How To Combine Electroporation With Nanoparticle Drug Delivery
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electroporation-Nanoparticle Integration: Background and Objectives
Electroporation and nanoparticle drug delivery represent two cutting-edge technologies in the field of targeted drug delivery and gene therapy. Electroporation, a technique that uses electric pulses to create temporary pores in cell membranes, has been widely studied for its potential to enhance the uptake of various therapeutic agents. Nanoparticle drug delivery, on the other hand, has emerged as a promising approach to improve drug efficacy and reduce side effects by encapsulating drugs in nano-sized carriers.
The integration of these two technologies has gained significant attention in recent years, as researchers seek to overcome the limitations of each method individually. Electroporation can enhance the cellular uptake of nanoparticles, potentially leading to more efficient drug delivery and improved therapeutic outcomes. This combination approach addresses key challenges in drug delivery, such as poor cellular penetration and limited bioavailability of certain drugs.
The evolution of this combined approach can be traced back to the early 2000s when researchers began exploring the synergistic effects of electroporation and nanoparticles. Initial studies focused on using electroporation to enhance the delivery of DNA-loaded nanoparticles for gene therapy applications. As the field progressed, the scope expanded to include various types of nanoparticles and therapeutic agents, ranging from small molecule drugs to proteins and RNA.
The primary objective of integrating electroporation with nanoparticle drug delivery is to develop more effective and targeted therapeutic strategies. This combination aims to achieve higher intracellular concentrations of drugs, improve the delivery of large or charged molecules that typically struggle to cross cell membranes, and enable more precise control over the spatial and temporal distribution of therapeutic agents within tissues.
Key technological goals include optimizing the parameters of electroporation to maximize nanoparticle uptake without causing cellular damage, designing nanoparticles that are compatible with electroporation protocols, and developing in vivo delivery systems that can effectively combine these two approaches. Additionally, researchers are working towards understanding the mechanisms underlying the enhanced delivery achieved through this combination, which is crucial for further improving and fine-tuning the technology.
The potential applications of this integrated approach span a wide range of medical fields, including cancer therapy, gene therapy, and the treatment of various genetic and acquired diseases. As research in this area continues to advance, it is expected to lead to the development of more sophisticated and effective drug delivery platforms, potentially revolutionizing the way we approach the treatment of complex diseases.
The integration of these two technologies has gained significant attention in recent years, as researchers seek to overcome the limitations of each method individually. Electroporation can enhance the cellular uptake of nanoparticles, potentially leading to more efficient drug delivery and improved therapeutic outcomes. This combination approach addresses key challenges in drug delivery, such as poor cellular penetration and limited bioavailability of certain drugs.
The evolution of this combined approach can be traced back to the early 2000s when researchers began exploring the synergistic effects of electroporation and nanoparticles. Initial studies focused on using electroporation to enhance the delivery of DNA-loaded nanoparticles for gene therapy applications. As the field progressed, the scope expanded to include various types of nanoparticles and therapeutic agents, ranging from small molecule drugs to proteins and RNA.
The primary objective of integrating electroporation with nanoparticle drug delivery is to develop more effective and targeted therapeutic strategies. This combination aims to achieve higher intracellular concentrations of drugs, improve the delivery of large or charged molecules that typically struggle to cross cell membranes, and enable more precise control over the spatial and temporal distribution of therapeutic agents within tissues.
Key technological goals include optimizing the parameters of electroporation to maximize nanoparticle uptake without causing cellular damage, designing nanoparticles that are compatible with electroporation protocols, and developing in vivo delivery systems that can effectively combine these two approaches. Additionally, researchers are working towards understanding the mechanisms underlying the enhanced delivery achieved through this combination, which is crucial for further improving and fine-tuning the technology.
The potential applications of this integrated approach span a wide range of medical fields, including cancer therapy, gene therapy, and the treatment of various genetic and acquired diseases. As research in this area continues to advance, it is expected to lead to the development of more sophisticated and effective drug delivery platforms, potentially revolutionizing the way we approach the treatment of complex diseases.
Market Analysis for Nanoparticle-Enhanced Drug Delivery
The market for nanoparticle-enhanced drug delivery systems combined with electroporation is experiencing significant growth and attracting substantial investment. This innovative approach addresses key challenges in traditional drug delivery methods, offering improved efficacy and reduced side effects. The global market for nanoparticle drug delivery is projected to reach $136 billion by 2026, with a compound annual growth rate of 8.5% from 2021 to 2026.
The combination of electroporation and nanoparticle drug delivery presents a particularly promising segment within this market. Electroporation enhances the permeability of cell membranes, allowing for more efficient delivery of nanoparticle-encapsulated drugs. This synergy is especially valuable in oncology, where targeted drug delivery is crucial for minimizing damage to healthy tissues.
