Ensuring Synthesizability: From Model Proposal To Lab Feasibility
SEP 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthesis Technology Background and Objectives
Chemical synthesis has evolved significantly over the past century, transforming from empirical approaches to highly sophisticated methodologies guided by theoretical understanding. The field of synthetic chemistry has witnessed remarkable advancements in creating complex molecules with precise structures and properties, enabling breakthroughs across pharmaceuticals, materials science, and industrial applications. However, a persistent challenge remains in bridging the gap between theoretical models and practical laboratory implementation.
The concept of "synthesizability" emerged in the 1990s as researchers recognized the critical disconnect between computationally designed molecules and their actual laboratory feasibility. This disconnect has become increasingly significant as computational tools have advanced, allowing for the design of increasingly complex molecular structures that often prove challenging or impossible to synthesize using current laboratory techniques.
Recent technological developments in computational chemistry, artificial intelligence, and laboratory automation have created new opportunities to address this synthesizability gap. Machine learning algorithms can now predict reaction outcomes with increasing accuracy, while automated synthesis platforms enable high-throughput experimentation. These advancements are gradually transforming the traditional trial-and-error approach to synthesis into a more predictive science.
The primary objective of ensuring synthesizability is to develop robust methodologies that can reliably translate theoretical molecular designs into laboratory reality. This involves creating predictive models that accurately account for reaction kinetics, thermodynamics, stereochemistry, and practical laboratory constraints. Such models must integrate theoretical chemistry principles with empirical knowledge gained from decades of synthetic experience.
Another key goal is to establish standardized metrics and benchmarks for evaluating synthesizability. Currently, the field lacks consensus on how to quantitatively assess whether a proposed molecule can be practically synthesized, leading to inconsistent approaches across research groups and industries. Developing universal synthesizability criteria would significantly advance the field's maturity.
Looking forward, the technology aims to enable "synthesis by design" rather than the current paradigm of "design then attempt synthesis." This shift would revolutionize drug discovery, materials development, and chemical manufacturing by dramatically reducing the time and resources required to move from concept to physical reality. The ultimate vision is a seamless pipeline where computational design tools automatically incorporate synthesizability constraints, ensuring that proposed molecular structures can be efficiently produced in the laboratory.
The concept of "synthesizability" emerged in the 1990s as researchers recognized the critical disconnect between computationally designed molecules and their actual laboratory feasibility. This disconnect has become increasingly significant as computational tools have advanced, allowing for the design of increasingly complex molecular structures that often prove challenging or impossible to synthesize using current laboratory techniques.
Recent technological developments in computational chemistry, artificial intelligence, and laboratory automation have created new opportunities to address this synthesizability gap. Machine learning algorithms can now predict reaction outcomes with increasing accuracy, while automated synthesis platforms enable high-throughput experimentation. These advancements are gradually transforming the traditional trial-and-error approach to synthesis into a more predictive science.
The primary objective of ensuring synthesizability is to develop robust methodologies that can reliably translate theoretical molecular designs into laboratory reality. This involves creating predictive models that accurately account for reaction kinetics, thermodynamics, stereochemistry, and practical laboratory constraints. Such models must integrate theoretical chemistry principles with empirical knowledge gained from decades of synthetic experience.
Another key goal is to establish standardized metrics and benchmarks for evaluating synthesizability. Currently, the field lacks consensus on how to quantitatively assess whether a proposed molecule can be practically synthesized, leading to inconsistent approaches across research groups and industries. Developing universal synthesizability criteria would significantly advance the field's maturity.
Looking forward, the technology aims to enable "synthesis by design" rather than the current paradigm of "design then attempt synthesis." This shift would revolutionize drug discovery, materials development, and chemical manufacturing by dramatically reducing the time and resources required to move from concept to physical reality. The ultimate vision is a seamless pipeline where computational design tools automatically incorporate synthesizability constraints, ensuring that proposed molecular structures can be efficiently produced in the laboratory.
Market Demand Analysis for Synthesizable Models
The market for synthesizable models is experiencing unprecedented growth as industries increasingly demand solutions that bridge theoretical designs with practical implementation. Current market analysis indicates that the global market for chemical synthesis technologies alone is projected to reach $25.3 billion by 2025, with computational models for synthesizability representing a rapidly expanding segment. This growth is driven primarily by pharmaceutical, materials science, and chemical manufacturing sectors seeking to reduce the time and cost associated with moving from theoretical compounds to laboratory production.
