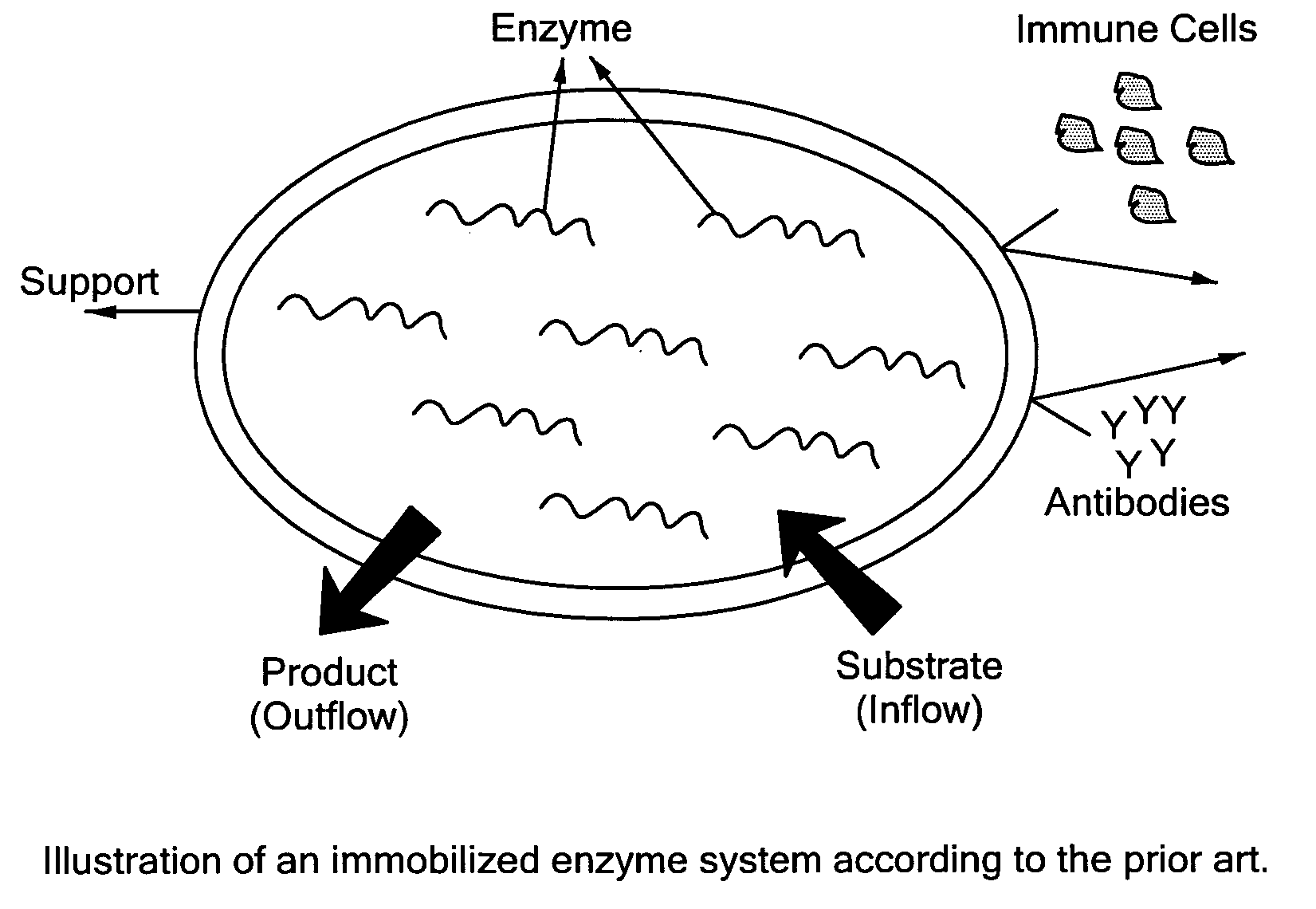
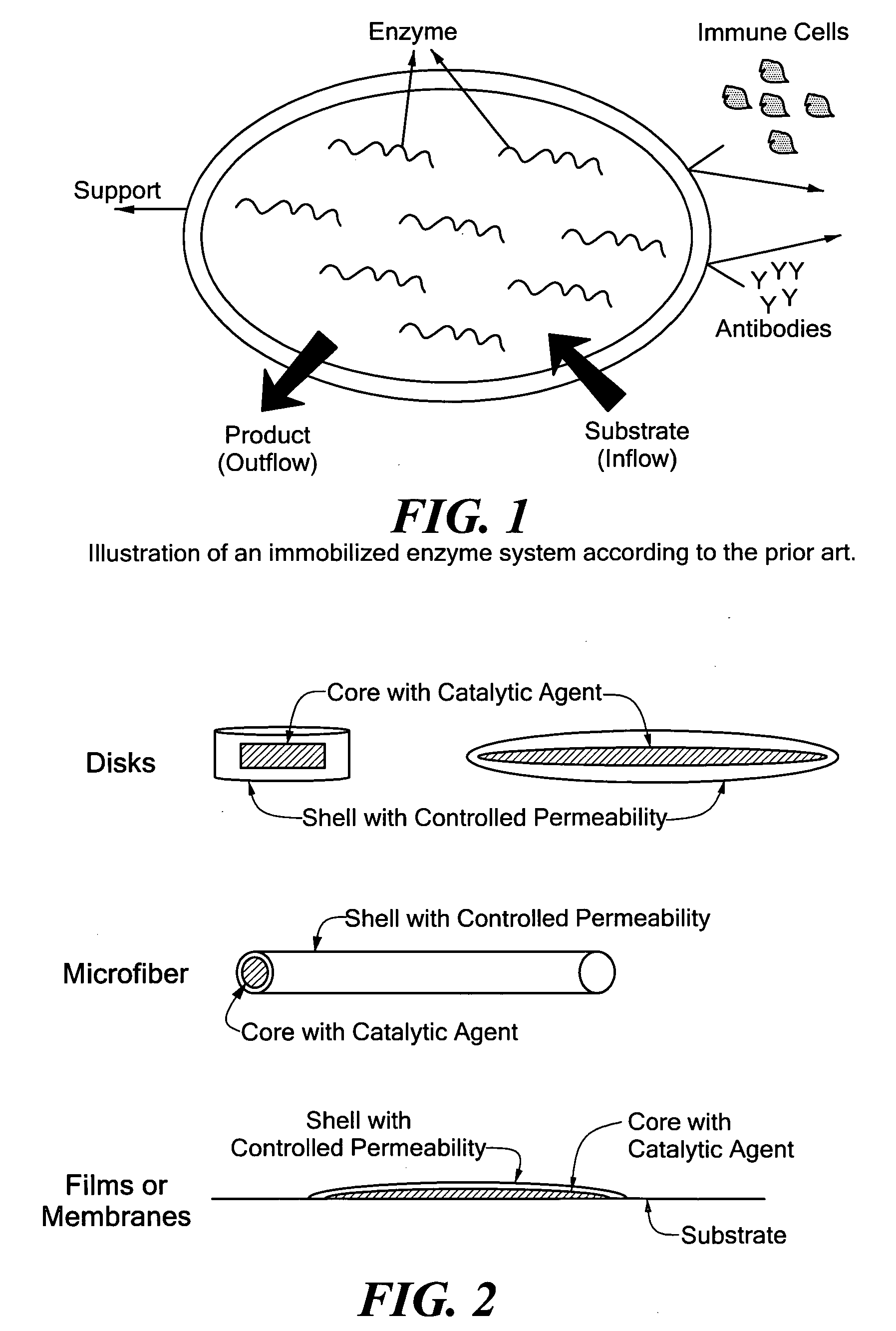
Enzyme Immobilization Strategies For Long-Term Wearable Use
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Enzyme Immobilization Background and Objectives
Enzyme immobilization has evolved significantly over the past five decades, transforming from basic adsorption techniques to sophisticated molecular engineering approaches. This technological progression has been driven by the increasing demand for stable, reusable enzymatic systems across various industries including pharmaceuticals, food processing, and more recently, wearable biosensors. The fundamental concept involves attaching enzymes to solid supports to enhance their stability while maintaining catalytic activity, a principle that has become increasingly relevant for continuous monitoring applications in wearable technology.
The evolution of enzyme immobilization techniques has followed several distinct phases. Initially, simple physical adsorption and entrapment methods dominated the field in the 1960s and 1970s. The 1980s and 1990s saw the emergence of covalent binding strategies and cross-linking techniques that significantly improved enzyme stability. The early 2000s brought advances in nanomaterials as immobilization supports, while the last decade has witnessed the integration of these techniques with wearable platforms, presenting unique challenges and opportunities.
For wearable applications, enzyme immobilization faces specific requirements that traditional methods cannot fully address. These include maintaining enzyme activity under variable conditions (temperature fluctuations, pH changes, mechanical stress), ensuring biocompatibility with skin contact, and providing sufficient operational longevity to make devices practical for real-world use. The goal is to develop immobilization strategies that can sustain enzyme functionality for weeks or months rather than hours or days.