Market demand is driven by several factors, including the rising prevalence of chronic diseases, increasing research and development in nanomedicine, and the growing need for targeted drug delivery systems. The oncology sector represents the largest market share, followed by neurology and cardiovascular applications. North America currently leads the market, with Asia-Pacific expected to show the fastest growth in the coming years.
Pharmaceutical and biotechnology companies are the primary end-users of this technology, with academic and research institutions also contributing significantly to market growth through ongoing research and clinical trials. The market is characterized by a high degree of innovation and collaboration between industry players and research institutions.
Key market trends include the development of multifunctional nanoparticles that combine diagnostic and therapeutic capabilities, known as theranostics. There is also a growing focus on personalized medicine, where nanoparticle-based drug delivery systems can be tailored to individual patient profiles for optimal efficacy.
Challenges in the market include regulatory hurdles, concerns about long-term safety, and the high cost of development and production. However, ongoing advancements in nanotechnology and increasing investment in research are expected to address these challenges over time.
The market landscape is competitive, with several major pharmaceutical companies and specialized nanotechnology firms actively developing and commercializing nanoparticle-based drug delivery systems. Strategic partnerships and collaborations are common, as companies seek to combine expertise in nanotechnology, drug development, and electroporation techniques.
In conclusion, the market for nanoparticle-enhanced drug delivery combined with electroporation shows strong growth potential. As research progresses and more products reach clinical trials and commercialization, this innovative approach is poised to significantly impact the pharmaceutical industry and improve patient outcomes across various therapeutic areas.
The combination of electroporation and nanoparticle drug delivery presents a particularly promising segment within this market. Electroporation enhances the permeability of cell membranes, allowing for more efficient delivery of nanoparticle-encapsulated drugs. This synergy is especially valuable in oncology, where targeted drug delivery is crucial for minimizing damage to healthy tissues.
Market demand is driven by several factors, including the rising prevalence of chronic diseases, increasing research and development in nanomedicine, and the growing need for targeted drug delivery systems. The oncology sector represents the largest market share, followed by neurology and cardiovascular applications. North America currently leads the market, with Asia-Pacific expected to show the fastest growth in the coming years.
Pharmaceutical and biotechnology companies are the primary end-users of this technology, with academic and research institutions also contributing significantly to market growth through ongoing research and clinical trials. The market is characterized by a high degree of innovation and collaboration between industry players and research institutions.
Key market trends include the development of multifunctional nanoparticles that combine diagnostic and therapeutic capabilities, known as theranostics. There is also a growing focus on personalized medicine, where nanoparticle-based drug delivery systems can be tailored to individual patient profiles for optimal efficacy.
Challenges in the market include regulatory hurdles, concerns about long-term safety, and the high cost of development and production. However, ongoing advancements in nanotechnology and increasing investment in research are expected to address these challenges over time.
The market landscape is competitive, with several major pharmaceutical companies and specialized nanotechnology firms actively developing and commercializing nanoparticle-based drug delivery systems. Strategic partnerships and collaborations are common, as companies seek to combine expertise in nanotechnology, drug development, and electroporation techniques.
In conclusion, the market for nanoparticle-enhanced drug delivery combined with electroporation shows strong growth potential. As research progresses and more products reach clinical trials and commercialization, this innovative approach is poised to significantly impact the pharmaceutical industry and improve patient outcomes across various therapeutic areas.
Current Challenges in Electroporation-Nanoparticle Synergy
Despite the promising potential of combining electroporation with nanoparticle drug delivery, several significant challenges hinder the widespread adoption and optimization of this synergistic approach. One of the primary obstacles is the complexity of designing nanoparticles that can effectively interact with electroporated cell membranes while maintaining their structural integrity and drug payload.
The precise control of electroporation parameters, such as electric field strength, pulse duration, and frequency, remains a critical challenge. These parameters must be carefully tuned to create transient pores in cell membranes without causing irreversible damage or cell death. Simultaneously, they need to facilitate the efficient entry of nanoparticles, which adds another layer of complexity to the optimization process.
Another significant hurdle is the limited understanding of the dynamic interactions between nanoparticles and electroporated membranes. The mechanisms by which nanoparticles traverse the transient pores and their behavior within the intracellular environment are not fully elucidated, making it difficult to design optimal nanoparticle formulations for this specific application.
The heterogeneity of target tissues and cell types presents an additional challenge. Different cell types may respond differently to electroporation and nanoparticle uptake, necessitating the development of versatile and adaptable protocols that can accommodate this variability while maintaining efficacy and safety.
Scaling up the combined electroporation-nanoparticle delivery system for clinical applications poses significant technical and logistical challenges. Ensuring uniform electroporation across larger tissue volumes and maintaining consistent nanoparticle distribution remain problematic, particularly for deep-seated or hard-to-reach tissues.
Moreover, the potential for off-target effects and unintended tissue damage due to the combination of electroporation and nanoparticles raises safety concerns. Mitigating these risks while maximizing therapeutic efficacy requires extensive research and careful protocol design.
