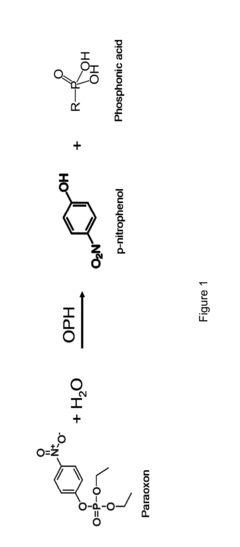
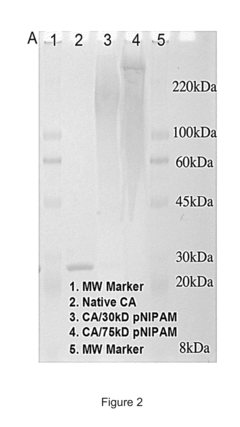
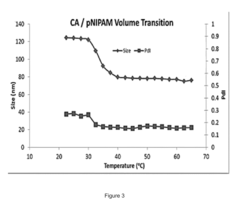
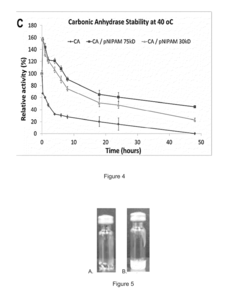
Stability Engineering Of Enzyme Catalysts For On-Body Energy Harvesting
SEP 3, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Enzyme Catalysts for Energy Harvesting: Background and Objectives
Enzyme catalysts have emerged as a promising frontier in sustainable energy generation, particularly for wearable and implantable devices. The historical trajectory of enzyme-based energy harvesting can be traced back to the early 2000s when researchers first demonstrated the feasibility of enzymatic biofuel cells. These initial systems, while groundbreaking, suffered from significant limitations in stability, power density, and operational longevity, restricting their practical applications.
The evolution of enzyme catalysis for energy harvesting has accelerated dramatically over the past decade, driven by advances in protein engineering, materials science, and nanotechnology. Particularly noteworthy has been the shift from simple glucose oxidase systems to more complex multi-enzymatic cascades capable of complete substrate oxidation, thereby maximizing energy extraction efficiency. This progression represents a fundamental paradigm shift from proof-of-concept demonstrations to engineered systems with practical utility.
On-body energy harvesting presents a unique set of technical challenges and opportunities. The human body offers abundant energy sources in the form of glucose, lactate, and other metabolites present in physiological fluids such as sweat, tears, and interstitial fluid. Harnessing these resources through enzymatic catalysis could potentially enable self-powered wearable health monitors, drug delivery systems, and other biomedical devices without the need for conventional batteries.
The primary technical objective in this field centers on stability engineering of enzyme catalysts to overcome their inherent limitations in the harsh conditions of on-body applications. Enzymes naturally evolved for intracellular environments must be re-engineered to maintain catalytic activity despite fluctuating temperatures, pH variations, mechanical stresses, and the presence of inhibitors in physiological fluids. Additionally, long-term operational stability measured in months rather than hours is essential for practical deployment.
Current research trajectories focus on several complementary approaches: directed evolution to enhance intrinsic enzyme stability, immobilization strategies to protect enzymes from denaturation, development of enzyme-nanomaterial hybrids with enhanced electron transfer capabilities, and biomimetic systems that capture the catalytic efficiency of enzymes while improving durability. These approaches collectively aim to bridge the gap between laboratory demonstrations and commercially viable technologies.
The ultimate goal of stability engineering for enzyme catalysts extends beyond incremental improvements to enable transformative applications in personalized healthcare, continuous physiological monitoring, and sustainable energy generation. Success in this domain would represent a significant step toward truly autonomous wearable technologies that leverage the body's own biochemistry as an inexhaustible power source.
The evolution of enzyme catalysis for energy harvesting has accelerated dramatically over the past decade, driven by advances in protein engineering, materials science, and nanotechnology. Particularly noteworthy has been the shift from simple glucose oxidase systems to more complex multi-enzymatic cascades capable of complete substrate oxidation, thereby maximizing energy extraction efficiency. This progression represents a fundamental paradigm shift from proof-of-concept demonstrations to engineered systems with practical utility.
On-body energy harvesting presents a unique set of technical challenges and opportunities. The human body offers abundant energy sources in the form of glucose, lactate, and other metabolites present in physiological fluids such as sweat, tears, and interstitial fluid. Harnessing these resources through enzymatic catalysis could potentially enable self-powered wearable health monitors, drug delivery systems, and other biomedical devices without the need for conventional batteries.
