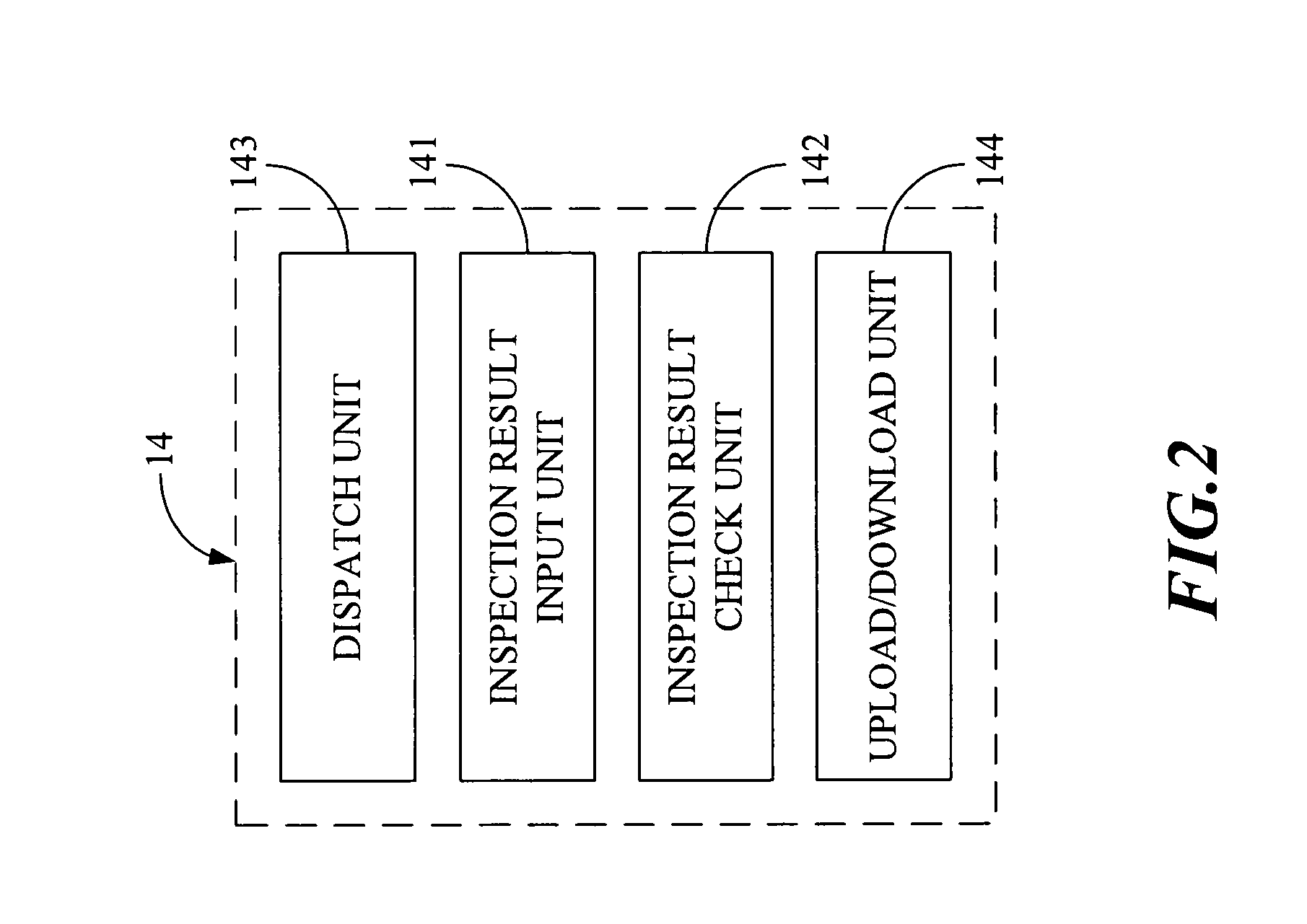
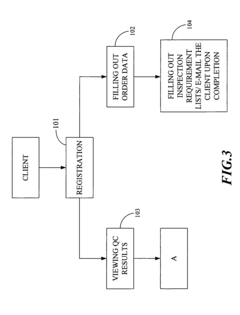
Quality Control Inspection and Maintenance Result Reporting System
PatentInactiveUS20080319814A1
Innovation
- A QC inspection and maintenance result reporting system utilizing a web server and inspection database, allowing clients and QC personnel to input and view order data, inspection results, and upload photographs through the internet, with features like determination units for privilege management, order input, data maintenance, and upload/download capabilities.
