Energy And Carbon Footprint Comparison With Anthraquinone Process
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Anthraquinone Process Energy Efficiency Background
The Anthraquinone Process has been the predominant industrial method for hydrogen peroxide (H₂O₂) production since its commercialization in the 1940s, replacing earlier electrochemical processes. This auto-oxidation process utilizes a working solution containing an alkylated anthraquinone that undergoes catalytic hydrogenation followed by oxidation with air to produce hydrogen peroxide. The process accounts for over 95% of global hydrogen peroxide production, estimated at approximately 5.5 million tons annually.
Energy efficiency has become increasingly critical in chemical manufacturing due to rising energy costs and environmental concerns. The Anthraquinone Process, while well-established, presents significant energy challenges. The process requires multiple energy-intensive steps including hydrogenation, oxidation, extraction, and purification. Particularly, the separation and purification stages demand substantial thermal energy for distillation and concentration of hydrogen peroxide solutions.
Historical data indicates that conventional Anthraquinone Process plants consume between 2.5-3.5 MWh of electricity and 5-8 GJ of steam per ton of 100% H₂O₂ produced. This translates to a total energy footprint of approximately 14-20 GJ per ton of product. The carbon footprint associated with this energy consumption varies significantly depending on regional energy sources but typically ranges from 1.2-2.0 tons of CO₂ equivalent per ton of hydrogen peroxide.
Process improvements over the past decades have focused on optimizing reaction conditions, developing more stable working solutions, and enhancing heat integration. Modern plants have achieved energy reductions of 15-25% compared to facilities built in the 1980s. However, fundamental thermodynamic limitations of the multi-step process continue to present efficiency barriers.
The energy profile of the Anthraquinone Process is characterized by its indirect production route. Rather than direct combination of hydrogen and oxygen (which would be theoretically more energy-efficient but presents significant safety challenges), the process uses anthraquinone as a carrier molecule. This safer but more complex pathway inherently increases energy requirements through additional processing steps and solvent handling.
Recent sustainability initiatives have driven research into further efficiency improvements, including advanced catalysts for lower-temperature hydrogenation, more efficient oxidation systems, and enhanced heat recovery configurations. Despite these advances, the fundamental energy requirements of the process remain substantial, prompting exploration of alternative production technologies with potentially lower energy and carbon footprints.
Energy efficiency has become increasingly critical in chemical manufacturing due to rising energy costs and environmental concerns. The Anthraquinone Process, while well-established, presents significant energy challenges. The process requires multiple energy-intensive steps including hydrogenation, oxidation, extraction, and purification. Particularly, the separation and purification stages demand substantial thermal energy for distillation and concentration of hydrogen peroxide solutions.
Historical data indicates that conventional Anthraquinone Process plants consume between 2.5-3.5 MWh of electricity and 5-8 GJ of steam per ton of 100% H₂O₂ produced. This translates to a total energy footprint of approximately 14-20 GJ per ton of product. The carbon footprint associated with this energy consumption varies significantly depending on regional energy sources but typically ranges from 1.2-2.0 tons of CO₂ equivalent per ton of hydrogen peroxide.
Process improvements over the past decades have focused on optimizing reaction conditions, developing more stable working solutions, and enhancing heat integration. Modern plants have achieved energy reductions of 15-25% compared to facilities built in the 1980s. However, fundamental thermodynamic limitations of the multi-step process continue to present efficiency barriers.
The energy profile of the Anthraquinone Process is characterized by its indirect production route. Rather than direct combination of hydrogen and oxygen (which would be theoretically more energy-efficient but presents significant safety challenges), the process uses anthraquinone as a carrier molecule. This safer but more complex pathway inherently increases energy requirements through additional processing steps and solvent handling.
Recent sustainability initiatives have driven research into further efficiency improvements, including advanced catalysts for lower-temperature hydrogenation, more efficient oxidation systems, and enhanced heat recovery configurations. Despite these advances, the fundamental energy requirements of the process remain substantial, prompting exploration of alternative production technologies with potentially lower energy and carbon footprints.
Market Demand for Low-Carbon Chemical Processes
The global chemical industry is experiencing a significant shift towards low-carbon processes, driven by increasing regulatory pressures, consumer demands for sustainable products, and corporate commitments to reduce environmental impacts. The hydrogen peroxide market, valued at approximately $3.5 billion in 2022, is projected to grow at a CAGR of 5.2% through 2030, with sustainability becoming a key differentiator among suppliers.