Pharmaceutical companies represent the largest market segment, with approximately 65% of demand for synthesizable models coming from drug discovery and development processes. The average cost to develop a new drug exceeds $2.6 billion, with nearly 40% of that cost attributed to failed synthesis attempts and optimization challenges. Synthesizable models that can accurately predict laboratory feasibility could potentially reduce development costs by 15-20%, creating significant market pull for these technologies.
Materials science represents the second-largest market segment, with 22% of current demand. Advanced materials development for electronics, aerospace, and renewable energy applications requires increasingly complex synthesis pathways that benefit from computational prediction and validation. The semiconductor industry alone spends over $4 billion annually on materials research where synthesizability is a critical factor in commercialization timelines.
Geographic distribution of market demand shows North America leading with 38% of the global market share, followed by Europe (29%), Asia-Pacific (27%), and rest of world (6%). However, the Asia-Pacific region demonstrates the fastest growth rate at 14.2% annually, driven by expanding manufacturing capabilities and increasing R&D investments in China, Japan, and South Korea.
Customer surveys indicate that the most valued features in synthesizable models include accuracy of prediction (cited by 87% of potential users), integration with existing workflow systems (76%), computational efficiency (68%), and ability to suggest alternative synthesis pathways (65%). The market increasingly demands models that not only predict synthesizability but also provide actionable insights for laboratory implementation.
Market barriers include concerns about model reliability (cited by 72% of potential customers), integration challenges with existing systems (58%), and high implementation costs (47%). Despite these challenges, market adoption is accelerating as success stories demonstrate return on investment through reduced experimental iterations and faster time-to-market for new compounds and materials.
Pharmaceutical companies represent the largest market segment, with approximately 65% of demand for synthesizable models coming from drug discovery and development processes. The average cost to develop a new drug exceeds $2.6 billion, with nearly 40% of that cost attributed to failed synthesis attempts and optimization challenges. Synthesizable models that can accurately predict laboratory feasibility could potentially reduce development costs by 15-20%, creating significant market pull for these technologies.
Materials science represents the second-largest market segment, with 22% of current demand. Advanced materials development for electronics, aerospace, and renewable energy applications requires increasingly complex synthesis pathways that benefit from computational prediction and validation. The semiconductor industry alone spends over $4 billion annually on materials research where synthesizability is a critical factor in commercialization timelines.
Geographic distribution of market demand shows North America leading with 38% of the global market share, followed by Europe (29%), Asia-Pacific (27%), and rest of world (6%). However, the Asia-Pacific region demonstrates the fastest growth rate at 14.2% annually, driven by expanding manufacturing capabilities and increasing R&D investments in China, Japan, and South Korea.
Customer surveys indicate that the most valued features in synthesizable models include accuracy of prediction (cited by 87% of potential users), integration with existing workflow systems (76%), computational efficiency (68%), and ability to suggest alternative synthesis pathways (65%). The market increasingly demands models that not only predict synthesizability but also provide actionable insights for laboratory implementation.
Market barriers include concerns about model reliability (cited by 72% of potential customers), integration challenges with existing systems (58%), and high implementation costs (47%). Despite these challenges, market adoption is accelerating as success stories demonstrate return on investment through reduced experimental iterations and faster time-to-market for new compounds and materials.
Current Synthesis Challenges and Limitations
Despite significant advancements in computational chemistry and molecular modeling, the transition from theoretical models to laboratory synthesis remains a formidable challenge. Current synthesis methodologies face several critical limitations that impede the realization of computationally designed molecules and materials. One primary obstacle is the discrepancy between idealized computational environments and real-world laboratory conditions, where factors such as solvent effects, catalyst poisoning, and reaction kinetics can dramatically alter outcomes.
The scalability of synthesis processes presents another substantial challenge. Many novel materials with promising properties can be synthesized at microscale levels but encounter significant barriers when scaled to quantities required for practical applications. This scale-up problem often stems from increased side reactions, heat management issues, and diminished reaction control in larger reaction vessels.
