How To Implement Microfluidic Electroporation For High Throughput
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Electroporation Background and Objectives
Microfluidic electroporation has emerged as a powerful technique for high-throughput cell manipulation, combining the precision of microfluidics with the efficiency of electroporation. This technology has evolved significantly over the past two decades, driven by the increasing demand for more efficient and controllable methods of introducing foreign molecules into cells.
The field of microfluidic electroporation originated from the convergence of two separate disciplines: microfluidics and electroporation. Microfluidics, which deals with the manipulation of fluids at the microscale, has been developing since the 1990s. Electroporation, on the other hand, has been used for cell transfection since the 1980s. The integration of these technologies began in the early 2000s, with pioneering work demonstrating the potential of microfluidic devices for enhancing electroporation efficiency.
As the field progressed, researchers focused on optimizing various aspects of microfluidic electroporation, including electrode design, channel geometry, and flow dynamics. The development of novel materials and fabrication techniques has also played a crucial role in advancing this technology. Recent years have seen a shift towards more sophisticated designs that allow for precise control over electric field distribution and cell exposure time.
The primary objective of implementing microfluidic electroporation for high throughput is to achieve efficient and uniform transfection of large cell populations while maintaining high cell viability. This goal encompasses several key aspects: maximizing the number of cells that can be processed per unit time, ensuring consistent electric field exposure across all cells, minimizing cell damage, and achieving high transfection efficiency.
Another important objective is to develop versatile platforms that can accommodate a wide range of cell types and molecules for transfection. This includes adapting the technology for use with difficult-to-transfect cell lines, primary cells, and even in vivo applications. Additionally, there is a growing interest in integrating microfluidic electroporation with other lab-on-a-chip technologies to create multifunctional platforms for cell analysis and manipulation.
The future trajectory of microfluidic electroporation is likely to focus on further miniaturization, automation, and integration with other technologies. This includes the development of high-density electrode arrays, the incorporation of advanced sensing and control systems, and the exploration of novel electrode materials and configurations. There is also a trend towards developing user-friendly, plug-and-play systems that can be easily adopted by researchers without specialized microfluidics expertise.
The field of microfluidic electroporation originated from the convergence of two separate disciplines: microfluidics and electroporation. Microfluidics, which deals with the manipulation of fluids at the microscale, has been developing since the 1990s. Electroporation, on the other hand, has been used for cell transfection since the 1980s. The integration of these technologies began in the early 2000s, with pioneering work demonstrating the potential of microfluidic devices for enhancing electroporation efficiency.
As the field progressed, researchers focused on optimizing various aspects of microfluidic electroporation, including electrode design, channel geometry, and flow dynamics. The development of novel materials and fabrication techniques has also played a crucial role in advancing this technology. Recent years have seen a shift towards more sophisticated designs that allow for precise control over electric field distribution and cell exposure time.
The primary objective of implementing microfluidic electroporation for high throughput is to achieve efficient and uniform transfection of large cell populations while maintaining high cell viability. This goal encompasses several key aspects: maximizing the number of cells that can be processed per unit time, ensuring consistent electric field exposure across all cells, minimizing cell damage, and achieving high transfection efficiency.
Another important objective is to develop versatile platforms that can accommodate a wide range of cell types and molecules for transfection. This includes adapting the technology for use with difficult-to-transfect cell lines, primary cells, and even in vivo applications. Additionally, there is a growing interest in integrating microfluidic electroporation with other lab-on-a-chip technologies to create multifunctional platforms for cell analysis and manipulation.
The future trajectory of microfluidic electroporation is likely to focus on further miniaturization, automation, and integration with other technologies. This includes the development of high-density electrode arrays, the incorporation of advanced sensing and control systems, and the exploration of novel electrode materials and configurations. There is also a trend towards developing user-friendly, plug-and-play systems that can be easily adopted by researchers without specialized microfluidics expertise.