Traditional hydrogen peroxide production via the anthraquinone process has faced mounting criticism due to its substantial energy consumption and carbon footprint. Industrial customers across sectors including pulp and paper, textile bleaching, wastewater treatment, and electronics manufacturing are increasingly prioritizing suppliers who can demonstrate reduced environmental impact in their production methods.
Market research indicates that 78% of chemical industry procurement managers now include carbon footprint metrics in their supplier evaluation criteria, compared to just 35% five years ago. This trend is particularly pronounced in Europe, where the EU's Carbon Border Adjustment Mechanism and stringent emissions regulations have created strong market incentives for low-carbon alternatives to conventional chemical processes.
The pulp and paper industry, which consumes approximately 60% of globally produced hydrogen peroxide, has set ambitious decarbonization targets, with major players committing to 30-50% reductions in supply chain emissions by 2030. This creates a substantial market pull for hydrogen peroxide produced through more energy-efficient and lower-carbon processes.
Emerging economies, particularly in Asia-Pacific, represent the fastest-growing markets for hydrogen peroxide, with projected demand increases of 7.8% annually through 2028. These regions are increasingly implementing stricter environmental regulations, creating new market opportunities for advanced production technologies with improved sustainability profiles.
Price premium analysis suggests that industrial customers are willing to pay 5-12% more for chemical products with verified lower carbon footprints, particularly when these can contribute to their own scope 3 emissions reduction targets. This willingness to pay has been increasing steadily since 2018, indicating strengthening market demand for greener alternatives to the anthraquinone process.
Market forecasts predict that by 2030, low-carbon hydrogen peroxide production methods could capture up to 25% of the global market share, representing a significant opportunity for early adopters of alternative technologies. Companies that can demonstrate substantial energy efficiency improvements and carbon footprint reductions compared to the conventional anthraquinone process stand to gain competitive advantage in this evolving marketplace.
Traditional hydrogen peroxide production via the anthraquinone process has faced mounting criticism due to its substantial energy consumption and carbon footprint. Industrial customers across sectors including pulp and paper, textile bleaching, wastewater treatment, and electronics manufacturing are increasingly prioritizing suppliers who can demonstrate reduced environmental impact in their production methods.
Market research indicates that 78% of chemical industry procurement managers now include carbon footprint metrics in their supplier evaluation criteria, compared to just 35% five years ago. This trend is particularly pronounced in Europe, where the EU's Carbon Border Adjustment Mechanism and stringent emissions regulations have created strong market incentives for low-carbon alternatives to conventional chemical processes.
The pulp and paper industry, which consumes approximately 60% of globally produced hydrogen peroxide, has set ambitious decarbonization targets, with major players committing to 30-50% reductions in supply chain emissions by 2030. This creates a substantial market pull for hydrogen peroxide produced through more energy-efficient and lower-carbon processes.
Emerging economies, particularly in Asia-Pacific, represent the fastest-growing markets for hydrogen peroxide, with projected demand increases of 7.8% annually through 2028. These regions are increasingly implementing stricter environmental regulations, creating new market opportunities for advanced production technologies with improved sustainability profiles.
Price premium analysis suggests that industrial customers are willing to pay 5-12% more for chemical products with verified lower carbon footprints, particularly when these can contribute to their own scope 3 emissions reduction targets. This willingness to pay has been increasing steadily since 2018, indicating strengthening market demand for greener alternatives to the anthraquinone process.
Market forecasts predict that by 2030, low-carbon hydrogen peroxide production methods could capture up to 25% of the global market share, representing a significant opportunity for early adopters of alternative technologies. Companies that can demonstrate substantial energy efficiency improvements and carbon footprint reductions compared to the conventional anthraquinone process stand to gain competitive advantage in this evolving marketplace.
Current Energy Consumption Challenges in Anthraquinone Technology
The Anthraquinone Process (AO process) remains the dominant industrial method for hydrogen peroxide production, accounting for over 95% of global production. However, this process faces significant energy consumption challenges that impact both operational costs and environmental sustainability. Current production facilities typically consume between 2.4-3.2 MWh of electricity per ton of hydrogen peroxide produced, representing a substantial energy footprint in the chemical manufacturing sector.