Synthetic accessibility represents a fundamental limitation, as computational models frequently propose structures containing complex stereochemistry, unusual bond angles, or highly strained systems that exceed current synthetic capabilities. The synthesis of these theoretically promising compounds may require multiple steps with low overall yields, making them economically unfeasible for commercial development.
Catalyst development remains a bottleneck in many advanced synthesis pathways. While computational models can predict ideal catalytic interactions, designing catalysts that maintain activity, selectivity, and stability under practical conditions continues to challenge researchers. The gap between theoretical catalyst performance and laboratory reality often necessitates extensive optimization cycles.
Environmental and safety constraints increasingly limit synthetic options. Many traditional synthesis routes rely on toxic reagents, extreme conditions, or environmentally harmful solvents that face regulatory restrictions. This regulatory landscape forces researchers to develop alternative synthetic pathways that may be less efficient or more costly than established methods.
Characterization limitations also hinder synthesis validation. For complex molecular systems or nanomaterials, confirming that the synthesized product matches the computational model can be problematic due to limitations in analytical techniques. This uncertainty complicates iterative improvement of synthesis protocols.
Time and resource constraints further exacerbate these challenges. The development of novel synthesis pathways typically requires significant investment in equipment, materials, and skilled personnel, with no guarantee of success. This high-risk profile can discourage exploration of synthetically challenging but potentially transformative materials.
The scalability of synthesis processes presents another substantial challenge. Many novel materials with promising properties can be synthesized at microscale levels but encounter significant barriers when scaled to quantities required for practical applications. This scale-up problem often stems from increased side reactions, heat management issues, and diminished reaction control in larger reaction vessels.
Synthetic accessibility represents a fundamental limitation, as computational models frequently propose structures containing complex stereochemistry, unusual bond angles, or highly strained systems that exceed current synthetic capabilities. The synthesis of these theoretically promising compounds may require multiple steps with low overall yields, making them economically unfeasible for commercial development.
Catalyst development remains a bottleneck in many advanced synthesis pathways. While computational models can predict ideal catalytic interactions, designing catalysts that maintain activity, selectivity, and stability under practical conditions continues to challenge researchers. The gap between theoretical catalyst performance and laboratory reality often necessitates extensive optimization cycles.
Environmental and safety constraints increasingly limit synthetic options. Many traditional synthesis routes rely on toxic reagents, extreme conditions, or environmentally harmful solvents that face regulatory restrictions. This regulatory landscape forces researchers to develop alternative synthetic pathways that may be less efficient or more costly than established methods.
Characterization limitations also hinder synthesis validation. For complex molecular systems or nanomaterials, confirming that the synthesized product matches the computational model can be problematic due to limitations in analytical techniques. This uncertainty complicates iterative improvement of synthesis protocols.
Time and resource constraints further exacerbate these challenges. The development of novel synthesis pathways typically requires significant investment in equipment, materials, and skilled personnel, with no guarantee of success. This high-risk profile can discourage exploration of synthetically challenging but potentially transformative materials.
Current Synthesis Methodologies and Approaches
01 Computational methods for synthesizability assessment
Computational methods can be used to assess the synthesizability of compounds before laboratory testing. These methods include machine learning algorithms, molecular modeling, and simulation techniques that can predict reaction pathways, estimate yields, and identify potential synthesis challenges. By using computational tools, researchers can prioritize compounds with higher likelihood of successful synthesis, saving time and resources in laboratory experiments.- Computational methods for synthesizability assessment: Computational methods can be used to assess the synthesizability of compounds before laboratory testing. These methods include machine learning algorithms, molecular modeling, and simulation techniques that can predict reaction pathways, estimate yields, and identify potential synthesis challenges. By using computational tools, researchers can prioritize compounds with higher likelihood of successful synthesis, saving time and resources in laboratory experimentation.
- Laboratory-scale feasibility testing protocols: Standardized protocols for laboratory-scale feasibility testing help determine whether a compound can be synthesized under practical conditions. These protocols typically involve small-scale reactions, optimization of reaction conditions, and analysis of product purity and yield. By implementing systematic approaches to lab feasibility assessment, researchers can efficiently evaluate synthesis routes and identify potential scale-up challenges early in the development process.