Market Analysis for High-Throughput Cell Transfection
The market for high-throughput cell transfection technologies, particularly those utilizing microfluidic electroporation, is experiencing significant growth driven by the increasing demand for efficient and scalable gene delivery methods in various research and therapeutic applications. This market segment is closely tied to the broader cell and gene therapy market, which is projected to expand rapidly in the coming years.
The primary drivers of market demand include the growing focus on personalized medicine, advancements in gene editing technologies such as CRISPR-Cas9, and the rising prevalence of genetic disorders and cancers. Pharmaceutical and biotechnology companies are increasingly investing in cell-based therapies, creating a strong need for high-throughput transfection methods that can efficiently modify large numbers of cells.
Academic research institutions and contract research organizations (CROs) also contribute significantly to the market demand. These entities require advanced transfection technologies for various applications, including drug discovery, functional genomics studies, and the development of disease models.
The market for high-throughput cell transfection is geographically diverse, with North America and Europe leading in terms of adoption and technological advancements. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by increasing investments in life sciences research and a growing biopharmaceutical industry.
Key market segments for high-throughput cell transfection technologies include gene therapy, cell therapy, and regenerative medicine. The gene therapy segment, in particular, is experiencing rapid growth due to recent regulatory approvals and a robust pipeline of gene-based therapeutics in clinical trials.
The adoption of microfluidic electroporation for high-throughput applications is gaining traction due to its advantages over traditional bulk electroporation methods. These benefits include higher transfection efficiency, reduced cell damage, and the ability to process large numbers of cells with minimal sample volumes.
Market challenges include the high cost of advanced transfection systems, regulatory hurdles in the development and commercialization of cell and gene therapies, and the need for specialized expertise to operate complex microfluidic devices. However, ongoing technological advancements and increasing collaborations between academic institutions and industry players are expected to address these challenges and drive market growth.
In conclusion, the market for high-throughput cell transfection, particularly using microfluidic electroporation, shows strong growth potential. The increasing demand for efficient gene delivery methods in various biomedical applications, coupled with technological advancements, is expected to drive market expansion in the coming years.
The primary drivers of market demand include the growing focus on personalized medicine, advancements in gene editing technologies such as CRISPR-Cas9, and the rising prevalence of genetic disorders and cancers. Pharmaceutical and biotechnology companies are increasingly investing in cell-based therapies, creating a strong need for high-throughput transfection methods that can efficiently modify large numbers of cells.
Academic research institutions and contract research organizations (CROs) also contribute significantly to the market demand. These entities require advanced transfection technologies for various applications, including drug discovery, functional genomics studies, and the development of disease models.
The market for high-throughput cell transfection is geographically diverse, with North America and Europe leading in terms of adoption and technological advancements. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by increasing investments in life sciences research and a growing biopharmaceutical industry.
Key market segments for high-throughput cell transfection technologies include gene therapy, cell therapy, and regenerative medicine. The gene therapy segment, in particular, is experiencing rapid growth due to recent regulatory approvals and a robust pipeline of gene-based therapeutics in clinical trials.
The adoption of microfluidic electroporation for high-throughput applications is gaining traction due to its advantages over traditional bulk electroporation methods. These benefits include higher transfection efficiency, reduced cell damage, and the ability to process large numbers of cells with minimal sample volumes.
Market challenges include the high cost of advanced transfection systems, regulatory hurdles in the development and commercialization of cell and gene therapies, and the need for specialized expertise to operate complex microfluidic devices. However, ongoing technological advancements and increasing collaborations between academic institutions and industry players are expected to address these challenges and drive market growth.
In conclusion, the market for high-throughput cell transfection, particularly using microfluidic electroporation, shows strong growth potential. The increasing demand for efficient gene delivery methods in various biomedical applications, coupled with technological advancements, is expected to drive market expansion in the coming years.
Current Challenges in Microfluidic Electroporation
Microfluidic electroporation has emerged as a promising technique for high-throughput cell manipulation, offering precise control over electric field distribution and cellular interactions. However, several challenges persist in its implementation, hindering widespread adoption and optimal performance.