Lastly, the regulatory landscape for such a combined approach is complex and evolving. Demonstrating the safety and efficacy of this dual-modality system to regulatory bodies presents unique challenges, as it involves both a physical method (electroporation) and a nanoscale drug delivery system, each with its own set of regulatory considerations.
Addressing these challenges requires interdisciplinary collaboration among experts in electroporation, nanotechnology, drug delivery, cell biology, and clinical medicine. Overcoming these hurdles will be crucial for realizing the full potential of combining electroporation with nanoparticle drug delivery in various therapeutic applications.
The precise control of electroporation parameters, such as electric field strength, pulse duration, and frequency, remains a critical challenge. These parameters must be carefully tuned to create transient pores in cell membranes without causing irreversible damage or cell death. Simultaneously, they need to facilitate the efficient entry of nanoparticles, which adds another layer of complexity to the optimization process.
Another significant hurdle is the limited understanding of the dynamic interactions between nanoparticles and electroporated membranes. The mechanisms by which nanoparticles traverse the transient pores and their behavior within the intracellular environment are not fully elucidated, making it difficult to design optimal nanoparticle formulations for this specific application.
The heterogeneity of target tissues and cell types presents an additional challenge. Different cell types may respond differently to electroporation and nanoparticle uptake, necessitating the development of versatile and adaptable protocols that can accommodate this variability while maintaining efficacy and safety.
Scaling up the combined electroporation-nanoparticle delivery system for clinical applications poses significant technical and logistical challenges. Ensuring uniform electroporation across larger tissue volumes and maintaining consistent nanoparticle distribution remain problematic, particularly for deep-seated or hard-to-reach tissues.
Moreover, the potential for off-target effects and unintended tissue damage due to the combination of electroporation and nanoparticles raises safety concerns. Mitigating these risks while maximizing therapeutic efficacy requires extensive research and careful protocol design.
Lastly, the regulatory landscape for such a combined approach is complex and evolving. Demonstrating the safety and efficacy of this dual-modality system to regulatory bodies presents unique challenges, as it involves both a physical method (electroporation) and a nanoscale drug delivery system, each with its own set of regulatory considerations.
Addressing these challenges requires interdisciplinary collaboration among experts in electroporation, nanotechnology, drug delivery, cell biology, and clinical medicine. Overcoming these hurdles will be crucial for realizing the full potential of combining electroporation with nanoparticle drug delivery in various therapeutic applications.
Existing Electroporation-Nanoparticle Combination Strategies
01 Electroporation-enhanced nanoparticle drug delivery
Electroporation is used to enhance the delivery of nanoparticle-based drugs by temporarily increasing cell membrane permeability. This technique allows for improved intracellular uptake of therapeutic agents, resulting in higher drug delivery efficiency. The combination of electroporation with nanoparticle formulations can significantly increase the effectiveness of various treatments, including cancer therapies and gene delivery.- Electroporation-enhanced nanoparticle delivery: Electroporation is used to enhance the delivery of nanoparticles carrying therapeutic agents. This technique creates temporary pores in cell membranes, allowing for increased uptake of nanoparticles and their cargo. The combination of electroporation with nanoparticle drug delivery systems significantly improves drug delivery efficiency, particularly for intracellular targets.
- Optimizing electroporation parameters: The efficiency of drug delivery using electroporation and nanoparticles can be improved by optimizing various parameters. These include electric field strength, pulse duration, number of pulses, and interval between pulses. Adjusting these parameters based on the specific nanoparticle properties and target tissue characteristics can lead to enhanced drug delivery efficiency.
- Nanoparticle design for electroporation-mediated delivery: The design of nanoparticles plays a crucial role in the efficiency of electroporation-mediated drug delivery. Factors such as size, surface charge, and composition of nanoparticles can be optimized to enhance their interaction with cell membranes during electroporation. This optimization can lead to improved cellular uptake and drug release, ultimately increasing delivery efficiency.
- Combination with other delivery enhancement techniques: Electroporation combined with nanoparticle drug delivery can be further enhanced by incorporating additional delivery techniques. These may include the use of cell-penetrating peptides, pH-responsive nanoparticles, or ultrasound-mediated delivery. The synergistic effects of these combined approaches can significantly improve drug delivery efficiency compared to electroporation alone.
- Tissue-specific electroporation protocols: Developing tissue-specific electroporation protocols can enhance the efficiency of nanoparticle drug delivery. Different tissues have varying electrical properties and cellular compositions, requiring tailored electroporation parameters. By optimizing these protocols for specific target tissues, such as tumors or skin, the efficiency of drug delivery can be significantly improved while minimizing damage to surrounding healthy tissues.