Standardization of Electroporation QC Protocols
Standardization of electroporation quality control (QC) protocols is crucial for ensuring reproducibility and reliability in various applications, including gene therapy, cell-based therapies, and genetic engineering. The current lack of uniformity in QC procedures across different laboratories and industries has led to challenges in comparing results and validating experimental outcomes.
To address this issue, a comprehensive framework for electroporation QC standardization is necessary. This framework should encompass several key elements, including the establishment of universal control samples, defined benchmarks for performance evaluation, and standardized reporting formats. These components will collectively contribute to a more robust and consistent approach to electroporation QC.
Control samples play a vital role in assessing the efficiency and reproducibility of electroporation procedures. Standardized positive and negative controls should be developed and widely adopted across the field. These controls should include well-characterized cell lines, plasmids, and electroporation conditions that produce consistent results. By implementing these universal controls, researchers and industry professionals can more accurately compare their results and troubleshoot potential issues.
Benchmarks for electroporation performance are essential for evaluating the success of the procedure. These benchmarks should cover various aspects, such as cell viability, transfection efficiency, and gene expression levels. Establishing clear thresholds for acceptable performance will enable researchers to quickly determine whether their electroporation experiments meet industry standards. Additionally, these benchmarks should be adaptable to different cell types and applications, ensuring their relevance across diverse research areas.
Standardized reporting formats are crucial for effective communication and data sharing within the scientific community. A uniform template for reporting electroporation QC results should be developed, including essential parameters such as cell type, electroporation device settings, buffer composition, and post-electroporation handling procedures. This standardized format will facilitate easier comparison of results between different laboratories and enable more efficient meta-analyses of electroporation data.
Implementation of these standardized protocols will require collaboration between academic institutions, industry partners, and regulatory bodies. Workshops, conferences, and online platforms can be utilized to disseminate information and gather feedback from the scientific community. Regular updates and revisions to the standardization framework will be necessary to keep pace with technological advancements and emerging applications in the field of electroporation.
To address this issue, a comprehensive framework for electroporation QC standardization is necessary. This framework should encompass several key elements, including the establishment of universal control samples, defined benchmarks for performance evaluation, and standardized reporting formats. These components will collectively contribute to a more robust and consistent approach to electroporation QC.
Control samples play a vital role in assessing the efficiency and reproducibility of electroporation procedures. Standardized positive and negative controls should be developed and widely adopted across the field. These controls should include well-characterized cell lines, plasmids, and electroporation conditions that produce consistent results. By implementing these universal controls, researchers and industry professionals can more accurately compare their results and troubleshoot potential issues.
Benchmarks for electroporation performance are essential for evaluating the success of the procedure. These benchmarks should cover various aspects, such as cell viability, transfection efficiency, and gene expression levels. Establishing clear thresholds for acceptable performance will enable researchers to quickly determine whether their electroporation experiments meet industry standards. Additionally, these benchmarks should be adaptable to different cell types and applications, ensuring their relevance across diverse research areas.
Standardized reporting formats are crucial for effective communication and data sharing within the scientific community. A uniform template for reporting electroporation QC results should be developed, including essential parameters such as cell type, electroporation device settings, buffer composition, and post-electroporation handling procedures. This standardized format will facilitate easier comparison of results between different laboratories and enable more efficient meta-analyses of electroporation data.
Implementation of these standardized protocols will require collaboration between academic institutions, industry partners, and regulatory bodies. Workshops, conferences, and online platforms can be utilized to disseminate information and gather feedback from the scientific community. Regular updates and revisions to the standardization framework will be necessary to keep pace with technological advancements and emerging applications in the field of electroporation.