One of the primary challenges is achieving uniform electric field distribution across microfluidic channels. The complex geometry of microfluidic devices can lead to non-uniform field strengths, resulting in inconsistent electroporation efficiency. This variability affects the reproducibility of results and limits the overall throughput of the system.
Cell viability remains a critical concern in microfluidic electroporation. The application of electric fields can cause irreversible damage to cell membranes, leading to cell death. Balancing the need for effective pore formation with maintaining cell viability is a delicate process that requires precise control over pulse parameters and duration.
Scaling up microfluidic electroporation systems for high-throughput applications presents significant engineering challenges. Increasing the number of parallel channels while maintaining consistent electric field distribution and flow conditions across all channels is technically demanding. Additionally, integrating multiple electrodes and ensuring their uniform performance adds complexity to device fabrication.
The optimization of flow dynamics within microfluidic channels is another hurdle. Achieving the ideal balance between flow rate, residence time, and electric field exposure is crucial for efficient electroporation. Turbulence and non-uniform flow patterns can lead to inconsistent results and reduced throughput.
Electrode fouling and bubble formation are persistent issues in microfluidic electroporation. The accumulation of cellular debris and electrochemical reactions at electrode surfaces can degrade performance over time. Bubble generation during electroporation can disrupt flow patterns and electric field distribution, compromising the efficiency and reliability of the process.
The integration of real-time monitoring and feedback systems remains a challenge. Developing robust sensors and control mechanisms that can operate at the microscale and provide instantaneous adjustments to electroporation parameters is technically complex but essential for optimizing performance and ensuring consistency.
Lastly, the biocompatibility of materials used in microfluidic electroporation devices is a ongoing concern. Ensuring that all components, including electrodes and channel materials, do not adversely affect cell viability or introduce contaminants is crucial for maintaining the integrity of biological samples and experimental results.
Addressing these challenges requires interdisciplinary approaches, combining expertise in microfluidics, electrical engineering, materials science, and cell biology. Overcoming these hurdles will be essential for realizing the full potential of microfluidic electroporation in high-throughput applications across various fields, including gene therapy, drug delivery, and cell-based assays.
One of the primary challenges is achieving uniform electric field distribution across microfluidic channels. The complex geometry of microfluidic devices can lead to non-uniform field strengths, resulting in inconsistent electroporation efficiency. This variability affects the reproducibility of results and limits the overall throughput of the system.
Cell viability remains a critical concern in microfluidic electroporation. The application of electric fields can cause irreversible damage to cell membranes, leading to cell death. Balancing the need for effective pore formation with maintaining cell viability is a delicate process that requires precise control over pulse parameters and duration.
Scaling up microfluidic electroporation systems for high-throughput applications presents significant engineering challenges. Increasing the number of parallel channels while maintaining consistent electric field distribution and flow conditions across all channels is technically demanding. Additionally, integrating multiple electrodes and ensuring their uniform performance adds complexity to device fabrication.
The optimization of flow dynamics within microfluidic channels is another hurdle. Achieving the ideal balance between flow rate, residence time, and electric field exposure is crucial for efficient electroporation. Turbulence and non-uniform flow patterns can lead to inconsistent results and reduced throughput.
Electrode fouling and bubble formation are persistent issues in microfluidic electroporation. The accumulation of cellular debris and electrochemical reactions at electrode surfaces can degrade performance over time. Bubble generation during electroporation can disrupt flow patterns and electric field distribution, compromising the efficiency and reliability of the process.
The integration of real-time monitoring and feedback systems remains a challenge. Developing robust sensors and control mechanisms that can operate at the microscale and provide instantaneous adjustments to electroporation parameters is technically complex but essential for optimizing performance and ensuring consistency.
Lastly, the biocompatibility of materials used in microfluidic electroporation devices is a ongoing concern. Ensuring that all components, including electrodes and channel materials, do not adversely affect cell viability or introduce contaminants is crucial for maintaining the integrity of biological samples and experimental results.