The primary technical objective in this field centers on stability engineering of enzyme catalysts to overcome their inherent limitations in the harsh conditions of on-body applications. Enzymes naturally evolved for intracellular environments must be re-engineered to maintain catalytic activity despite fluctuating temperatures, pH variations, mechanical stresses, and the presence of inhibitors in physiological fluids. Additionally, long-term operational stability measured in months rather than hours is essential for practical deployment.
Current research trajectories focus on several complementary approaches: directed evolution to enhance intrinsic enzyme stability, immobilization strategies to protect enzymes from denaturation, development of enzyme-nanomaterial hybrids with enhanced electron transfer capabilities, and biomimetic systems that capture the catalytic efficiency of enzymes while improving durability. These approaches collectively aim to bridge the gap between laboratory demonstrations and commercially viable technologies.
The ultimate goal of stability engineering for enzyme catalysts extends beyond incremental improvements to enable transformative applications in personalized healthcare, continuous physiological monitoring, and sustainable energy generation. Success in this domain would represent a significant step toward truly autonomous wearable technologies that leverage the body's own biochemistry as an inexhaustible power source.
Market Analysis for On-Body Energy Harvesting Technologies
The on-body energy harvesting market is experiencing significant growth, driven by the expanding wearable technology sector and increasing demand for sustainable power solutions. Current market valuations indicate that the global on-body energy harvesting market reached approximately 500 million USD in 2022 and is projected to grow at a compound annual growth rate of 18-20% through 2030, potentially reaching 2.5 billion USD by the end of the decade.
The primary market segments for enzyme-based on-body energy harvesting technologies include healthcare monitoring devices, fitness trackers, smart textiles, and implantable medical devices. The healthcare segment currently dominates the market share, accounting for nearly 45% of applications, as continuous health monitoring requires reliable power sources that can operate autonomously for extended periods.
Consumer demand is increasingly focused on devices that eliminate the need for frequent battery replacement or recharging. Market surveys indicate that 78% of wearable device users consider battery life a critical factor in purchasing decisions, creating a strong value proposition for enzyme-catalyzed energy harvesting solutions that can generate power from bodily fluids such as sweat or interstitial fluid.
Regional market analysis shows North America leading with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region is expected to demonstrate the fastest growth rate due to increasing adoption of wearable technologies and substantial investments in biotechnology research.
Key market drivers include the miniaturization of electronic components, advancements in enzyme engineering techniques, growing consumer awareness about sustainable technologies, and increasing healthcare costs driving demand for remote patient monitoring. The integration of enzyme catalysts with flexible electronics represents a particularly promising market opportunity, with potential applications extending beyond conventional wearables to smart bandages and electronic skin.
Market barriers include concerns about enzyme stability under variable body conditions, manufacturing scalability challenges, regulatory hurdles for biocatalytic materials in contact with skin, and competition from alternative energy harvesting technologies such as piezoelectric and thermoelectric systems. The cost factor remains significant, with enzyme-based systems currently more expensive than conventional batteries for many applications.
Customer segmentation reveals three primary markets: medical device manufacturers seeking long-term power solutions for continuous monitoring devices, consumer electronics companies looking to differentiate their wearable products, and military/defense organizations requiring reliable power sources for field operations. Each segment has distinct requirements regarding power output, stability, and cost parameters that must be addressed through targeted enzyme engineering approaches.
The primary market segments for enzyme-based on-body energy harvesting technologies include healthcare monitoring devices, fitness trackers, smart textiles, and implantable medical devices. The healthcare segment currently dominates the market share, accounting for nearly 45% of applications, as continuous health monitoring requires reliable power sources that can operate autonomously for extended periods.
Consumer demand is increasingly focused on devices that eliminate the need for frequent battery replacement or recharging. Market surveys indicate that 78% of wearable device users consider battery life a critical factor in purchasing decisions, creating a strong value proposition for enzyme-catalyzed energy harvesting solutions that can generate power from bodily fluids such as sweat or interstitial fluid.
Regional market analysis shows North America leading with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region is expected to demonstrate the fastest growth rate due to increasing adoption of wearable technologies and substantial investments in biotechnology research.