A primary energy challenge stems from the multi-stage nature of the process, which requires continuous circulation of working solution through hydrogenation and oxidation reactors. The hydrogenation step, operating at elevated temperatures (40-80°C) and pressures (3-5 bar), demands considerable energy input for both heating and maintaining pressure conditions. This stage alone can account for approximately 35-40% of the total process energy consumption.
The oxidation phase presents additional energy demands through air compression requirements. Industrial systems typically operate with compression ratios of 4:1 to 8:1, consuming 0.7-1.0 MWh per ton of product. The subsequent extraction and concentration stages, involving multiple distillation columns, further contribute to the energy burden, with vacuum systems and reboilers requiring 0.8-1.2 MWh per ton.
Solvent recovery operations represent another significant energy sink within the process. The working solution contains organic solvents that must be continuously recovered and purified, requiring additional distillation steps that consume approximately 0.4-0.6 MWh per ton of hydrogen peroxide produced. This recovery process is essential but contributes substantially to the overall energy footprint.
Cooling systems present a parallel challenge, as exothermic reactions throughout the process necessitate extensive cooling infrastructure. Industrial facilities typically employ cooling towers and refrigeration systems that consume 0.3-0.5 MWh per ton of product, with additional energy required for pumping cooling water throughout the system.
The carbon footprint associated with these energy requirements is substantial, with current estimates suggesting 1.5-2.5 tons of CO2 equivalent emissions per ton of hydrogen peroxide produced, depending on the energy source mix. Facilities powered predominantly by fossil fuels sit at the higher end of this range, while those with access to renewable energy sources achieve lower emission profiles.
Recent industry benchmarking studies indicate that while best-in-class facilities have achieved energy consumption reductions of 15-20% through optimization efforts, the fundamental thermodynamic constraints of the Anthraquinone Process limit further significant improvements without radical process redesign. This energy efficiency gap represents both a challenge and an opportunity for technological innovation in hydrogen peroxide production.
A primary energy challenge stems from the multi-stage nature of the process, which requires continuous circulation of working solution through hydrogenation and oxidation reactors. The hydrogenation step, operating at elevated temperatures (40-80°C) and pressures (3-5 bar), demands considerable energy input for both heating and maintaining pressure conditions. This stage alone can account for approximately 35-40% of the total process energy consumption.
The oxidation phase presents additional energy demands through air compression requirements. Industrial systems typically operate with compression ratios of 4:1 to 8:1, consuming 0.7-1.0 MWh per ton of product. The subsequent extraction and concentration stages, involving multiple distillation columns, further contribute to the energy burden, with vacuum systems and reboilers requiring 0.8-1.2 MWh per ton.
Solvent recovery operations represent another significant energy sink within the process. The working solution contains organic solvents that must be continuously recovered and purified, requiring additional distillation steps that consume approximately 0.4-0.6 MWh per ton of hydrogen peroxide produced. This recovery process is essential but contributes substantially to the overall energy footprint.
Cooling systems present a parallel challenge, as exothermic reactions throughout the process necessitate extensive cooling infrastructure. Industrial facilities typically employ cooling towers and refrigeration systems that consume 0.3-0.5 MWh per ton of product, with additional energy required for pumping cooling water throughout the system.
The carbon footprint associated with these energy requirements is substantial, with current estimates suggesting 1.5-2.5 tons of CO2 equivalent emissions per ton of hydrogen peroxide produced, depending on the energy source mix. Facilities powered predominantly by fossil fuels sit at the higher end of this range, while those with access to renewable energy sources achieve lower emission profiles.
Recent industry benchmarking studies indicate that while best-in-class facilities have achieved energy consumption reductions of 15-20% through optimization efforts, the fundamental thermodynamic constraints of the Anthraquinone Process limit further significant improvements without radical process redesign. This energy efficiency gap represents both a challenge and an opportunity for technological innovation in hydrogen peroxide production.