Addressing these challenges requires interdisciplinary approaches, combining expertise in microfluidics, electrical engineering, materials science, and cell biology. Overcoming these hurdles will be essential for realizing the full potential of microfluidic electroporation in high-throughput applications across various fields, including gene therapy, drug delivery, and cell-based assays.
Existing High-Throughput Electroporation Solutions
01 High-throughput microfluidic electroporation systems
Advanced microfluidic devices are designed to perform high-throughput electroporation, allowing for the rapid and efficient transfection of large numbers of cells. These systems often incorporate parallel processing channels, automated sample handling, and precise control over electric field parameters to maximize throughput while maintaining cell viability.- High-throughput microfluidic electroporation systems: Advanced microfluidic devices are designed for high-throughput electroporation, enabling efficient transfection of large numbers of cells. These systems often incorporate parallel processing channels, automated sample handling, and precise control of electric field parameters to maximize throughput while maintaining cell viability.
- Continuous flow electroporation in microfluidic channels: Continuous flow electroporation techniques in microfluidic channels allow for increased throughput by processing cells in a steady stream. This approach enables the treatment of large cell populations efficiently, with the potential for real-time monitoring and adjustment of electroporation parameters.
- Integration of cell sorting and electroporation: Microfluidic platforms that combine cell sorting capabilities with electroporation enhance overall throughput by allowing selective transfection of specific cell populations. This integration streamlines workflow and improves efficiency in applications such as gene editing and cell therapy production.
- Optimization of electrode design for improved throughput: Novel electrode configurations and materials are developed to enhance electroporation efficiency and throughput in microfluidic devices. These designs aim to create more uniform electric fields, reduce cell damage, and allow for higher processing speeds.
- Automation and scalability of microfluidic electroporation: Automated microfluidic electroporation systems are developed to increase throughput and reproducibility. These platforms often incorporate robotics, machine learning algorithms for parameter optimization, and modular designs that allow for easy scaling of operations from research to industrial applications.
02 Continuous flow electroporation in microfluidic channels
Continuous flow electroporation techniques utilize microfluidic channels to expose cells to electric fields as they flow through the device. This approach enables high-throughput processing by eliminating the need for batch operations, allowing for continuous cell transfection and potentially increasing overall efficiency and yield.Expand Specific Solutions03 Integration of cell sorting and electroporation
Microfluidic platforms that combine cell sorting capabilities with electroporation enhance throughput by allowing for selective transfection of specific cell populations. These integrated systems can isolate target cells based on various criteria before subjecting them to electroporation, improving overall efficiency and reducing processing time.Expand Specific Solutions04 Optimization of electrode design and configuration
Innovative electrode designs and configurations within microfluidic devices can significantly improve electroporation throughput. This includes the use of 3D electrodes, interdigitated electrode arrays, and novel materials to enhance electric field distribution and cell membrane permeabilization efficiency across a larger volume of sample.Expand Specific Solutions05 Automation and integration with lab-on-a-chip systems
Microfluidic electroporation throughput can be enhanced by integrating the process into automated lab-on-a-chip systems. These platforms incorporate sample preparation, electroporation, and post-processing steps into a single, automated workflow, reducing manual handling and increasing overall throughput and reproducibility.Expand Specific Solutions
Key Players in Microfluidic Electroporation Industry
The microfluidic electroporation for high throughput implementation is in an emerging phase, with growing market potential due to its applications in cell therapy and gene editing. The technology is advancing rapidly, but still evolving towards full maturity. Key players like Cytequest, Bio-Rad Laboratories, and Inscripta are driving innovation in this space. Established institutions such as the University of California and MIT are contributing significant research. The competitive landscape includes both specialized startups and large biotech companies, indicating a dynamic market with opportunities for technological breakthroughs and commercialization.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed a microfluidic electroporation platform that combines their expertise in gene transfer technologies with advanced microfluidics. Their system utilizes a disposable microfluidic chip with multiple electroporation chambers, allowing for parallel processing of samples. The platform incorporates precise fluid handling and electrode designs to ensure uniform electric field distribution across the chip. Bio-Rad's technology also features automated sample loading and recovery, minimizing manual handling and reducing contamination risks. The system is designed to be compatible with a wide range of cell types and can be optimized for various transfection protocols[2][5].