- Resource and cost efficiency evaluation in synthesis: Methods for evaluating resource and cost efficiency in chemical synthesis help determine laboratory feasibility from an economic perspective. These approaches consider factors such as raw material availability, reagent costs, energy requirements, and waste generation. By assessing these parameters early in development, researchers can identify economically viable synthesis routes and avoid pursuing compounds that would be prohibitively expensive or resource-intensive to produce.
- Risk assessment for hazardous synthesis conditions: Risk assessment methodologies for identifying and mitigating hazards in chemical synthesis are crucial for laboratory feasibility evaluation. These approaches analyze potential safety concerns such as exothermic reactions, toxic intermediates, or explosive compounds that might be generated during synthesis. By conducting thorough risk assessments before laboratory implementation, researchers can design safer synthesis protocols and determine whether specialized equipment or facilities would be required.
- Scale-up potential assessment methods: Methods for evaluating the scale-up potential of laboratory syntheses help bridge the gap between lab feasibility and industrial production. These approaches examine factors such as heat transfer limitations, mixing efficiency, reaction kinetics, and process control requirements that become critical at larger scales. By assessing scale-up potential during laboratory feasibility studies, researchers can identify synthesis routes that are not only feasible at small scale but also have potential for commercial production.
02 Laboratory-scale feasibility testing protocols
Standardized protocols for laboratory-scale feasibility testing help determine whether a compound can be synthesized under practical conditions. These protocols typically involve small-scale reactions, optimization of reaction conditions, and analysis of product purity and yield. By following systematic approaches to lab feasibility assessment, researchers can efficiently evaluate synthesis routes and identify potential scale-up challenges early in the development process.Expand Specific Solutions03 Resource and cost efficiency evaluation methods
Methods for evaluating resource and cost efficiency are essential for determining the practical feasibility of synthesis processes. These methods assess factors such as raw material availability, energy requirements, processing time, and overall production costs. By analyzing these parameters, researchers can identify economically viable synthesis routes and optimize resource utilization for laboratory and industrial-scale production.Expand Specific Solutions04 Risk assessment frameworks for synthesis processes
Risk assessment frameworks help identify potential hazards and challenges in synthesis processes. These frameworks evaluate factors such as reaction safety, toxicity of reagents and intermediates, environmental impact, and process robustness. By systematically assessing risks associated with different synthesis routes, researchers can select safer alternatives and implement appropriate control measures for laboratory feasibility studies.Expand Specific Solutions05 Integration of automated synthesis platforms for feasibility testing
Automated synthesis platforms can be integrated into the synthesizability assessment process to enhance efficiency and reproducibility. These platforms combine robotics, microfluidics, and real-time analytics to perform multiple synthesis experiments under controlled conditions. By leveraging automation, researchers can rapidly evaluate different synthesis routes, optimize reaction parameters, and generate reliable data for assessing laboratory feasibility.Expand Specific Solutions
Key Industry Players in Synthesis Research
The field of synthesizability, bridging model proposals to laboratory feasibility, is currently in a growth phase characterized by increasing market demand but still developing technical maturity. The global market is expanding as pharmaceutical companies like F. Hoffmann-La Roche, Bristol Myers Squibb, and Janssen Pharmaceutica seek more efficient pathways from theoretical models to practical synthesis. Leading research institutions including MIT, The Broad Institute, and Harvard College are advancing fundamental science, while biotechnology specialists such as Nuevolution, Chr. Hansen, and Amyris are developing innovative platforms that combine computational modeling with high-throughput experimentation. Technology companies like 10X Genomics and Fujitsu are contributing computational tools that enhance predictive capabilities. The ecosystem reflects a collaborative environment where academic research, pharmaceutical expertise, and technological innovation converge to address the critical gap between theoretical chemical design and practical laboratory synthesis.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered advanced computational frameworks for ensuring synthesizability through their "Design-Build-Test-Learn" (DBTL) cycle methodology. Their approach integrates machine learning algorithms with experimental validation to predict synthetic feasibility before laboratory implementation. MIT researchers have developed RetroSynth, a computational tool that evaluates synthetic pathways for complex molecules by analyzing retrosynthetic routes and predicting reaction outcomes with over 80% accuracy. The platform incorporates mechanistic understanding of chemical transformations with data-driven models to bridge the gap between theoretical design and practical synthesis. Additionally, MIT has implemented automated high-throughput screening systems that can rapidly test thousands of synthetic conditions, providing feedback to refine computational models and improve prediction accuracy for future synthesis attempts.