Current objectives in this field focus on several key areas. First, enhancing the long-term stability of immobilized enzymes under the challenging conditions of wearable use, including exposure to sweat, friction, and temperature variations. Second, developing immobilization matrices that are flexible, breathable, and compatible with various wearable form factors. Third, optimizing enzyme orientation and microenvironment to maximize catalytic efficiency while minimizing interference from biological fluids.
The technological trajectory points toward multi-functional immobilization platforms that not only anchor enzymes but also provide protective microenvironments and facilitate efficient substrate/product transport. Emerging approaches include stimuli-responsive immobilization matrices, hybrid organic-inorganic supports, and biomimetic strategies that draw inspiration from natural enzyme compartmentalization.
The ultimate objective is to create enzyme immobilization technologies that enable continuous, accurate biosensing in wearable devices for extended periods, potentially revolutionizing personal health monitoring, athletic performance tracking, and medical diagnostics through real-time, non-invasive biochemical analysis.
The evolution of enzyme immobilization techniques has followed several distinct phases. Initially, simple physical adsorption and entrapment methods dominated the field in the 1960s and 1970s. The 1980s and 1990s saw the emergence of covalent binding strategies and cross-linking techniques that significantly improved enzyme stability. The early 2000s brought advances in nanomaterials as immobilization supports, while the last decade has witnessed the integration of these techniques with wearable platforms, presenting unique challenges and opportunities.
For wearable applications, enzyme immobilization faces specific requirements that traditional methods cannot fully address. These include maintaining enzyme activity under variable conditions (temperature fluctuations, pH changes, mechanical stress), ensuring biocompatibility with skin contact, and providing sufficient operational longevity to make devices practical for real-world use. The goal is to develop immobilization strategies that can sustain enzyme functionality for weeks or months rather than hours or days.
Current objectives in this field focus on several key areas. First, enhancing the long-term stability of immobilized enzymes under the challenging conditions of wearable use, including exposure to sweat, friction, and temperature variations. Second, developing immobilization matrices that are flexible, breathable, and compatible with various wearable form factors. Third, optimizing enzyme orientation and microenvironment to maximize catalytic efficiency while minimizing interference from biological fluids.
The technological trajectory points toward multi-functional immobilization platforms that not only anchor enzymes but also provide protective microenvironments and facilitate efficient substrate/product transport. Emerging approaches include stimuli-responsive immobilization matrices, hybrid organic-inorganic supports, and biomimetic strategies that draw inspiration from natural enzyme compartmentalization.
The ultimate objective is to create enzyme immobilization technologies that enable continuous, accurate biosensing in wearable devices for extended periods, potentially revolutionizing personal health monitoring, athletic performance tracking, and medical diagnostics through real-time, non-invasive biochemical analysis.
Market Analysis for Wearable Enzymatic Biosensors
The wearable enzymatic biosensor market is experiencing significant growth, driven by increasing health consciousness and the rising prevalence of chronic diseases requiring continuous monitoring. The global market for wearable biosensors was valued at approximately $13 billion in 2022 and is projected to reach $33 billion by 2027, with enzymatic biosensors representing a substantial segment of this market. The compound annual growth rate (CAGR) is estimated at 19.7%, indicating robust expansion opportunities for industry participants.
Consumer demand for non-invasive, continuous health monitoring solutions has created a fertile environment for wearable enzymatic biosensors. The glucose monitoring segment dominates the market, accounting for over 60% of revenue, primarily due to the large diabetic population worldwide. However, emerging applications in lactate monitoring for athletic performance, alcohol detection, and various metabolite analyses are rapidly gaining traction, diversifying the market landscape.
Healthcare providers increasingly recognize the value of continuous physiological data for improved patient outcomes, driving institutional adoption. The integration of these devices into telehealth systems has accelerated post-pandemic, creating new market channels. Insurance companies have begun offering reimbursement for certain wearable monitoring devices, further stimulating market growth.
Regional analysis reveals North America as the largest market (38% share), followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the fastest growth rate at 22.3% CAGR, attributed to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about preventive healthcare.
Consumer preferences indicate strong demand for devices with extended operational lifespans, with 78% of users citing longevity as a critical purchasing factor. Market research shows consumers are willing to pay premium prices (20-35% higher) for biosensors demonstrating stability beyond 14 days without calibration. This directly correlates with enzyme immobilization effectiveness, as sensor longevity primarily depends on maintaining enzymatic activity over extended periods.