Strengths: Disposable chip design for easy handling, automated sample processing, and versatility across cell types. Weaknesses: Potentially higher consumable costs due to disposable chips.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a high-throughput microfluidic electroporation system that utilizes a novel electrode configuration and flow-through design. The system incorporates parallel microchannels with integrated electrodes, allowing for continuous-flow electroporation of cells. This approach enables processing of large sample volumes while maintaining high transfection efficiency. The device employs precise control over electric field strength and pulse duration, optimizing conditions for various cell types. Additionally, MIT's system integrates on-chip cell sorting capabilities, enhancing overall throughput and allowing for immediate analysis of transfected cells[1][3].
Strengths: High-throughput capability, precise control over electroporation parameters, and integrated cell sorting. Weaknesses: Potential complexity in fabrication and scalability for industrial applications.
Core Innovations in Microfluidic Electroporation
Method and Apparatus for High Throughput High Efficiency Transfection of Cells
PatentPendingUS20240084236A1
Innovation
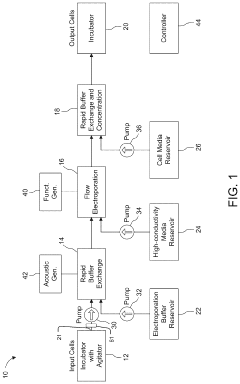
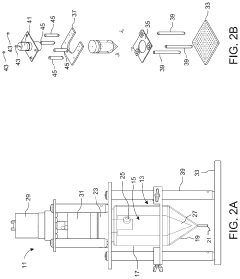
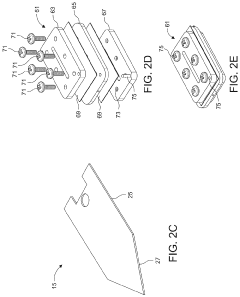
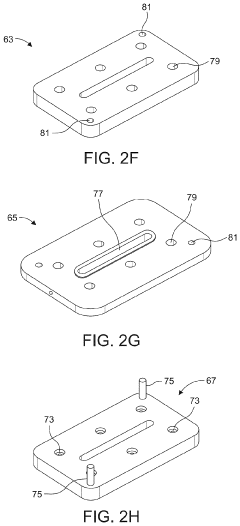
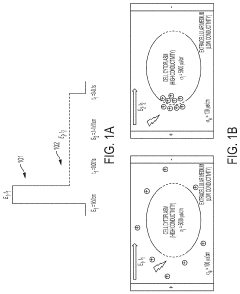
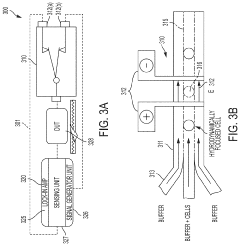
- A microfluidic hydrodynamic sheath flow configuration with automated systems for continuous, high-throughput electroporation, minimizing exposure to non-ideal buffers and preventing direct contact with electrodes, using multiple microfluidic channels and electrodes to maintain cells under cell-culture conditions.