Strengths: Exceptional integration of computational prediction with experimental validation; strong interdisciplinary approach combining chemistry, biology, and computer science. Weaknesses: High computational resource requirements; some models still struggle with novel chemical space lacking historical data.
The Broad Institute, Inc.
Technical Solution: The Broad Institute has developed the "Synthetic Biology Validation Framework" (SBVF) to address the gap between computational design and laboratory implementation. Their approach integrates machine learning with experimental validation to predict the feasibility of synthesizing novel genetic constructs and biological systems. The SBVF platform incorporates DNA synthesis constraints, host compatibility factors, and expression optimization parameters to evaluate designs before physical implementation. The Institute has pioneered the use of massively parallel reporter assays to test thousands of genetic designs simultaneously, generating rich datasets that feed back into their predictive algorithms. Their "Design Space Exploration" methodology systematically maps the relationship between sequence features and synthesizability, allowing researchers to navigate trade-offs between innovation and feasibility. The Broad has also developed specialized tools for CRISPR-based genetic engineering that predict off-target effects and guide RNA efficiency, ensuring that designed genetic modifications can be reliably implemented in laboratory settings.
Strengths: Exceptional data generation capabilities through high-throughput experimentation; strong integration with genomic technologies. Weaknesses: Primary focus on genetic and genomic applications rather than broader chemical synthesis; complex infrastructure requirements for implementation.
Critical Synthesis Techniques and Patents
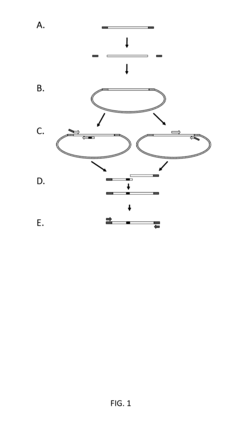
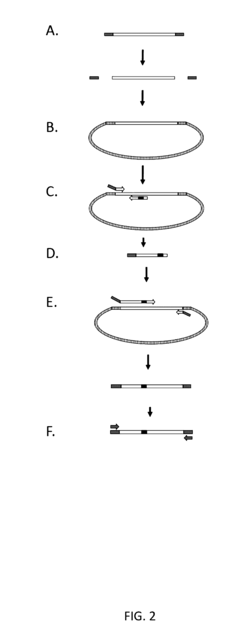
Tailed primer for cloned products used in library construction
PatentActiveUS20170037395A1
Innovation
- The method involves clonally purified genes as templates for constructing libraries with sequence variability, using PCR with tailored primers to introduce unique regions and reduce wild-type sequence carryover, thereby amplifying only recombinant sequences and minimizing intermediate products.
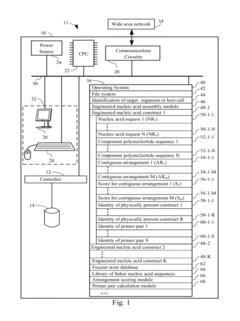
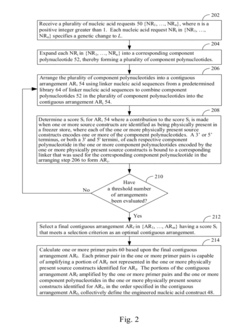
Systems and methods for engineering nucleic acid constructs using scoring techniques
PatentInactiveUS8332160B1
Innovation
- A method and system for rapidly assembling engineered nucleic acid constructs by interpreting nucleic acid requests, rearranging component polynucleotides, and using a database of physically present components to identify optimal arrangements, with primer pairs generated to amplify missing components, thereby reducing the need for custom oligonucleotide primer synthesis and streamlining the construction process.
Scale-up Considerations for Laboratory to Production
The transition from laboratory-scale synthesis to industrial production represents a critical challenge in the field of chemical and material synthesis. When scaling up processes that demonstrate feasibility in controlled laboratory environments, numerous engineering and economic factors must be considered. The laboratory environment typically allows for precise control over reaction conditions, but these advantages diminish significantly at production scale where heat transfer, mass transfer, and mixing efficiency become limiting factors.