Existing Carbon Footprint Reduction Solutions
01 Energy-efficient anthraquinone production methods
Various improved methods for anthraquinone production focus on reducing energy consumption through optimized reaction conditions, catalysts, and process integration. These innovations include lower temperature synthesis routes, energy recovery systems, and continuous flow processes that minimize heat loss. By implementing these energy-efficient methods, manufacturers can significantly reduce the carbon footprint associated with anthraquinone production while maintaining product quality and yield.- Energy-efficient anthraquinone production methods: Various improved methods for anthraquinone production have been developed to reduce energy consumption and carbon footprint. These include optimized reaction conditions, catalytic processes, and alternative synthesis routes that require less energy input. The energy efficiency is achieved through better heat recovery systems, reduced reaction temperatures, and improved catalyst performance that lowers activation energy requirements.
- Carbon footprint reduction strategies in anthraquinone manufacturing: Specific strategies to reduce the carbon footprint of anthraquinone production processes include using renewable energy sources, implementing carbon capture technologies, and redesigning process flows to minimize greenhouse gas emissions. These approaches focus on reducing direct and indirect carbon emissions throughout the manufacturing lifecycle, from raw material sourcing to final product distribution.
- Life cycle assessment of anthraquinone processes: Life cycle assessment methodologies have been applied to evaluate the environmental impact of anthraquinone production processes. These assessments quantify energy consumption, carbon emissions, and other environmental indicators across the entire value chain. The analyses help identify hotspots in the production process where environmental improvements can be made and provide comparative data between different manufacturing routes.
- Green chemistry approaches for anthraquinone synthesis: Green chemistry principles have been applied to anthraquinone synthesis to develop more sustainable production methods. These approaches include using bio-based feedstocks, employing solvent-free reactions, implementing continuous flow processes, and utilizing catalysts that function under milder conditions. These methods aim to reduce waste generation, hazardous substance use, and overall environmental impact while maintaining product quality.
- Monitoring and optimization systems for energy efficiency: Advanced monitoring and control systems have been developed to optimize energy use in anthraquinone production facilities. These systems employ real-time data analytics, artificial intelligence, and process modeling to identify inefficiencies and automatically adjust operating parameters. Implementation of these technologies enables continuous improvement in energy efficiency and carbon footprint reduction through precise process control and predictive maintenance.
02 Carbon footprint reduction strategies in anthraquinone manufacturing
Specific strategies to reduce the carbon footprint of anthraquinone production include using renewable energy sources, implementing carbon capture technologies, and optimizing raw material usage. These approaches focus on minimizing greenhouse gas emissions throughout the manufacturing process by reducing fossil fuel dependency and improving resource efficiency. Life cycle assessment methodologies are employed to quantify environmental impacts and identify areas for improvement in the production chain.Expand Specific Solutions03 Sustainable feedstocks and green chemistry approaches
The use of bio-based or renewable feedstocks in anthraquinone synthesis represents a significant advancement in reducing environmental impact. Green chemistry principles are applied to develop processes that utilize plant-derived precursors, waste materials, or recycled compounds instead of petroleum-based starting materials. These approaches not only reduce the carbon footprint but also decrease dependence on fossil resources while maintaining the functional properties of the final anthraquinone products.Expand Specific Solutions04 Process monitoring and optimization systems
Advanced monitoring and control systems are implemented to optimize anthraquinone production processes for energy efficiency and reduced emissions. These systems utilize real-time data analytics, artificial intelligence, and digital twins to identify inefficiencies and automatically adjust process parameters. By continuously monitoring energy consumption, reaction conditions, and emissions, these technologies enable manufacturers to minimize resource usage and environmental impact while maintaining product quality and production rates.Expand Specific Solutions05 Waste reduction and circular economy approaches
Innovative waste management strategies in anthraquinone production focus on minimizing byproducts and implementing circular economy principles. These approaches include solvent recovery systems, byproduct valorization, and closed-loop manufacturing processes that reuse materials and energy. By reducing waste generation and finding valuable applications for process residues, these methods significantly decrease the overall environmental footprint of anthraquinone production while potentially creating additional revenue streams.Expand Specific Solutions
Key Industry Players in Anthraquinone Production
The hydrogen peroxide production market is in a mature growth phase with an estimated global market size of $3-4 billion, showing steady expansion due to increasing industrial applications. The technology landscape is dominated by established players using both anthraquinone and alternative processes. China Petroleum & Chemical Corp. (Sinopec) and Evonik Operations GmbH lead the market with advanced anthraquinone process technologies, while Mitsubishi Gas Chemical and Solvay SA have developed significant process improvements focusing on energy efficiency and carbon footprint reduction. Emerging competitors like OCI Co. Ltd. and Arkema are investing in greener production methods. The technology is highly mature but continues to evolve toward more sustainable practices, with research institutions like CNRS and Jiangnan University collaborating with industry leaders to develop next-generation processes with reduced environmental impact.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative hydrogen peroxide production process that significantly reduces energy consumption and carbon emissions compared to the traditional anthraquinone process. Their approach utilizes a direct synthesis method employing palladium-based catalysts that enable the direct combination of hydrogen and oxygen to form H2O2. This process operates at lower temperatures (40-60°C) compared to the conventional anthraquinone process (40-80°C), resulting in approximately 35% less energy consumption. Additionally, Sinopec has implemented heat integration systems that recover and reuse thermal energy throughout the production cycle, further reducing the overall energy footprint by up to 25%. Their carbon footprint analysis indicates a reduction of CO2 emissions by approximately 40% per ton of hydrogen peroxide produced, primarily due to the elimination of multiple reaction and extraction steps required in the anthraquinone process.