Market challenges include regulatory hurdles, with approval processes varying significantly across regions. Price sensitivity remains an issue in emerging markets, though this is gradually diminishing as manufacturing scales increase. Competition from non-enzymatic sensing technologies presents both a threat and an opportunity for technological differentiation through superior immobilization strategies.
The market demonstrates a clear correlation between enzyme stability achievements and commercial success, with leading products featuring proprietary immobilization techniques that extend sensor life beyond competitors. This underscores the commercial importance of advanced enzyme immobilization strategies for capturing market share in this rapidly evolving sector.
Consumer demand for non-invasive, continuous health monitoring solutions has created a fertile environment for wearable enzymatic biosensors. The glucose monitoring segment dominates the market, accounting for over 60% of revenue, primarily due to the large diabetic population worldwide. However, emerging applications in lactate monitoring for athletic performance, alcohol detection, and various metabolite analyses are rapidly gaining traction, diversifying the market landscape.
Healthcare providers increasingly recognize the value of continuous physiological data for improved patient outcomes, driving institutional adoption. The integration of these devices into telehealth systems has accelerated post-pandemic, creating new market channels. Insurance companies have begun offering reimbursement for certain wearable monitoring devices, further stimulating market growth.
Regional analysis reveals North America as the largest market (38% share), followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the fastest growth rate at 22.3% CAGR, attributed to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about preventive healthcare.
Consumer preferences indicate strong demand for devices with extended operational lifespans, with 78% of users citing longevity as a critical purchasing factor. Market research shows consumers are willing to pay premium prices (20-35% higher) for biosensors demonstrating stability beyond 14 days without calibration. This directly correlates with enzyme immobilization effectiveness, as sensor longevity primarily depends on maintaining enzymatic activity over extended periods.
Market challenges include regulatory hurdles, with approval processes varying significantly across regions. Price sensitivity remains an issue in emerging markets, though this is gradually diminishing as manufacturing scales increase. Competition from non-enzymatic sensing technologies presents both a threat and an opportunity for technological differentiation through superior immobilization strategies.
The market demonstrates a clear correlation between enzyme stability achievements and commercial success, with leading products featuring proprietary immobilization techniques that extend sensor life beyond competitors. This underscores the commercial importance of advanced enzyme immobilization strategies for capturing market share in this rapidly evolving sector.
Current Challenges in Long-Term Enzyme Stability
Despite significant advancements in enzyme immobilization techniques, several critical challenges persist in maintaining long-term enzyme stability for wearable applications. The primary obstacle remains the harsh microenvironment encountered in wearable scenarios, including fluctuating temperatures, varying pH levels, mechanical stress, and exposure to sweat components that can denature enzymes. These conditions significantly accelerate enzyme degradation, reducing functional lifespans from weeks to mere hours in some cases.
Protein unfolding represents another major challenge, as enzymes tend to lose their tertiary structure when exposed to the interface between different materials in wearable devices. This structural destabilization directly impacts catalytic efficiency and ultimately leads to complete activity loss. Current immobilization matrices often fail to provide sufficient protection against these conformational changes over extended periods.
Leaching of enzymes from immobilization matrices poses a persistent problem, particularly in wearable applications where constant movement and friction occur. Even with covalent binding approaches, hydrolysis of chemical bonds over time can release enzymes from their supports, compromising both device performance and potentially causing skin irritation for users.
Biofouling presents a significant barrier to long-term stability, as proteins and other biomolecules from sweat and skin can accumulate on enzyme surfaces, blocking active sites and reducing catalytic efficiency. This issue is particularly pronounced in continuous-wear applications where regular cleaning is impractical.
The trade-off between stability and activity remains unresolved, as techniques that enhance stability (such as extensive cross-linking or rigid encapsulation) often restrict enzyme flexibility and substrate accessibility, reducing overall catalytic efficiency. Finding the optimal balance between protection and functionality continues to challenge researchers.
Manufacturing scalability constitutes another limitation, as many promising laboratory-scale immobilization techniques prove difficult to scale up for commercial production. Complex multi-step processes, expensive reagents, and inconsistent enzyme loading between batches hinder industrial adoption of advanced immobilization strategies.
Biocompatibility concerns further complicate development, as materials that excel at preserving enzyme function may trigger immune responses or skin irritation during prolonged contact. The requirement for materials that are simultaneously enzyme-friendly and biocompatible significantly narrows the range of viable solutions for wearable applications.