Regulatory Compliance in Electroporation QC
Regulatory compliance in electroporation quality control (QC) is a critical aspect of ensuring the safety and efficacy of this biotechnology application. As electroporation becomes increasingly prevalent in various fields, including gene therapy, cell-based therapies, and vaccine development, regulatory bodies have established stringent guidelines to govern its use and quality control processes.
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have developed comprehensive frameworks for the regulation of electroporation-based products and processes. These guidelines encompass various aspects of electroporation QC, including equipment validation, process standardization, and reporting requirements.
One of the key regulatory requirements is the implementation of robust quality management systems (QMS) for electroporation processes. This includes the establishment of standard operating procedures (SOPs) that detail every step of the electroporation process, from sample preparation to post-treatment analysis. These SOPs must be validated and regularly reviewed to ensure compliance with current good manufacturing practices (cGMP).
Equipment used in electroporation must undergo rigorous validation and calibration processes. Regulatory bodies require documentation of equipment specifications, maintenance records, and performance qualifications. This ensures that the electroporation devices consistently deliver the intended electrical pulses and maintain the required level of precision throughout their operational lifespan.
Control measures play a crucial role in regulatory compliance for electroporation QC. Regulatory agencies mandate the use of appropriate positive and negative controls in each electroporation experiment. These controls help validate the efficacy of the electroporation process and detect any potential issues that may affect the quality of the final product.
Benchmarking is another essential component of regulatory compliance in electroporation QC. Manufacturers and researchers are required to establish and adhere to predefined benchmarks for various parameters, such as cell viability, transfection efficiency, and product yield. These benchmarks serve as quality indicators and help ensure consistency across different batches and production runs.
Reporting formats for electroporation QC data are subject to strict regulatory requirements. Detailed documentation of all QC procedures, results, and deviations is mandatory. This includes maintaining comprehensive batch records, equipment logs, and analytical data. Many regulatory agencies now require electronic data capture systems that comply with 21 CFR Part 11 regulations, ensuring data integrity and traceability.
Risk assessment and mitigation strategies are integral to regulatory compliance in electroporation QC. Manufacturers must identify potential risks associated with the electroporation process and implement appropriate control measures. This may include strategies to prevent cross-contamination, minimize variability in electrical field strength, and ensure the sterility of the final product.
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have developed comprehensive frameworks for the regulation of electroporation-based products and processes. These guidelines encompass various aspects of electroporation QC, including equipment validation, process standardization, and reporting requirements.
One of the key regulatory requirements is the implementation of robust quality management systems (QMS) for electroporation processes. This includes the establishment of standard operating procedures (SOPs) that detail every step of the electroporation process, from sample preparation to post-treatment analysis. These SOPs must be validated and regularly reviewed to ensure compliance with current good manufacturing practices (cGMP).
Equipment used in electroporation must undergo rigorous validation and calibration processes. Regulatory bodies require documentation of equipment specifications, maintenance records, and performance qualifications. This ensures that the electroporation devices consistently deliver the intended electrical pulses and maintain the required level of precision throughout their operational lifespan.
Control measures play a crucial role in regulatory compliance for electroporation QC. Regulatory agencies mandate the use of appropriate positive and negative controls in each electroporation experiment. These controls help validate the efficacy of the electroporation process and detect any potential issues that may affect the quality of the final product.
Benchmarking is another essential component of regulatory compliance in electroporation QC. Manufacturers and researchers are required to establish and adhere to predefined benchmarks for various parameters, such as cell viability, transfection efficiency, and product yield. These benchmarks serve as quality indicators and help ensure consistency across different batches and production runs.
Reporting formats for electroporation QC data are subject to strict regulatory requirements. Detailed documentation of all QC procedures, results, and deviations is mandatory. This includes maintaining comprehensive batch records, equipment logs, and analytical data. Many regulatory agencies now require electronic data capture systems that comply with 21 CFR Part 11 regulations, ensuring data integrity and traceability.
Risk assessment and mitigation strategies are integral to regulatory compliance in electroporation QC. Manufacturers must identify potential risks associated with the electroporation process and implement appropriate control measures. This may include strategies to prevent cross-contamination, minimize variability in electrical field strength, and ensure the sterility of the final product.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!