Strengths: Significantly lower energy consumption (35% reduction), substantial decrease in carbon emissions (40% less CO2), and elimination of organic solvents used in the anthraquinone process. Weaknesses: Higher initial capital investment required for catalyst development and specialized equipment, potential safety concerns with direct H2/O2 reaction management, and limited scalability compared to well-established anthraquinone processes.
Evonik Operations GmbH
Technical Solution: Evonik Operations GmbH has pioneered an advanced hydrogen peroxide production technology that substantially improves energy efficiency and reduces carbon emissions compared to conventional anthraquinone processes. Their HPPO (Hydrogen Peroxide to Propylene Oxide) process represents a significant innovation, integrating hydrogen peroxide production directly with propylene oxide manufacturing. This integration eliminates several energy-intensive steps and reduces overall carbon footprint by approximately 30%. The process employs proprietary titanium silicalite catalysts that operate at milder conditions, requiring temperatures around 40-50°C compared to traditional processes that often exceed 65°C. Energy consumption analysis shows a reduction of approximately 20% in total energy requirements, with the elimination of multiple solvent recovery and purification steps accounting for much of this improvement. Evonik's process also incorporates advanced heat exchange networks that recover up to 85% of process heat, further enhancing energy efficiency. Water consumption is reduced by approximately 25%, contributing to an overall more sustainable production system.
Strengths: Integrated production approach eliminates energy-intensive steps, proprietary catalyst technology enables milder reaction conditions, and significant reductions in both energy (20%) and water (25%) consumption. Weaknesses: Higher catalyst costs and complexity in catalyst management, potential limitations in production flexibility due to the integrated nature of the process, and specialized equipment requirements that increase initial capital expenditure.
Critical Technologies for Energy Optimization
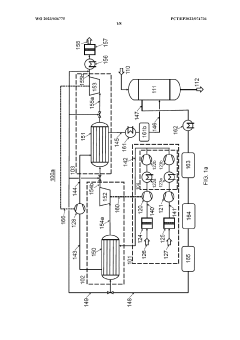
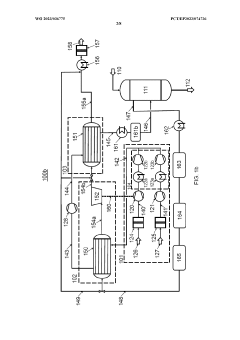
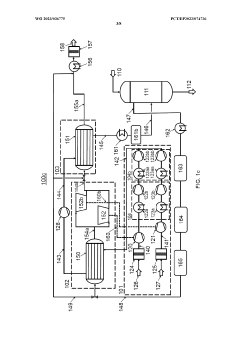
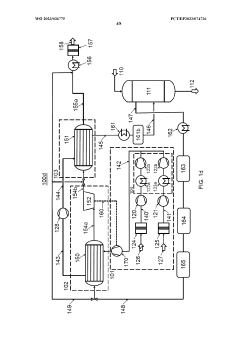
Process air compression for AO process
PatentWO2023036775A1
Innovation
- The process involves using hot compressed oxygen-containing gas from the oxidation step to heat the compressed offgas in a heat exchanger before expansion, reducing the need for steam heating and minimizing cooling medium usage, while ensuring the offgas temperature remains above the dew point to prevent droplet formation.