Key market drivers include the miniaturization of electronic components, advancements in enzyme engineering techniques, growing consumer awareness about sustainable technologies, and increasing healthcare costs driving demand for remote patient monitoring. The integration of enzyme catalysts with flexible electronics represents a particularly promising market opportunity, with potential applications extending beyond conventional wearables to smart bandages and electronic skin.
Market barriers include concerns about enzyme stability under variable body conditions, manufacturing scalability challenges, regulatory hurdles for biocatalytic materials in contact with skin, and competition from alternative energy harvesting technologies such as piezoelectric and thermoelectric systems. The cost factor remains significant, with enzyme-based systems currently more expensive than conventional batteries for many applications.
Customer segmentation reveals three primary markets: medical device manufacturers seeking long-term power solutions for continuous monitoring devices, consumer electronics companies looking to differentiate their wearable products, and military/defense organizations requiring reliable power sources for field operations. Each segment has distinct requirements regarding power output, stability, and cost parameters that must be addressed through targeted enzyme engineering approaches.
Current Challenges in Enzyme Stability Engineering
Despite significant advancements in enzyme engineering, several critical challenges persist in developing stable enzyme catalysts for on-body energy harvesting applications. The primary obstacle remains the harsh microenvironment of on-body applications, characterized by fluctuating temperatures, varying pH levels, presence of sweat components, and mechanical stress. These conditions severely compromise enzyme stability and catalytic efficiency over extended periods.
Thermal stability presents a particular challenge as body temperature fluctuations can lead to enzyme denaturation. Current stabilization techniques like directed evolution and computational design have shown limited success in maintaining activity across the temperature range experienced in wearable contexts (typically 25-40°C with potential spikes).
Operational stability under mechanical stress poses another significant hurdle. On-body applications subject enzymes to continuous movement, friction, and pressure, accelerating protein unfolding and aggregation. Existing immobilization strategies often reduce catalytic efficiency while attempting to enhance mechanical stability, creating an unresolved engineering trade-off.
Long-term storage stability remains problematic, with most engineered enzymes showing significant activity loss after weeks or months, falling short of the shelf-life requirements for commercial wearable devices. Current preservation methods using stabilizing agents often interfere with subsequent catalytic performance.
The integration of enzymes with electronic components introduces additional compatibility challenges. Interface engineering between biological catalysts and electronic materials frequently results in enzyme deactivation due to unfavorable interactions with conductive materials or exposure to mild electrical fields.
Scalable production represents another major obstacle. Laboratory-scale enzyme engineering techniques have not translated effectively to industrial-scale production while maintaining consistent stability profiles. This manufacturing gap significantly impedes commercialization efforts.
Multi-enzyme cascade systems, which could potentially enhance energy harvesting efficiency, face synchronization difficulties when individual enzymes exhibit different stability profiles under on-body conditions. Current approaches fail to ensure balanced degradation rates across enzyme networks.
Additionally, the biocompatibility requirements for on-body applications impose restrictions on stabilization strategies. Many effective stabilizing agents and immobilization materials that enhance enzyme longevity are unsuitable for prolonged skin contact, limiting the available engineering toolbox.
Addressing these challenges requires interdisciplinary approaches combining protein engineering, materials science, and wearable technology expertise to develop next-generation stable enzyme catalysts capable of reliable on-body energy harvesting.
Thermal stability presents a particular challenge as body temperature fluctuations can lead to enzyme denaturation. Current stabilization techniques like directed evolution and computational design have shown limited success in maintaining activity across the temperature range experienced in wearable contexts (typically 25-40°C with potential spikes).
Operational stability under mechanical stress poses another significant hurdle. On-body applications subject enzymes to continuous movement, friction, and pressure, accelerating protein unfolding and aggregation. Existing immobilization strategies often reduce catalytic efficiency while attempting to enhance mechanical stability, creating an unresolved engineering trade-off.
Long-term storage stability remains problematic, with most engineered enzymes showing significant activity loss after weeks or months, falling short of the shelf-life requirements for commercial wearable devices. Current preservation methods using stabilizing agents often interfere with subsequent catalytic performance.
The integration of enzymes with electronic components introduces additional compatibility challenges. Interface engineering between biological catalysts and electronic materials frequently results in enzyme deactivation due to unfavorable interactions with conductive materials or exposure to mild electrical fields.
Scalable production represents another major obstacle. Laboratory-scale enzyme engineering techniques have not translated effectively to industrial-scale production while maintaining consistent stability profiles. This manufacturing gap significantly impedes commercialization efforts.