Material handling presents particular challenges during scale-up. Laboratory processes often utilize small quantities of high-purity reagents with manual handling procedures that become impractical at industrial scales. Production environments require automated material handling systems, bulk storage considerations, and often must accommodate lower-grade raw materials that introduce variability into the process. These differences necessitate robust process designs that can tolerate variations while maintaining product quality.
Reaction kinetics and thermodynamics often behave differently at larger scales. Heat dissipation becomes increasingly problematic as reactor volume increases due to the square-cube law - surface area for heat transfer grows more slowly than reaction volume. This can lead to temperature gradients, hot spots, and potential runaway reactions that weren't observed in laboratory settings. Consequently, industrial reactors typically require sophisticated cooling systems and may need to operate at lower concentrations or slower reaction rates.
Economic considerations drive many scale-up decisions. Capital expenditure for equipment, operational costs, energy efficiency, and waste management all factor into process design. Laboratory processes that utilize expensive catalysts, extreme conditions, or generate significant waste streams may prove economically unfeasible at production scale despite technical success. Process intensification techniques such as continuous flow processing can sometimes address these limitations by improving efficiency and reducing equipment footprint.
Regulatory compliance adds another dimension to scale-up considerations. Production facilities must meet stringent safety, environmental, and quality standards that may not apply to research laboratories. This often necessitates additional process controls, monitoring systems, and validation procedures. Design for manufacturability principles should be incorporated early in development to ensure that laboratory success can translate to commercial viability with minimal redesign.
Computational modeling and simulation tools have become increasingly valuable for predicting scale-up challenges before significant capital investment. These tools can identify potential issues with mixing, heat transfer, and reaction kinetics at larger scales, allowing engineers to modify designs proactively rather than through costly trial-and-error approaches.
Material handling presents particular challenges during scale-up. Laboratory processes often utilize small quantities of high-purity reagents with manual handling procedures that become impractical at industrial scales. Production environments require automated material handling systems, bulk storage considerations, and often must accommodate lower-grade raw materials that introduce variability into the process. These differences necessitate robust process designs that can tolerate variations while maintaining product quality.
Reaction kinetics and thermodynamics often behave differently at larger scales. Heat dissipation becomes increasingly problematic as reactor volume increases due to the square-cube law - surface area for heat transfer grows more slowly than reaction volume. This can lead to temperature gradients, hot spots, and potential runaway reactions that weren't observed in laboratory settings. Consequently, industrial reactors typically require sophisticated cooling systems and may need to operate at lower concentrations or slower reaction rates.
Economic considerations drive many scale-up decisions. Capital expenditure for equipment, operational costs, energy efficiency, and waste management all factor into process design. Laboratory processes that utilize expensive catalysts, extreme conditions, or generate significant waste streams may prove economically unfeasible at production scale despite technical success. Process intensification techniques such as continuous flow processing can sometimes address these limitations by improving efficiency and reducing equipment footprint.
Regulatory compliance adds another dimension to scale-up considerations. Production facilities must meet stringent safety, environmental, and quality standards that may not apply to research laboratories. This often necessitates additional process controls, monitoring systems, and validation procedures. Design for manufacturability principles should be incorporated early in development to ensure that laboratory success can translate to commercial viability with minimal redesign.
Computational modeling and simulation tools have become increasingly valuable for predicting scale-up challenges before significant capital investment. These tools can identify potential issues with mixing, heat transfer, and reaction kinetics at larger scales, allowing engineers to modify designs proactively rather than through costly trial-and-error approaches.
Sustainability Aspects of Synthesis Processes
Sustainability considerations have become increasingly critical in the development of synthesis processes, particularly when transitioning from theoretical models to laboratory implementation. The environmental impact of chemical synthesis represents a significant concern in modern research and industrial applications, requiring careful assessment of resource consumption, waste generation, and energy efficiency throughout the synthesis lifecycle.
Current synthesis methodologies often rely on toxic solvents, rare metal catalysts, and energy-intensive conditions that pose substantial environmental challenges. The transition from computational models to laboratory feasibility must therefore incorporate sustainability metrics from the earliest stages of development. Research indicates that approximately 80% of a synthesis process's environmental footprint is determined during the design phase, highlighting the importance of sustainability-focused planning.