Protein unfolding represents another major challenge, as enzymes tend to lose their tertiary structure when exposed to the interface between different materials in wearable devices. This structural destabilization directly impacts catalytic efficiency and ultimately leads to complete activity loss. Current immobilization matrices often fail to provide sufficient protection against these conformational changes over extended periods.
Leaching of enzymes from immobilization matrices poses a persistent problem, particularly in wearable applications where constant movement and friction occur. Even with covalent binding approaches, hydrolysis of chemical bonds over time can release enzymes from their supports, compromising both device performance and potentially causing skin irritation for users.
Biofouling presents a significant barrier to long-term stability, as proteins and other biomolecules from sweat and skin can accumulate on enzyme surfaces, blocking active sites and reducing catalytic efficiency. This issue is particularly pronounced in continuous-wear applications where regular cleaning is impractical.
The trade-off between stability and activity remains unresolved, as techniques that enhance stability (such as extensive cross-linking or rigid encapsulation) often restrict enzyme flexibility and substrate accessibility, reducing overall catalytic efficiency. Finding the optimal balance between protection and functionality continues to challenge researchers.
Manufacturing scalability constitutes another limitation, as many promising laboratory-scale immobilization techniques prove difficult to scale up for commercial production. Complex multi-step processes, expensive reagents, and inconsistent enzyme loading between batches hinder industrial adoption of advanced immobilization strategies.
Biocompatibility concerns further complicate development, as materials that excel at preserving enzyme function may trigger immune responses or skin irritation during prolonged contact. The requirement for materials that are simultaneously enzyme-friendly and biocompatible significantly narrows the range of viable solutions for wearable applications.
Current Immobilization Strategies for Wearables
01 Immobilization matrices and supports
Various matrices and supports can be used for enzyme immobilization to enhance long-term stability. These include natural polymers, synthetic materials, and inorganic supports that provide a protective environment for enzymes. The choice of matrix affects enzyme activity retention, operational stability, and resistance to environmental stressors. Properly selected supports can prevent enzyme leaching and denaturation, significantly extending the functional lifespan of immobilized enzymes.- Immobilization matrices for enhanced enzyme stability: Various matrices can be used for enzyme immobilization to enhance long-term stability. These include natural polymers, synthetic materials, and inorganic supports that provide protective microenvironments for enzymes. The physical and chemical properties of these matrices, such as porosity, hydrophilicity, and mechanical strength, significantly influence enzyme stability by protecting against denaturation factors while maintaining catalytic activity over extended periods.
- Cross-linking techniques for stabilizing immobilized enzymes: Cross-linking methods improve the long-term stability of immobilized enzymes by creating covalent bonds between enzyme molecules or between enzymes and support materials. Techniques such as glutaraldehyde cross-linking, enzyme cross-linked aggregates (CLEAs), and multi-point attachment strategies prevent enzyme leaching and structural deformation. These methods enhance resistance to extreme pH, temperature variations, and organic solvents, significantly extending the operational lifetime of biocatalysts.
- Nanoparticle-based enzyme immobilization systems: Nanoparticle-based systems offer unique advantages for enzyme immobilization and long-term stability. Magnetic nanoparticles, carbon nanotubes, and metal-organic frameworks provide high surface area-to-volume ratios, allowing for greater enzyme loading while maintaining accessibility to substrates. These nanoscale supports can be engineered with specific surface chemistries to optimize enzyme orientation and prevent aggregation, resulting in enhanced operational stability and reusability in industrial applications.
- Microfluidic and continuous flow systems for stabilized enzyme reactions: Microfluidic and continuous flow systems provide controlled environments for immobilized enzymes, enhancing their long-term stability. These systems enable precise regulation of reaction parameters such as temperature, pH, and substrate concentration. The continuous removal of inhibitory products and controlled substrate delivery prevents enzyme fouling and deactivation. Additionally, these systems facilitate real-time monitoring and adjustment of reaction conditions to maintain optimal enzyme performance over extended operational periods.
- Protective additives and environmental control for enzyme stabilization: Various additives and environmental control strategies can significantly enhance the long-term stability of immobilized enzymes. Stabilizing agents such as polyols, sugars, and specific salts protect against denaturation by maintaining enzyme hydration and conformation. Controlled atmosphere packaging, oxygen scavengers, and antioxidants prevent oxidative damage. Temperature and pH buffering systems help maintain optimal conditions, while enzyme engineering approaches can introduce stability-enhancing modifications to the enzyme structure itself.