Multi-enzyme cascade systems, which could potentially enhance energy harvesting efficiency, face synchronization difficulties when individual enzymes exhibit different stability profiles under on-body conditions. Current approaches fail to ensure balanced degradation rates across enzyme networks.
Additionally, the biocompatibility requirements for on-body applications impose restrictions on stabilization strategies. Many effective stabilizing agents and immobilization materials that enhance enzyme longevity are unsuitable for prolonged skin contact, limiting the available engineering toolbox.
Addressing these challenges requires interdisciplinary approaches combining protein engineering, materials science, and wearable technology expertise to develop next-generation stable enzyme catalysts capable of reliable on-body energy harvesting.
Current Approaches to Enzyme Stability Enhancement
01 Enzyme immobilization techniques
Various immobilization techniques can be employed to enhance enzyme stability. These include covalent binding, adsorption, entrapment, and cross-linking methods. Immobilization provides structural rigidity to enzymes, protecting them against denaturation due to heat, pH changes, and organic solvents. This approach also enables enzyme reuse and continuous operation in industrial processes, significantly improving catalyst longevity and operational stability.- Enzyme stabilization through immobilization techniques: Immobilization of enzymes on various supports enhances their stability by protecting them from environmental factors. This technique involves attaching enzymes to solid carriers, which prevents denaturation and extends their catalytic lifespan. Immobilized enzymes show improved resistance to temperature fluctuations, pH changes, and organic solvents, making them more suitable for industrial applications. The immobilization process can involve physical adsorption, covalent binding, or entrapment methods.
- Protein engineering for enhanced enzyme stability: Genetic modification and protein engineering techniques can be used to create enzyme variants with improved stability characteristics. These approaches include site-directed mutagenesis, directed evolution, and rational design to modify amino acid sequences. The engineered enzymes exhibit enhanced resistance to thermal denaturation, oxidative stress, and proteolytic degradation. These modifications can significantly extend the functional lifetime of enzyme catalysts in various industrial processes.
- Formulation with stabilizing additives and excipients: The addition of specific compounds to enzyme formulations can significantly enhance their stability. These stabilizing agents include polyols, sugars, amino acids, and certain salts that help maintain the enzyme's native conformation. Additives can prevent protein aggregation, protect against oxidative damage, and create favorable microenvironments. Optimized formulations with these stabilizers extend shelf-life and maintain catalytic activity under challenging conditions.
- Temperature and pH optimization for enzyme stability: Controlling environmental conditions such as temperature and pH is crucial for maintaining enzyme stability. Each enzyme has optimal temperature and pH ranges where its structure remains intact and functional. Techniques for stabilizing enzymes include buffer system optimization, controlled cooling protocols, and thermal adaptation methods. Understanding the relationship between these parameters and enzyme conformational stability allows for process designs that maximize catalyst longevity.
- Cross-linking and chemical modification strategies: Chemical modification of enzymes through cross-linking or addition of functional groups can significantly improve their stability. Cross-linking enzyme aggregates (CLEAs) or crystals (CLECs) creates robust biocatalysts resistant to denaturation. Other chemical modifications include PEGylation, glycosylation, and acetylation, which can shield vulnerable sites on the enzyme surface. These approaches enhance resistance to organic solvents, extreme pH, and elevated temperatures while maintaining catalytic efficiency.