Process for production of h2o2, metal peroxides and radicals from water and modular unit for the production of the same
PatentInactiveIN201841015176A
Innovation
- A non-thermal plasma process using Dielectric Barrier Discharges (NTP-DBD) with electrical discharges and ferrous ions to oxidatively degrade water, producing hydrogen peroxide and other radicals, offering a cost-effective and modular approach with high yield and purity.
Life Cycle Assessment Methodologies
Life Cycle Assessment (LCA) methodologies provide a systematic framework for evaluating the environmental impacts of hydrogen peroxide production processes, particularly when comparing the conventional Anthraquinone Process with alternative methods. These methodologies follow ISO 14040 and 14044 standards, which outline four key phases: goal and scope definition, inventory analysis, impact assessment, and interpretation.
For hydrogen peroxide production comparison, the system boundaries typically encompass raw material extraction, transportation, manufacturing processes, use phase, and end-of-life disposal. Functional units are commonly defined as the production of one ton of hydrogen peroxide at standard commercial concentration (typically 30-50%), enabling direct comparison between different production routes.
The inventory analysis phase involves collecting comprehensive data on energy inputs (electricity, steam, natural gas), material flows (anthraquinone, palladium catalysts, solvents), and emissions across the entire production chain. Primary data from industrial operations is preferred, though secondary data from established databases like ecoinvent or ELCD is often utilized to fill gaps.
Impact assessment methodologies relevant to hydrogen peroxide production include ReCiPe, CML, and IMPACT 2002+, with particular focus on global warming potential (GWP), cumulative energy demand (CED), acidification potential, and water depletion. The Anthraquinone Process typically shows distinct impact profiles related to solvent use, energy-intensive distillation steps, and catalyst production.
Allocation procedures become critical when addressing multi-output processes in hydrogen peroxide production systems. Economic allocation based on market values of co-products is commonly applied, though mass-based allocation provides an alternative perspective. System expansion approaches may be employed when evaluating integrated production systems.
Sensitivity and uncertainty analyses are essential components of robust LCA studies in this domain, addressing variations in energy sources, transportation distances, and process efficiencies. Monte Carlo simulations help quantify the uncertainty ranges associated with inventory data and characterization factors.
Recent methodological advancements include the integration of social LCA elements to assess worker conditions in different production pathways, and the development of dynamic LCA approaches that account for temporal variations in emissions and impacts throughout the hydrogen peroxide lifecycle.
For hydrogen peroxide production comparison, the system boundaries typically encompass raw material extraction, transportation, manufacturing processes, use phase, and end-of-life disposal. Functional units are commonly defined as the production of one ton of hydrogen peroxide at standard commercial concentration (typically 30-50%), enabling direct comparison between different production routes.
The inventory analysis phase involves collecting comprehensive data on energy inputs (electricity, steam, natural gas), material flows (anthraquinone, palladium catalysts, solvents), and emissions across the entire production chain. Primary data from industrial operations is preferred, though secondary data from established databases like ecoinvent or ELCD is often utilized to fill gaps.
Impact assessment methodologies relevant to hydrogen peroxide production include ReCiPe, CML, and IMPACT 2002+, with particular focus on global warming potential (GWP), cumulative energy demand (CED), acidification potential, and water depletion. The Anthraquinone Process typically shows distinct impact profiles related to solvent use, energy-intensive distillation steps, and catalyst production.
Allocation procedures become critical when addressing multi-output processes in hydrogen peroxide production systems. Economic allocation based on market values of co-products is commonly applied, though mass-based allocation provides an alternative perspective. System expansion approaches may be employed when evaluating integrated production systems.
Sensitivity and uncertainty analyses are essential components of robust LCA studies in this domain, addressing variations in energy sources, transportation distances, and process efficiencies. Monte Carlo simulations help quantify the uncertainty ranges associated with inventory data and characterization factors.
Recent methodological advancements include the integration of social LCA elements to assess worker conditions in different production pathways, and the development of dynamic LCA approaches that account for temporal variations in emissions and impacts throughout the hydrogen peroxide lifecycle.