02 Optimization of electroporation parameters
The efficiency of drug delivery using electroporation combined with nanoparticles can be optimized by adjusting various parameters. These include electric field strength, pulse duration, number of pulses, and electrode configuration. Fine-tuning these parameters can lead to improved cellular uptake of nanoparticles while minimizing cell damage, resulting in enhanced drug delivery efficiency.Expand Specific Solutions03 Nanoparticle design for electroporation-mediated delivery
The design and composition of nanoparticles play a crucial role in their effectiveness when combined with electroporation. Factors such as size, surface charge, and targeting ligands can be optimized to enhance cellular uptake and drug release. Specialized nanoparticle formulations can improve stability, reduce aggregation, and increase the overall efficiency of drug delivery when used in conjunction with electroporation.Expand Specific Solutions04 Tissue-specific electroporation protocols
Developing tissue-specific electroporation protocols can enhance the efficiency of nanoparticle drug delivery to target organs or tissues. By tailoring the electroporation parameters and nanoparticle properties to specific tissue types, such as tumors or skin, the drug delivery efficiency can be significantly improved. This approach allows for more precise and effective treatments while minimizing side effects in non-target tissues.Expand Specific Solutions05 Combination with other drug delivery enhancement techniques
Combining electroporation and nanoparticle drug delivery with other enhancement techniques can further improve drug delivery efficiency. These may include the use of ultrasound, magnetic fields, or pH-responsive nanoparticles. Such multi-modal approaches can synergistically enhance drug penetration, cellular uptake, and therapeutic efficacy, leading to more effective treatments for various diseases and conditions.Expand Specific Solutions
Key Players in Electroporation-Nanoparticle Drug Delivery
The field of combining electroporation with nanoparticle drug delivery is in a dynamic growth phase, characterized by increasing market size and evolving technological maturity. This emerging sector sits at the intersection of nanotechnology and biomedical engineering, with significant potential for revolutionizing targeted drug delivery. Key players like Alcon AG, Novartis AG, and Boston Scientific Ltd. are investing heavily in research and development, driving innovation in this space. Universities such as Chongqing University and the University of Southern California are contributing to the advancement of this technology through academic research. The market is witnessing a blend of established pharmaceutical companies and innovative startups, indicating a competitive landscape with diverse approaches to solving challenges in this field.
The Regents of the University of California
Technical Solution: The University of California has pioneered a multifaceted approach to combining electroporation with nanoparticle drug delivery. Their research teams have developed a system that utilizes biodegradable, polymer-based nanoparticles loaded with therapeutic agents. These nanoparticles are designed with specific surface charges to enhance their interaction with cell membranes during electroporation [4]. The university's approach involves a two-step process: first, the nanoparticles are administered to the target area, then a customized electroporation device applies precisely calibrated electric pulses to increase cellular uptake. This method has shown a significant improvement in drug penetration depth, with studies reporting up to a 5-fold increase in drug concentration within tumor tissues compared to nanoparticle administration alone [5]. Additionally, the research team has developed a real-time monitoring system that adjusts electroporation parameters based on tissue impedance, ensuring optimal pore formation while minimizing cellular damage [6].
Strengths: Highly customizable for different drug types and tissue targets, with real-time optimization capabilities. Weaknesses: Complex system that may require extensive training for clinical implementation, and potential variability in results across different tissue types.
Novartis AG
Technical Solution: Novartis AG has developed a sophisticated approach to combining electroporation with nanoparticle drug delivery, leveraging its extensive pharmaceutical expertise. Their system, named ElectroNanoTx, utilizes lipid-based nanoparticles (LNPs) optimized for electroporation-assisted delivery. These LNPs are designed with a unique composition that enhances their stability during electroporation while facilitating rapid intracellular release upon entry [11]. Novartis has also developed a proprietary electroporation device that employs a multi-electrode array, allowing for precise targeting of specific tissue layers. This technology has shown particular promise in dermal and transdermal drug delivery, with studies demonstrating up to an 8-fold increase in drug penetration compared to passive diffusion methods [12]. Additionally, Novartis has incorporated AI-driven algorithms into their system to optimize electroporation parameters in real-time based on tissue characteristics and drug properties, potentially improving treatment efficacy and reducing variability between patients [13].
Strengths: Highly optimized nanoparticle formulations, precise tissue targeting capabilities, and AI-enhanced treatment customization. Weaknesses: Potentially high cost due to advanced technology integration, and may require regulatory approval for both the drug and device components.
Innovative Approaches in Electroporation-Nanoparticle Integration
Drug delivery device
PatentInactiveEP1818046A1
Innovation
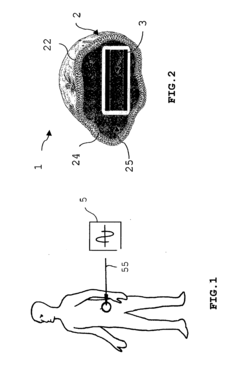
- A drug delivery device comprising a membrane with a micro- or nano-capacitor that generates an electric potential to induce electroporation, allowing controlled release of drugs through the membrane, which can be targeted to specific cells using affinity-conjugated molecules and enhanced with magnetic particles for traceability and localized release.