02 Protein engineering for enhanced stability
Protein engineering approaches involve modifying enzyme structures at the molecular level to improve their stability. This includes directed evolution, rational design, and site-directed mutagenesis to create variants with improved thermostability, pH tolerance, and resistance to chemical denaturants. These engineered enzymes can withstand harsh industrial conditions while maintaining catalytic efficiency, making them valuable for various biotechnological applications.Expand Specific Solutions03 Stabilizing additives and formulations
Various additives can be incorporated into enzyme formulations to enhance stability. These include polyols, sugars, salts, and specific buffer systems that help maintain the enzyme's native conformation. Stabilizing agents like glycerol, sorbitol, and trehalose prevent denaturation by forming hydrogen bonds with the enzyme surface. Proper formulation design can significantly extend shelf-life and improve performance under challenging environmental conditions.Expand Specific Solutions04 Enzyme stabilization in extreme conditions
Specialized approaches are developed to maintain enzyme activity under extreme conditions such as high temperatures, extreme pH, organic solvents, or high salt concentrations. These include isolation of extremophilic enzymes from organisms naturally adapted to harsh environments, chemical modification of enzyme surfaces, and development of protective microenvironments. These strategies are particularly important for industrial applications where process conditions are often far from physiological.Expand Specific Solutions05 Encapsulation and carrier systems
Encapsulation technologies protect enzymes by housing them within protective matrices or carrier systems. These include microencapsulation in polymeric materials, incorporation into liposomes, nanoparticles, or hydrogels. Such systems shield enzymes from harsh external environments while allowing substrate diffusion. Additionally, these carrier systems can be designed with stimuli-responsive properties for controlled release and activity, further enhancing the practical applications of enzyme catalysts in various fields.Expand Specific Solutions
Leading Organizations in Enzymatic Energy Harvesting
The enzyme catalyst stability engineering for on-body energy harvesting is in an emerging growth phase, with the market expected to expand significantly as wearable technology adoption increases. Current market size remains modest but projections indicate substantial growth potential as energy harvesting technologies become critical for sustainable wearable devices. The technological landscape shows varying maturity levels, with academic institutions like MIT, Johns Hopkins University, and Georgia State University Research Foundation leading fundamental research, while companies such as BASF Corp., Danisco, and Roche Diabetes Care are advancing commercial applications. Specialized firms like AgroSpheres and Geyser Batteries are developing novel enzyme stabilization techniques, while established players like FUJIFILM Wako and Asahi Kasei are leveraging their chemical expertise to improve enzyme performance in challenging on-body environments.
The Johns Hopkins University
Technical Solution: Johns Hopkins has developed a comprehensive platform for enzyme stabilization in wearable biofuel cells focused on harvesting energy from human perspiration. Their approach centers on a triple-protection strategy: (1) genetic engineering of oxidoreductase enzymes to introduce disulfide bonds and surface-charged residues that enhance thermostability, (2) chemical modification using crosslinking agents that preserve enzyme structure while allowing substrate diffusion, and (3) immobilization within biocompatible hydrogel matrices that mimic natural cellular environments. The university's researchers have demonstrated lactate oxidase and pyruvate oxidase systems with remarkable stability at skin temperature (33-37°C) and pH variations (pH 4-7) typical of sweat conditions. Their latest prototypes maintain over 85% activity after 14 days of continuous operation in simulated sweat conditions, with power outputs sufficient for low-power biomedical sensors.
Strengths: Excellent enzyme stability under variable physiological conditions; biocompatible materials suitable for prolonged skin contact; comprehensive approach combining genetic, chemical and physical stabilization. Weaknesses: Limited power output compared to conventional energy sources; potential challenges with enzyme regeneration in long-term applications; sensitivity to certain components in sweat that may inhibit enzymatic activity.
Roche Diabetes Care, Inc.
Technical Solution: Roche Diabetes Care has leveraged its extensive expertise in glucose monitoring to develop stabilized glucose-oxidizing enzyme systems for on-body energy harvesting. Their proprietary enzyme stabilization platform incorporates specially engineered glucose oxidase variants with enhanced thermal and pH stability. The company has developed a multi-layer immobilization approach where enzymes are encapsulated within protective osmium-based redox polymers that facilitate electron transfer while shielding the enzyme from environmental stressors. Their latest wearable energy harvesting prototype integrates with continuous glucose monitoring systems, utilizing the same interstitial fluid sampling mechanism to power the sensor itself. The system achieves remarkable stability with less than 10% activity loss over 7 days of continuous operation at body temperature, generating consistent power output of 50-150 μW/cm² depending on glucose concentration in interstitial fluid.
Strengths: Seamless integration with existing glucose monitoring technology; highly optimized glucose oxidase variants with superior stability; established manufacturing capabilities for enzyme-based medical devices. Weaknesses: Limited to glucose as the primary fuel source; potential regulatory hurdles for dual-purpose (monitoring and power generation) devices; performance dependent on patient's glucose levels.
Key Innovations in Enzyme Immobilization and Protection
Stabilization of biomolecules by attachment of responsive polymers and sensors thereof
PatentActiveUS20150376594A1
Innovation
- The method involves covalently attaching stimulus-responsive polymers to enzymes using controlled radical polymerization, forming reversible nanoparticle structures that provide structural support and maintain enzyme stability by collapsing around the enzyme at elevated temperatures, preventing denaturation.