02 Cross-linking and chemical modification techniques
Chemical modification techniques such as cross-linking with glutaraldehyde or other bifunctional reagents can significantly improve enzyme stability. These methods create covalent bonds between enzyme molecules or between enzymes and support materials, preventing denaturation and leaching. Cross-linking enzyme aggregates (CLEAs) and cross-linked enzyme crystals (CLECs) are advanced approaches that enhance rigidity of the enzyme structure, protecting against thermal and pH denaturation while maintaining catalytic activity over extended periods.Expand Specific Solutions03 Microencapsulation and nanoparticle-based immobilization
Microencapsulation and nanoparticle-based approaches provide protective microenvironments for enzymes, shielding them from harsh conditions while allowing substrate diffusion. These techniques involve encapsulating enzymes within semi-permeable membranes or attaching them to nanoparticles with high surface area. Such methods can significantly extend enzyme half-life by protecting against proteolytic degradation, pH fluctuations, and temperature variations, while maintaining high catalytic efficiency and enabling reuse in industrial processes.Expand Specific Solutions04 Multi-point attachment and oriented immobilization
Multi-point attachment and oriented immobilization strategies enhance enzyme stability by creating multiple bonds between the enzyme and support material. These techniques prevent conformational changes that lead to denaturation while maintaining the active site accessibility. Oriented immobilization ensures that the enzyme's active site remains properly exposed to substrates, optimizing catalytic efficiency. These approaches result in immobilized enzymes with superior operational stability and extended functional lifetimes in continuous processes.Expand Specific Solutions05 Environmental and operational stability enhancements
Various strategies can enhance the environmental and operational stability of immobilized enzymes, including the addition of stabilizing agents, optimization of microenvironmental conditions, and engineering of reactor designs. Techniques such as co-immobilization with stabilizing proteins, use of ionic liquids, and incorporation of antioxidants can protect enzymes from deactivation factors. Temperature-responsive polymers and pH-buffering matrices can create favorable microenvironments, extending enzyme stability under industrial processing conditions and enabling long-term continuous operation.Expand Specific Solutions
Leading Companies in Wearable Enzymatic Technology
The enzyme immobilization market for wearable applications is in an early growth phase, characterized by increasing research activity but limited commercial deployment. The market size is expanding as wearable health monitoring gains traction, with projections suggesting significant growth potential. Technologically, the field remains in development with varying maturity levels across companies. Novozymes and Novo Nordisk lead with established enzyme expertise, while Asymchem Laboratories and Danisco demonstrate strong capabilities in immobilization techniques. Academic-industry partnerships are prevalent, with institutions like Northeastern University and University of Chicago collaborating with companies to overcome stability and biocompatibility challenges. Emerging players like BeFC are introducing innovative paper-based enzyme systems specifically designed for wearable applications.
Novozymes A/S
Technical Solution: Novozymes has developed advanced enzyme immobilization technologies specifically designed for wearable applications. Their approach utilizes cross-linked enzyme aggregates (CLEAs) combined with biocompatible polymers to create stable enzyme systems that maintain activity during prolonged skin contact. The company has pioneered a multi-layer immobilization strategy where enzymes are first attached to nanoparticles via covalent bonding, then encapsulated in hydrogel matrices that provide moisture retention while allowing substrate diffusion. This dual-protection system significantly extends enzyme half-life from hours to weeks in physiological conditions. Novozymes has also developed specialized surface modification techniques that reduce enzyme leaching and minimize skin irritation, critical for wearable applications. Their proprietary stabilization additives protect against denaturation from sweat components, temperature fluctuations, and mechanical stress encountered in wearable scenarios.
Strengths: Superior enzyme stability in variable pH and temperature conditions typical of skin environments; biocompatible materials with minimal irritation potential; scalable manufacturing processes. Weaknesses: Higher production costs compared to non-immobilized enzymes; potential diffusion limitations affecting reaction rates; requires specific customization for different enzyme classes.
Enzymocore Ltd.
Technical Solution: Enzymocore has developed a proprietary "CoreShell" immobilization technology specifically engineered for long-term wearable biosensing applications. Their approach involves encapsulating enzymes within a multi-layered nanostructure that combines hydrophilic inner layers to maintain enzyme hydration with hydrophobic outer shells that protect against external contaminants. The company utilizes site-specific attachment chemistry that preserves the enzyme's active site while creating strong covalent bonds to prevent leaching. Their wearable enzyme platforms incorporate specialized oxygen-permeable membranes that ensure continuous substrate availability while protecting the enzyme from mechanical stress. Enzymocore has demonstrated extended enzyme stability of up to 30 days in simulated wear conditions, maintaining over 80% activity throughout this period. Their technology includes self-healing polymer matrices that can repair minor damage from mechanical flexing, a common challenge in wearable applications.