High Throughput, Feedback-Controlled Electroporation Microdevice for Efficient Molecular Delivery into Single Cells
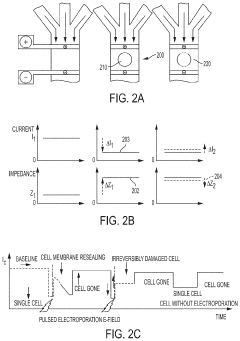
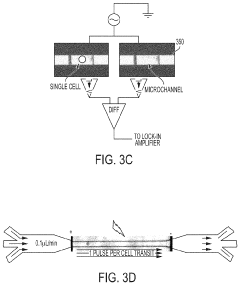
PatentActiveUS20210139825A1
Innovation
- A microfluidic electroporation system with a feedback-controlled, intelligent system that uses impedance monitoring and adjustable electrical signals to detect and permeabilize individual cells, ensuring optimal permeabilization without cell death, and then delivers molecules using a controlled delivery signal.
Scalability and Integration Considerations
Scaling up microfluidic electroporation for high throughput applications presents several key considerations. The integration of multiple electroporation units on a single chip is crucial for increasing throughput. This can be achieved through parallel processing channels, where multiple samples are electroporated simultaneously. However, maintaining uniform electric field distribution across all channels becomes challenging as the number of units increases. Advanced chip designs incorporating electrode arrays and optimized channel geometries can help mitigate this issue.
Another important aspect is the automation and control of fluid handling. High-throughput systems require precise and rapid sample loading, electroporation, and collection. Microfluidic pumps, valves, and mixers need to be seamlessly integrated to ensure consistent and efficient operation. The development of standardized interfaces between microfluidic chips and external equipment is essential for plug-and-play functionality and ease of use in research and industrial settings.
Power supply scaling is a critical factor in high-throughput microfluidic electroporation. As the number of electroporation units increases, the power requirements grow significantly. Designing modular power supply systems that can be easily scaled up to accommodate larger chip arrays is necessary. Additionally, implementing intelligent power management strategies can optimize energy consumption and prevent overheating.
Data acquisition and processing capabilities must also scale with the increased throughput. Real-time monitoring of electroporation parameters across multiple channels requires high-speed data acquisition systems. Integrating on-chip sensors for measuring electrical and biological parameters can provide valuable feedback for process optimization. Advanced data processing algorithms and machine learning techniques can be employed to analyze the large volumes of data generated by high-throughput systems.
Lastly, considerations for sample preparation and post-electroporation processing must be addressed. Developing integrated systems that combine sample preparation, electroporation, and downstream analysis can significantly enhance overall throughput. This may involve incorporating cell sorting, culturing, or analytical modules directly onto the microfluidic platform. Such integration can reduce handling steps, minimize sample loss, and improve the consistency of results across large sample sets.
Another important aspect is the automation and control of fluid handling. High-throughput systems require precise and rapid sample loading, electroporation, and collection. Microfluidic pumps, valves, and mixers need to be seamlessly integrated to ensure consistent and efficient operation. The development of standardized interfaces between microfluidic chips and external equipment is essential for plug-and-play functionality and ease of use in research and industrial settings.
Power supply scaling is a critical factor in high-throughput microfluidic electroporation. As the number of electroporation units increases, the power requirements grow significantly. Designing modular power supply systems that can be easily scaled up to accommodate larger chip arrays is necessary. Additionally, implementing intelligent power management strategies can optimize energy consumption and prevent overheating.
Data acquisition and processing capabilities must also scale with the increased throughput. Real-time monitoring of electroporation parameters across multiple channels requires high-speed data acquisition systems. Integrating on-chip sensors for measuring electrical and biological parameters can provide valuable feedback for process optimization. Advanced data processing algorithms and machine learning techniques can be employed to analyze the large volumes of data generated by high-throughput systems.
Lastly, considerations for sample preparation and post-electroporation processing must be addressed. Developing integrated systems that combine sample preparation, electroporation, and downstream analysis can significantly enhance overall throughput. This may involve incorporating cell sorting, culturing, or analytical modules directly onto the microfluidic platform. Such integration can reduce handling steps, minimize sample loss, and improve the consistency of results across large sample sets.
Bioethical Implications of High-Throughput Cell Modification
The rapid advancement of high-throughput cell modification techniques, particularly microfluidic electroporation, raises significant bioethical concerns that warrant careful consideration. As these technologies enable the manipulation of large numbers of cells with unprecedented efficiency, they challenge existing ethical frameworks and necessitate a reevaluation of our approach to biological research and medical applications.