Methods for improving protein properties
PatentActiveEP2171057A2
Innovation
- The method involves engineering enzymes by testing singly-substituted protein variants to identify favorable substitutions that enhance catalytic activity and stability, altering the net surface charge and surface charge distribution of enzymes like metalloproteases to create variants with improved performance and stability in detergent formulations.
Biocompatibility and Safety Considerations for On-Body Applications
The integration of enzyme catalysts into on-body energy harvesting systems necessitates rigorous biocompatibility and safety assessments. These wearable biocatalytic systems operate at the interface between technology and human physiology, requiring careful consideration of potential biological interactions and adverse effects.
Skin contact represents the primary interface for on-body enzyme catalytic systems, making dermal compatibility a critical concern. Enzymes and their stabilizing matrices must not trigger irritation, sensitization, or allergic responses. Recent studies have demonstrated that enzyme immobilization techniques using biocompatible polymers like polyethylene glycol (PEG) and hyaluronic acid derivatives significantly reduce immunogenic potential while maintaining catalytic activity in physiological conditions.
Leachability of system components presents another significant safety challenge. Enzyme catalysts, cofactors, stabilizers, and degradation products must remain contained within the device architecture to prevent unintended absorption through the skin. Advanced encapsulation technologies utilizing semi-permeable membranes have shown promise in creating selective barriers that retain catalytic components while allowing substrate and product diffusion.
Long-term exposure effects require thorough investigation, as on-body energy harvesting systems are designed for extended wear periods. Chronic toxicity assessments must evaluate potential cumulative effects from prolonged contact with enzyme preparations and their stabilizing matrices. Current research indicates that site-specific enzyme immobilization techniques can reduce protein unfolding and subsequent exposure of potentially immunogenic epitopes, thereby enhancing long-term biocompatibility profiles.
Microbial contamination risk represents a unique challenge for enzyme-based systems operating in the variable conditions of the human body surface. The nutrient-rich microenvironment created by sweat and skin secretions can potentially support microbial growth. Incorporation of antimicrobial peptides and biocompatible preservatives has emerged as a promising approach to maintain system sterility without compromising enzyme stability or catalytic efficiency.
Regulatory frameworks for on-body enzyme catalytic systems remain in development, with current guidelines primarily adapted from medical device and cosmetic product regulations. The FDA and EMA have begun establishing specific protocols for biocompatibility testing of wearable biocatalytic systems, focusing on cytotoxicity, sensitization, and irritation potential. Compliance with ISO 10993 standards for biological evaluation of medical devices provides a foundational framework for safety assessment.
Environmental factors such as temperature fluctuations, pH variations from sweat, and mechanical stress from body movement can potentially alter enzyme behavior and stability profiles, potentially generating unexpected degradation products. Recent advances in computational modeling now enable prediction of enzyme structural changes under various environmental stressors, facilitating proactive safety assessment and design optimization.
Skin contact represents the primary interface for on-body enzyme catalytic systems, making dermal compatibility a critical concern. Enzymes and their stabilizing matrices must not trigger irritation, sensitization, or allergic responses. Recent studies have demonstrated that enzyme immobilization techniques using biocompatible polymers like polyethylene glycol (PEG) and hyaluronic acid derivatives significantly reduce immunogenic potential while maintaining catalytic activity in physiological conditions.
Leachability of system components presents another significant safety challenge. Enzyme catalysts, cofactors, stabilizers, and degradation products must remain contained within the device architecture to prevent unintended absorption through the skin. Advanced encapsulation technologies utilizing semi-permeable membranes have shown promise in creating selective barriers that retain catalytic components while allowing substrate and product diffusion.
Long-term exposure effects require thorough investigation, as on-body energy harvesting systems are designed for extended wear periods. Chronic toxicity assessments must evaluate potential cumulative effects from prolonged contact with enzyme preparations and their stabilizing matrices. Current research indicates that site-specific enzyme immobilization techniques can reduce protein unfolding and subsequent exposure of potentially immunogenic epitopes, thereby enhancing long-term biocompatibility profiles.
Microbial contamination risk represents a unique challenge for enzyme-based systems operating in the variable conditions of the human body surface. The nutrient-rich microenvironment created by sweat and skin secretions can potentially support microbial growth. Incorporation of antimicrobial peptides and biocompatible preservatives has emerged as a promising approach to maintain system sterility without compromising enzyme stability or catalytic efficiency.