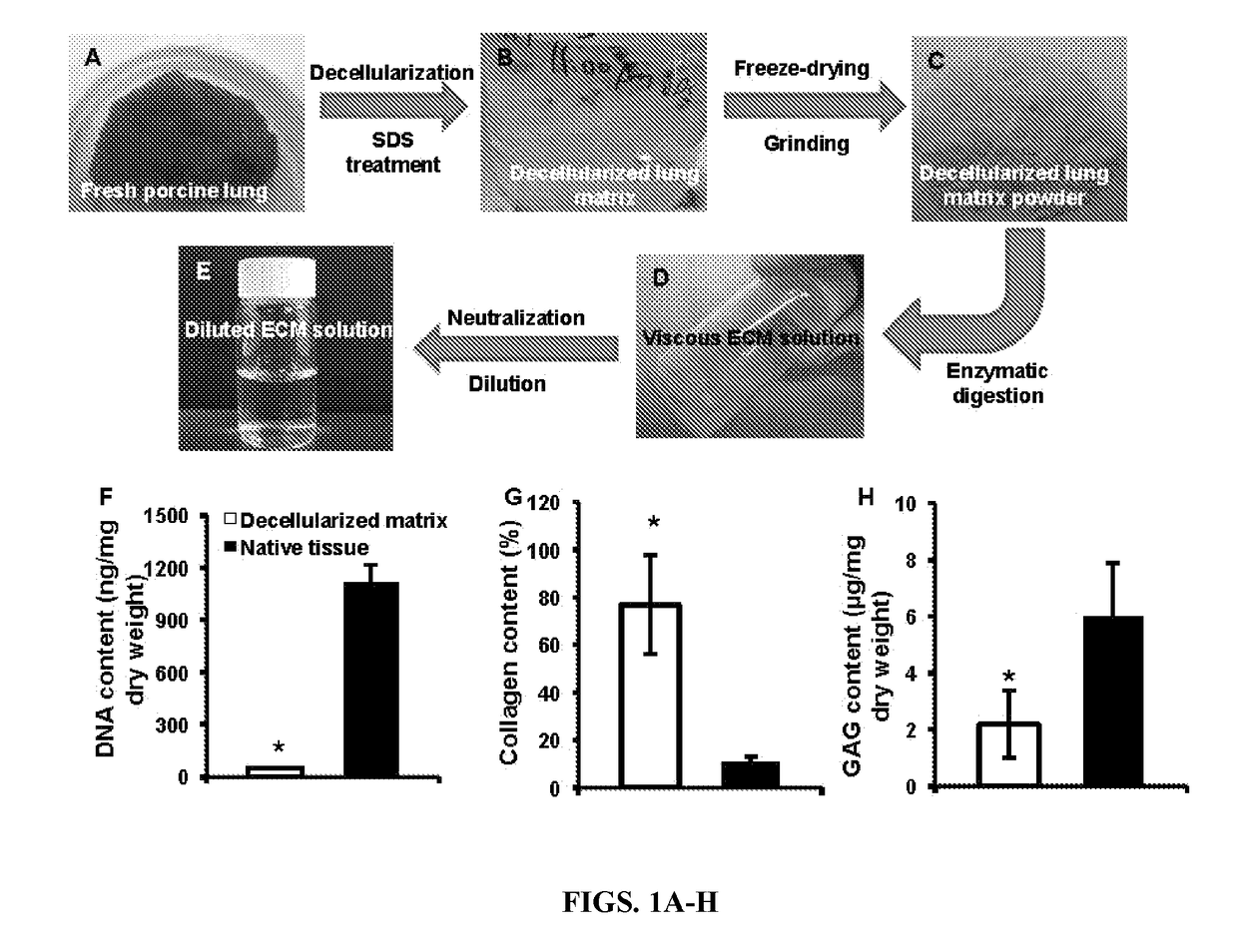
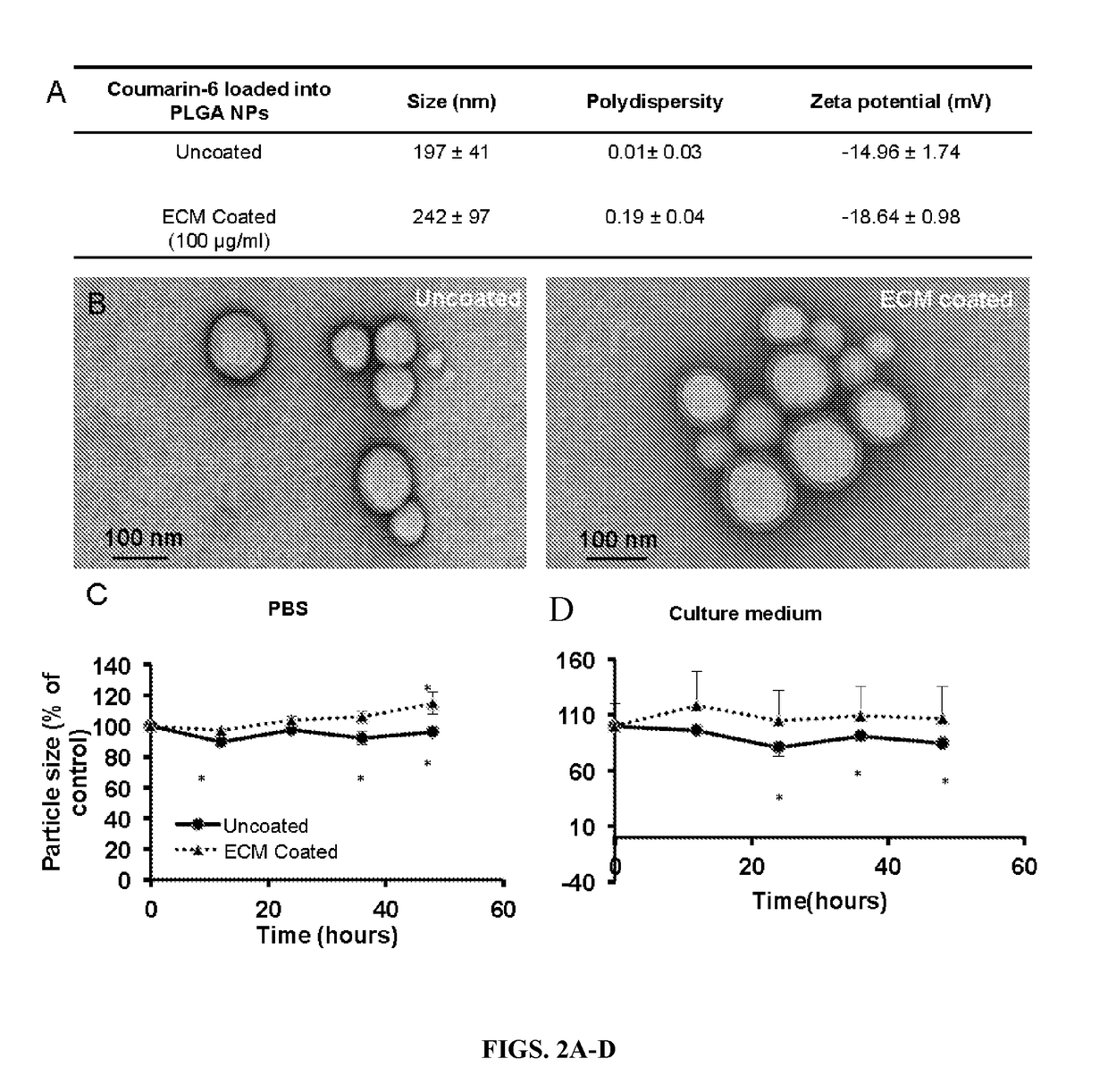
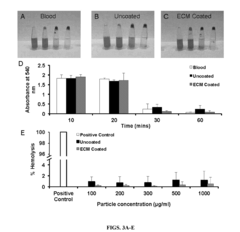
Nanoparticles containing extracellular matrix for drug delivery
PatentActiveUS20180207107A1
Innovation
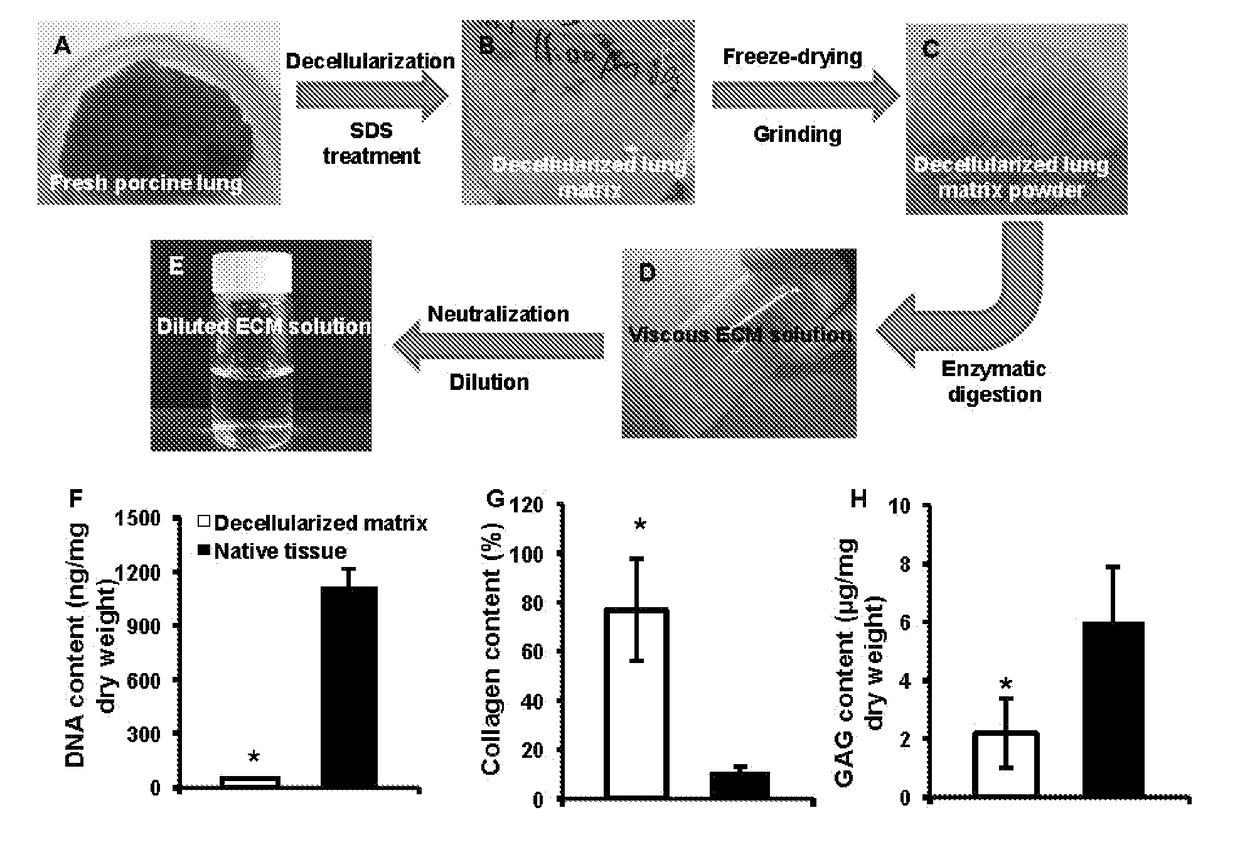
- Development of nanoparticles coated with decellularized extracellular matrix (ECM), specifically from lung tissue, which enhances uptake and retention in lung cells and delays payload release, improving pharmacokinetics and therapeutic efficacy.
Safety and Efficacy Considerations
The combination of electroporation with nanoparticle drug delivery presents both promising opportunities and potential risks that must be carefully considered. Safety is paramount in this approach, as electroporation involves the application of electrical pulses to temporarily increase cell membrane permeability. While this technique can enhance the uptake of nanoparticles carrying therapeutic agents, it also poses risks of cellular damage if not properly controlled.
One key safety consideration is the optimization of electrical parameters, including pulse strength, duration, and frequency. These must be finely tuned to achieve effective membrane permeabilization without causing irreversible cell damage or unwanted tissue effects. Additionally, the potential for localized heating and tissue damage at the site of electrode placement needs to be carefully monitored and mitigated.