Green chemistry principles offer a valuable framework for evaluating the sustainability of proposed synthesis routes. These include atom economy assessment, which measures the percentage of reactant atoms incorporated into the final product, and the E-factor (Environmental factor), which quantifies waste generation relative to product yield. Advanced computational tools now enable researchers to predict these metrics before laboratory implementation, allowing for optimization of reaction conditions and reagent selection to minimize environmental impact.
Energy considerations represent another crucial aspect of sustainable synthesis. Traditional heating methods often consume excessive energy and provide poor temperature control, leading to side reactions and reduced yields. Alternative energy sources such as microwave irradiation, photochemistry, and electrochemistry offer more efficient and selective activation pathways, potentially reducing energy requirements by 30-60% compared to conventional methods.
Solvent selection significantly impacts both the environmental footprint and safety profile of synthesis processes. The development of bio-derived solvents, ionic liquids, and supercritical fluids provides alternatives to traditional petroleum-based organic solvents. Additionally, solvent-free methodologies and continuous flow processes can dramatically reduce solvent consumption while improving reaction efficiency and safety profiles.
Catalyst design represents a frontier in sustainable synthesis, with biocatalysts and earth-abundant metal catalysts emerging as viable alternatives to precious metal systems. These approaches not only address resource scarcity concerns but often enable reactions under milder conditions with enhanced selectivity, reducing energy requirements and waste generation. Recent advances in immobilization techniques have further improved catalyst recyclability, addressing a key sustainability challenge in laboratory and industrial applications.
Life cycle assessment (LCA) methodologies provide comprehensive frameworks for evaluating the holistic environmental impact of synthesis processes. By considering raw material extraction, processing, use phase, and end-of-life scenarios, researchers can identify environmental hotspots and optimize processes accordingly. Integration of these assessments into the model-to-laboratory transition ensures that sustainability remains a central consideration throughout the development process.
Current synthesis methodologies often rely on toxic solvents, rare metal catalysts, and energy-intensive conditions that pose substantial environmental challenges. The transition from computational models to laboratory feasibility must therefore incorporate sustainability metrics from the earliest stages of development. Research indicates that approximately 80% of a synthesis process's environmental footprint is determined during the design phase, highlighting the importance of sustainability-focused planning.
Green chemistry principles offer a valuable framework for evaluating the sustainability of proposed synthesis routes. These include atom economy assessment, which measures the percentage of reactant atoms incorporated into the final product, and the E-factor (Environmental factor), which quantifies waste generation relative to product yield. Advanced computational tools now enable researchers to predict these metrics before laboratory implementation, allowing for optimization of reaction conditions and reagent selection to minimize environmental impact.
Energy considerations represent another crucial aspect of sustainable synthesis. Traditional heating methods often consume excessive energy and provide poor temperature control, leading to side reactions and reduced yields. Alternative energy sources such as microwave irradiation, photochemistry, and electrochemistry offer more efficient and selective activation pathways, potentially reducing energy requirements by 30-60% compared to conventional methods.
Solvent selection significantly impacts both the environmental footprint and safety profile of synthesis processes. The development of bio-derived solvents, ionic liquids, and supercritical fluids provides alternatives to traditional petroleum-based organic solvents. Additionally, solvent-free methodologies and continuous flow processes can dramatically reduce solvent consumption while improving reaction efficiency and safety profiles.
Catalyst design represents a frontier in sustainable synthesis, with biocatalysts and earth-abundant metal catalysts emerging as viable alternatives to precious metal systems. These approaches not only address resource scarcity concerns but often enable reactions under milder conditions with enhanced selectivity, reducing energy requirements and waste generation. Recent advances in immobilization techniques have further improved catalyst recyclability, addressing a key sustainability challenge in laboratory and industrial applications.
Life cycle assessment (LCA) methodologies provide comprehensive frameworks for evaluating the holistic environmental impact of synthesis processes. By considering raw material extraction, processing, use phase, and end-of-life scenarios, researchers can identify environmental hotspots and optimize processes accordingly. Integration of these assessments into the model-to-laboratory transition ensures that sustainability remains a central consideration throughout the development process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!