Regulatory frameworks for on-body enzyme catalytic systems remain in development, with current guidelines primarily adapted from medical device and cosmetic product regulations. The FDA and EMA have begun establishing specific protocols for biocompatibility testing of wearable biocatalytic systems, focusing on cytotoxicity, sensitization, and irritation potential. Compliance with ISO 10993 standards for biological evaluation of medical devices provides a foundational framework for safety assessment.
Environmental factors such as temperature fluctuations, pH variations from sweat, and mechanical stress from body movement can potentially alter enzyme behavior and stability profiles, potentially generating unexpected degradation products. Recent advances in computational modeling now enable prediction of enzyme structural changes under various environmental stressors, facilitating proactive safety assessment and design optimization.
Scalability and Manufacturing Challenges for Wearable Biocatalysts
The transition from laboratory-scale enzyme catalyst systems to commercially viable wearable biocatalysts presents significant manufacturing challenges. Current production methods for enzyme-based energy harvesting systems remain largely confined to small-scale laboratory settings, with limited scalability for mass production. The primary bottleneck lies in maintaining enzyme stability and activity during large-scale manufacturing processes, which often involve conditions that can denature or deactivate these sensitive biomolecules.
Traditional enzyme immobilization techniques that work effectively at laboratory scale often suffer from inconsistency when scaled up. Batch-to-batch variations in enzyme activity, stability, and loading density become more pronounced at industrial scales, creating significant quality control challenges. Additionally, the specialized equipment required for precise temperature and pH control during manufacturing adds substantial capital costs to production facilities.
Material sourcing represents another critical challenge, as high-purity enzymes suitable for on-body applications remain expensive when procured in commercial quantities. The supply chain for specialized enzymes is often fragmented, with limited suppliers capable of meeting both quality and quantity requirements for wearable technology applications. This creates potential bottlenecks in production scaling and increases dependency on specific suppliers.
Integration of biocatalysts with electronic components and flexible substrates presents unique manufacturing hurdles. Current assembly processes for wearable electronics are not optimized for handling biologically active components, requiring significant modifications to existing production lines. The development of automated manufacturing processes that can precisely deposit and encapsulate enzyme catalysts while maintaining their biological activity remains technically challenging.
Quality control and standardization pose additional complications. Unlike electronic components with well-established testing protocols, enzyme-based systems require specialized biological assays to verify functionality. These testing methods are often time-consuming and difficult to implement in high-throughput manufacturing environments. The lack of industry standards for biocatalyst performance in wearable applications further complicates quality assurance processes.
Cost-effective packaging solutions that maintain enzyme stability while allowing substrate access represent another manufacturing challenge. Current encapsulation technologies that effectively protect enzymes from environmental degradation are often expensive and difficult to implement at scale. The development of economical, mass-producible packaging solutions that balance protection with functionality remains a significant research priority for commercialization efforts.
Traditional enzyme immobilization techniques that work effectively at laboratory scale often suffer from inconsistency when scaled up. Batch-to-batch variations in enzyme activity, stability, and loading density become more pronounced at industrial scales, creating significant quality control challenges. Additionally, the specialized equipment required for precise temperature and pH control during manufacturing adds substantial capital costs to production facilities.
Material sourcing represents another critical challenge, as high-purity enzymes suitable for on-body applications remain expensive when procured in commercial quantities. The supply chain for specialized enzymes is often fragmented, with limited suppliers capable of meeting both quality and quantity requirements for wearable technology applications. This creates potential bottlenecks in production scaling and increases dependency on specific suppliers.
Integration of biocatalysts with electronic components and flexible substrates presents unique manufacturing hurdles. Current assembly processes for wearable electronics are not optimized for handling biologically active components, requiring significant modifications to existing production lines. The development of automated manufacturing processes that can precisely deposit and encapsulate enzyme catalysts while maintaining their biological activity remains technically challenging.
Quality control and standardization pose additional complications. Unlike electronic components with well-established testing protocols, enzyme-based systems require specialized biological assays to verify functionality. These testing methods are often time-consuming and difficult to implement in high-throughput manufacturing environments. The lack of industry standards for biocatalyst performance in wearable applications further complicates quality assurance processes.
Cost-effective packaging solutions that maintain enzyme stability while allowing substrate access represent another manufacturing challenge. Current encapsulation technologies that effectively protect enzymes from environmental degradation are often expensive and difficult to implement at scale. The development of economical, mass-producible packaging solutions that balance protection with functionality remains a significant research priority for commercialization efforts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!