The safety profile of the nanoparticles themselves is another critical factor. The size, shape, and surface properties of nanoparticles can significantly influence their interaction with biological systems. Potential toxicity, immunogenicity, and long-term accumulation in organs must be thoroughly evaluated. The use of biocompatible and biodegradable materials in nanoparticle construction can help address some of these concerns.
Efficacy considerations in combining electroporation with nanoparticle drug delivery are equally important. The primary goal is to achieve enhanced therapeutic outcomes compared to conventional drug delivery methods. This requires optimizing the synergy between electroporation parameters and nanoparticle characteristics to maximize drug uptake and targeted delivery.
One key aspect of efficacy is the ability to achieve uniform and consistent nanoparticle distribution within the target tissue. Electroporation can help overcome some of the barriers to nanoparticle penetration, but the heterogeneity of tissue structures and the variability in local electric field distribution can lead to non-uniform delivery. Advanced electrode designs and pulsing protocols may help address this challenge.
The timing and sequence of nanoparticle administration relative to electroporation application is another critical factor affecting efficacy. Optimal protocols need to be developed to ensure that nanoparticles are present in the extracellular space when cell membranes are most permeable. This may involve strategies such as pre-injection of nanoparticles followed by electroporation or simultaneous administration.
Furthermore, the potential for enhanced intracellular trafficking and endosomal escape of nanoparticles due to electroporation needs to be explored. This could lead to improved delivery of cargo to specific intracellular targets, potentially increasing therapeutic efficacy. However, the impact of electroporation on nanoparticle stability and drug release kinetics must also be carefully evaluated to ensure that the therapeutic payload remains intact and is released at the desired rate and location.
One key safety consideration is the optimization of electrical parameters, including pulse strength, duration, and frequency. These must be finely tuned to achieve effective membrane permeabilization without causing irreversible cell damage or unwanted tissue effects. Additionally, the potential for localized heating and tissue damage at the site of electrode placement needs to be carefully monitored and mitigated.
The safety profile of the nanoparticles themselves is another critical factor. The size, shape, and surface properties of nanoparticles can significantly influence their interaction with biological systems. Potential toxicity, immunogenicity, and long-term accumulation in organs must be thoroughly evaluated. The use of biocompatible and biodegradable materials in nanoparticle construction can help address some of these concerns.