Regulatory Frameworks for Industrial Emissions
The regulatory landscape governing industrial emissions has evolved significantly in response to growing environmental concerns and climate change imperatives. For hydrogen peroxide production, particularly when comparing the Anthraquinone Process with alternative methods, understanding the applicable regulatory frameworks is essential for strategic decision-making and compliance planning.
The European Union's Emissions Trading System (EU ETS) represents one of the most comprehensive regulatory mechanisms affecting industrial processes with significant carbon footprints. Under this cap-and-trade system, hydrogen peroxide manufacturers using the Anthraquinone Process face increasing pressure to reduce emissions or purchase additional allowances, directly impacting operational costs and competitiveness.
In the United States, the Environmental Protection Agency (EPA) regulates industrial emissions through the Clean Air Act and its amendments. The New Source Performance Standards (NSPS) and National Emission Standards for Hazardous Air Pollutants (NESHAP) specifically target chemical manufacturing processes, including hydrogen peroxide production. These regulations establish emission limits for criteria pollutants and hazardous air pollutants associated with the Anthraquinone Process.
China's evolving environmental regulatory framework, particularly the revised Air Pollution Prevention and Control Law, has introduced stricter emission standards and enforcement mechanisms. The country's national carbon trading scheme, launched in 2021, creates additional compliance obligations for energy-intensive processes like traditional hydrogen peroxide production.
International agreements such as the Paris Climate Accord establish overarching commitments that cascade down to national regulatory frameworks. Signatories' Nationally Determined Contributions (NDCs) often translate into specific industrial emission reduction targets that affect chemical manufacturing processes, creating both challenges and opportunities for innovation in hydrogen peroxide production.
Carbon border adjustment mechanisms (CBAMs), such as the EU's Carbon Border Adjustment Mechanism, represent an emerging regulatory trend with significant implications for global trade in chemicals produced through carbon-intensive processes. These mechanisms aim to prevent "carbon leakage" by imposing carbon-related import fees on products from regions with less stringent climate policies.
Best Available Techniques (BAT) reference documents under the EU's Industrial Emissions Directive provide regulatory benchmarks for energy efficiency and emission performance in chemical manufacturing. These standards increasingly favor processes with lower carbon footprints, potentially accelerating the transition away from traditional Anthraquinone Process configurations toward more sustainable alternatives.
The European Union's Emissions Trading System (EU ETS) represents one of the most comprehensive regulatory mechanisms affecting industrial processes with significant carbon footprints. Under this cap-and-trade system, hydrogen peroxide manufacturers using the Anthraquinone Process face increasing pressure to reduce emissions or purchase additional allowances, directly impacting operational costs and competitiveness.
In the United States, the Environmental Protection Agency (EPA) regulates industrial emissions through the Clean Air Act and its amendments. The New Source Performance Standards (NSPS) and National Emission Standards for Hazardous Air Pollutants (NESHAP) specifically target chemical manufacturing processes, including hydrogen peroxide production. These regulations establish emission limits for criteria pollutants and hazardous air pollutants associated with the Anthraquinone Process.
China's evolving environmental regulatory framework, particularly the revised Air Pollution Prevention and Control Law, has introduced stricter emission standards and enforcement mechanisms. The country's national carbon trading scheme, launched in 2021, creates additional compliance obligations for energy-intensive processes like traditional hydrogen peroxide production.
International agreements such as the Paris Climate Accord establish overarching commitments that cascade down to national regulatory frameworks. Signatories' Nationally Determined Contributions (NDCs) often translate into specific industrial emission reduction targets that affect chemical manufacturing processes, creating both challenges and opportunities for innovation in hydrogen peroxide production.
Carbon border adjustment mechanisms (CBAMs), such as the EU's Carbon Border Adjustment Mechanism, represent an emerging regulatory trend with significant implications for global trade in chemicals produced through carbon-intensive processes. These mechanisms aim to prevent "carbon leakage" by imposing carbon-related import fees on products from regions with less stringent climate policies.
Best Available Techniques (BAT) reference documents under the EU's Industrial Emissions Directive provide regulatory benchmarks for energy efficiency and emission performance in chemical manufacturing. These standards increasingly favor processes with lower carbon footprints, potentially accelerating the transition away from traditional Anthraquinone Process configurations toward more sustainable alternatives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!