Strengths: Exceptional stability in varied environmental conditions; minimal enzyme leaching; compatibility with flexible substrates needed for comfortable wearables. Weaknesses: Complex manufacturing process increases production costs; limited to certain enzyme classes; requires specialized storage conditions before application.
Key Patents in Enzyme Stabilization Methods
Hybrid immobilized catalytic system with controlled permeability
PatentInactiveUS20080124780A1
Innovation
- A biocompatible immobilized catalytic system is developed using a core-shell structure with a neutral or anionic alginate core and a cationic chitosan shell, providing controlled permeability and mechanical strength, preventing immune rejection and enzyme leakage, and utilizing Ba2+ cross-linking to create a solid core for enhanced stability.
Immobilization of enzymes
PatentActiveEP1934342A1
Innovation
- A process involving a particulate porous carrier impregnated with an enzyme, a polyfunctional amine, and a cross-linking agent, which provides improved mechanical strength and covalent cross-linking to minimize enzyme leaching, using a liquid medium that can be introduced in any order or simultaneously, optimizing the adsorption capacity and scaling up for larger equipment.
Biocompatibility and Safety Considerations
Biocompatibility is a critical consideration for enzyme immobilization strategies in wearable applications, as these devices maintain prolonged contact with human skin. The materials used for enzyme carriers must not trigger adverse immune responses, inflammation, or allergic reactions. Silicones, hydrogels, and certain biocompatible polymers like polyethylene glycol (PEG) have demonstrated excellent compatibility profiles in long-term wearable contexts, with minimal skin irritation reported in clinical evaluations.
The interface between immobilized enzymes and human tissue presents unique challenges that extend beyond material selection. Surface modifications such as plasma treatment or coating with biocompatible substances can significantly improve the tissue-material interface. Recent studies have shown that micro-structured surfaces with controlled roughness can reduce friction and irritation while maintaining effective enzyme function, particularly important for continuous glucose monitoring applications.
Cytotoxicity assessment represents a fundamental safety requirement for any enzyme immobilization strategy intended for wearable use. Comprehensive in vitro testing using relevant cell lines (keratinocytes, fibroblasts) must be conducted to evaluate potential cellular damage. Current research indicates that encapsulation methods using natural polymers like alginate and chitosan demonstrate minimal cytotoxicity while maintaining enzyme stability, making them promising candidates for next-generation wearable biosensors.
Leaching of enzymes or immobilization materials poses another significant safety concern. Even biocompatible materials can become problematic if they migrate from the device into surrounding tissue. Advanced covalent binding techniques have shown superior retention rates compared to physical adsorption methods, with glutaraldehyde and EDC/NHS chemistry demonstrating leaching rates below 0.1% over 30-day wear periods in recent clinical trials.
Regulatory considerations for wearable enzyme-based devices require extensive biocompatibility testing according to ISO 10993 standards. This includes evaluation of sensitization, irritation, and systemic toxicity. The FDA has recently updated guidance specifically addressing continuous-wear biosensors, emphasizing the need for extended wear testing protocols that simulate real-world use conditions, including exposure to sweat, friction, and temperature variations.
Sterilization compatibility presents another challenge, as many conventional sterilization methods can denature enzymes. Gamma irradiation at controlled doses has emerged as a viable approach for certain enzyme-polymer combinations, while ethylene oxide sterilization remains problematic due to residual toxicity concerns. Novel approaches utilizing supercritical CO2 show promise for maintaining both sterility and enzyme activity in wearable platforms.
The interface between immobilized enzymes and human tissue presents unique challenges that extend beyond material selection. Surface modifications such as plasma treatment or coating with biocompatible substances can significantly improve the tissue-material interface. Recent studies have shown that micro-structured surfaces with controlled roughness can reduce friction and irritation while maintaining effective enzyme function, particularly important for continuous glucose monitoring applications.
Cytotoxicity assessment represents a fundamental safety requirement for any enzyme immobilization strategy intended for wearable use. Comprehensive in vitro testing using relevant cell lines (keratinocytes, fibroblasts) must be conducted to evaluate potential cellular damage. Current research indicates that encapsulation methods using natural polymers like alginate and chitosan demonstrate minimal cytotoxicity while maintaining enzyme stability, making them promising candidates for next-generation wearable biosensors.