Efficacy considerations in combining electroporation with nanoparticle drug delivery are equally important. The primary goal is to achieve enhanced therapeutic outcomes compared to conventional drug delivery methods. This requires optimizing the synergy between electroporation parameters and nanoparticle characteristics to maximize drug uptake and targeted delivery.
One key aspect of efficacy is the ability to achieve uniform and consistent nanoparticle distribution within the target tissue. Electroporation can help overcome some of the barriers to nanoparticle penetration, but the heterogeneity of tissue structures and the variability in local electric field distribution can lead to non-uniform delivery. Advanced electrode designs and pulsing protocols may help address this challenge.
The timing and sequence of nanoparticle administration relative to electroporation application is another critical factor affecting efficacy. Optimal protocols need to be developed to ensure that nanoparticles are present in the extracellular space when cell membranes are most permeable. This may involve strategies such as pre-injection of nanoparticles followed by electroporation or simultaneous administration.
Furthermore, the potential for enhanced intracellular trafficking and endosomal escape of nanoparticles due to electroporation needs to be explored. This could lead to improved delivery of cargo to specific intracellular targets, potentially increasing therapeutic efficacy. However, the impact of electroporation on nanoparticle stability and drug release kinetics must also be carefully evaluated to ensure that the therapeutic payload remains intact and is released at the desired rate and location.
Regulatory Landscape for Combined Delivery Technologies
The regulatory landscape for combined delivery technologies involving electroporation and nanoparticle drug delivery is complex and evolving. Regulatory agencies worldwide are grappling with the challenges posed by these innovative approaches, which blur the lines between traditional drug delivery methods and medical devices.
In the United States, the Food and Drug Administration (FDA) has established a framework for regulating combination products, which includes technologies that combine drugs, devices, and/or biological products. The Office of Combination Products (OCP) plays a crucial role in determining the primary mode of action and assigning the lead center for review. For electroporation-nanoparticle combinations, this often involves coordination between the Center for Drug Evaluation and Research (CDER) and the Center for Devices and Radiological Health (CDRH).
The European Medicines Agency (EMA) has also developed guidelines for combination products, emphasizing the need for integrated assessment of safety, quality, and efficacy. The European approach focuses on the overall risk-benefit profile of the combined technology, requiring manufacturers to provide comprehensive data on both the drug and device components.
Regulatory bodies are particularly concerned with the safety aspects of these combined technologies. The potential for enhanced cellular uptake of nanoparticles through electroporation raises questions about long-term toxicity and biodistribution. As a result, regulatory agencies often require extensive preclinical studies and long-term follow-up data in clinical trials.
Another key regulatory consideration is the standardization of manufacturing processes for both the nanoparticles and the electroporation devices. Agencies are increasingly demanding robust quality control measures to ensure consistency and reliability in the production of these complex delivery systems.
The regulatory pathway for these combined technologies often involves a more extensive and time-consuming approval process compared to traditional drug or device applications. This is due to the need for additional data on the interaction between the nanoparticles and the electroporation process, as well as the potential for unforeseen synergistic effects.
Globally, there is a trend towards harmonization of regulatory approaches for these advanced delivery technologies. Initiatives such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are working to develop consistent guidelines across different regions, aiming to streamline the approval process and facilitate global market access.
In the United States, the Food and Drug Administration (FDA) has established a framework for regulating combination products, which includes technologies that combine drugs, devices, and/or biological products. The Office of Combination Products (OCP) plays a crucial role in determining the primary mode of action and assigning the lead center for review. For electroporation-nanoparticle combinations, this often involves coordination between the Center for Drug Evaluation and Research (CDER) and the Center for Devices and Radiological Health (CDRH).
The European Medicines Agency (EMA) has also developed guidelines for combination products, emphasizing the need for integrated assessment of safety, quality, and efficacy. The European approach focuses on the overall risk-benefit profile of the combined technology, requiring manufacturers to provide comprehensive data on both the drug and device components.
Regulatory bodies are particularly concerned with the safety aspects of these combined technologies. The potential for enhanced cellular uptake of nanoparticles through electroporation raises questions about long-term toxicity and biodistribution. As a result, regulatory agencies often require extensive preclinical studies and long-term follow-up data in clinical trials.
Another key regulatory consideration is the standardization of manufacturing processes for both the nanoparticles and the electroporation devices. Agencies are increasingly demanding robust quality control measures to ensure consistency and reliability in the production of these complex delivery systems.
The regulatory pathway for these combined technologies often involves a more extensive and time-consuming approval process compared to traditional drug or device applications. This is due to the need for additional data on the interaction between the nanoparticles and the electroporation process, as well as the potential for unforeseen synergistic effects.
Globally, there is a trend towards harmonization of regulatory approaches for these advanced delivery technologies. Initiatives such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are working to develop consistent guidelines across different regions, aiming to streamline the approval process and facilitate global market access.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!