One primary ethical concern is the potential for unintended consequences resulting from large-scale genetic modifications. The ability to alter the genetic makeup of vast numbers of cells simultaneously increases the risk of introducing unforeseen mutations or disrupting complex biological systems. This raises questions about the long-term effects on individual organisms and broader ecosystems, as well as the ethical implications of creating genetically modified organisms at an unprecedented scale.
Privacy and consent issues also come to the forefront with high-throughput cell modification techniques. As these technologies become more accessible, there is a growing need to establish robust protocols for obtaining informed consent from donors of biological materials. The potential for identifying individuals based on genetic information derived from modified cells raises concerns about data protection and the right to genetic privacy.
The equitable distribution of benefits arising from high-throughput cell modification technologies presents another ethical challenge. As these techniques hold promise for developing new therapies and medical treatments, ensuring fair access to the resulting advancements becomes crucial. This includes addressing disparities in healthcare access and considering the potential for these technologies to exacerbate existing socioeconomic inequalities.
Furthermore, the rapid pace of technological advancement in this field outstrips the development of regulatory frameworks and ethical guidelines. This lag creates a potential for misuse or exploitation of high-throughput cell modification techniques, highlighting the need for proactive policy-making and international cooperation to establish ethical standards and oversight mechanisms.
The concept of human enhancement and the blurring of lines between therapy and enhancement also emerge as significant ethical considerations. High-throughput cell modification techniques could potentially be used to enhance human capabilities beyond normal functioning, raising questions about the nature of humanity and the ethical boundaries of scientific intervention in human biology.
In conclusion, while high-throughput cell modification techniques offer immense potential for scientific and medical advancements, they also present complex bioethical challenges that require careful consideration and ongoing dialogue among scientists, ethicists, policymakers, and the public. Addressing these ethical implications is crucial for ensuring that the development and application of these powerful technologies align with societal values and respect for human dignity.
One primary ethical concern is the potential for unintended consequences resulting from large-scale genetic modifications. The ability to alter the genetic makeup of vast numbers of cells simultaneously increases the risk of introducing unforeseen mutations or disrupting complex biological systems. This raises questions about the long-term effects on individual organisms and broader ecosystems, as well as the ethical implications of creating genetically modified organisms at an unprecedented scale.
Privacy and consent issues also come to the forefront with high-throughput cell modification techniques. As these technologies become more accessible, there is a growing need to establish robust protocols for obtaining informed consent from donors of biological materials. The potential for identifying individuals based on genetic information derived from modified cells raises concerns about data protection and the right to genetic privacy.
The equitable distribution of benefits arising from high-throughput cell modification technologies presents another ethical challenge. As these techniques hold promise for developing new therapies and medical treatments, ensuring fair access to the resulting advancements becomes crucial. This includes addressing disparities in healthcare access and considering the potential for these technologies to exacerbate existing socioeconomic inequalities.
Furthermore, the rapid pace of technological advancement in this field outstrips the development of regulatory frameworks and ethical guidelines. This lag creates a potential for misuse or exploitation of high-throughput cell modification techniques, highlighting the need for proactive policy-making and international cooperation to establish ethical standards and oversight mechanisms.
The concept of human enhancement and the blurring of lines between therapy and enhancement also emerge as significant ethical considerations. High-throughput cell modification techniques could potentially be used to enhance human capabilities beyond normal functioning, raising questions about the nature of humanity and the ethical boundaries of scientific intervention in human biology.
In conclusion, while high-throughput cell modification techniques offer immense potential for scientific and medical advancements, they also present complex bioethical challenges that require careful consideration and ongoing dialogue among scientists, ethicists, policymakers, and the public. Addressing these ethical implications is crucial for ensuring that the development and application of these powerful technologies align with societal values and respect for human dignity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!