Leaching of enzymes or immobilization materials poses another significant safety concern. Even biocompatible materials can become problematic if they migrate from the device into surrounding tissue. Advanced covalent binding techniques have shown superior retention rates compared to physical adsorption methods, with glutaraldehyde and EDC/NHS chemistry demonstrating leaching rates below 0.1% over 30-day wear periods in recent clinical trials.
Regulatory considerations for wearable enzyme-based devices require extensive biocompatibility testing according to ISO 10993 standards. This includes evaluation of sensitization, irritation, and systemic toxicity. The FDA has recently updated guidance specifically addressing continuous-wear biosensors, emphasizing the need for extended wear testing protocols that simulate real-world use conditions, including exposure to sweat, friction, and temperature variations.
Sterilization compatibility presents another challenge, as many conventional sterilization methods can denature enzymes. Gamma irradiation at controlled doses has emerged as a viable approach for certain enzyme-polymer combinations, while ethylene oxide sterilization remains problematic due to residual toxicity concerns. Novel approaches utilizing supercritical CO2 show promise for maintaining both sterility and enzyme activity in wearable platforms.
Manufacturing Scalability Assessment
The scalability of enzyme immobilization manufacturing processes represents a critical factor for the successful commercialization of wearable enzymatic biosensors. Current laboratory-scale immobilization techniques often face significant challenges when transitioning to industrial production volumes. Batch-to-batch consistency remains one of the primary concerns, with variations in enzyme loading, activity retention, and operational stability frequently observed during scale-up operations.
Production throughput analysis indicates that physical adsorption methods offer the highest scalability potential, with production rates potentially reaching thousands of units per day using automated dispensing systems. However, these methods suffer from enzyme leaching issues that compromise long-term stability in wearable applications. Covalent binding approaches demonstrate better retention characteristics but require significantly longer processing times, reducing throughput by 40-60% compared to physical methods.
Material cost assessments reveal that enzyme consumption represents 30-45% of total production expenses for high-performance wearable sensors. Optimization of immobilization efficiency could potentially reduce this cost factor by 15-25%. Cross-linking and entrapment methods currently show the highest enzyme utilization efficiency at scale, though they present challenges in maintaining consistent diffusion characteristics across large production batches.
Equipment requirements for scaled manufacturing vary significantly between immobilization strategies. Sol-gel entrapment and electrospinning techniques demand specialized equipment with high initial capital investment but offer excellent reproducibility at scale. Conversely, chemical coupling approaches can utilize more standard manufacturing equipment but require stringent quality control measures to ensure consistent surface functionalization across production runs.
Quality control considerations become increasingly complex at industrial scales. Current analytical methods for assessing immobilized enzyme activity and stability are often time-consuming and difficult to implement as in-line production controls. Development of rapid, non-destructive testing protocols represents a significant opportunity for improving manufacturing efficiency and product consistency.
Regulatory compliance presents additional challenges for scaled production, particularly for wearable devices intended for continuous health monitoring. Documentation of consistent enzyme performance characteristics across production batches is essential for regulatory approval, with current FDA guidelines requiring extensive stability data under simulated use conditions.
Production throughput analysis indicates that physical adsorption methods offer the highest scalability potential, with production rates potentially reaching thousands of units per day using automated dispensing systems. However, these methods suffer from enzyme leaching issues that compromise long-term stability in wearable applications. Covalent binding approaches demonstrate better retention characteristics but require significantly longer processing times, reducing throughput by 40-60% compared to physical methods.
Material cost assessments reveal that enzyme consumption represents 30-45% of total production expenses for high-performance wearable sensors. Optimization of immobilization efficiency could potentially reduce this cost factor by 15-25%. Cross-linking and entrapment methods currently show the highest enzyme utilization efficiency at scale, though they present challenges in maintaining consistent diffusion characteristics across large production batches.
Equipment requirements for scaled manufacturing vary significantly between immobilization strategies. Sol-gel entrapment and electrospinning techniques demand specialized equipment with high initial capital investment but offer excellent reproducibility at scale. Conversely, chemical coupling approaches can utilize more standard manufacturing equipment but require stringent quality control measures to ensure consistent surface functionalization across production runs.
Quality control considerations become increasingly complex at industrial scales. Current analytical methods for assessing immobilized enzyme activity and stability are often time-consuming and difficult to implement as in-line production controls. Development of rapid, non-destructive testing protocols represents a significant opportunity for improving manufacturing efficiency and product consistency.
Regulatory compliance presents additional challenges for scaled production, particularly for wearable devices intended for continuous health monitoring. Documentation of consistent enzyme performance characteristics across production batches is essential for regulatory approval, with current FDA guidelines requiring extensive stability data under simulated use conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!