Evaluating Sodium Acetate's Role in Decarbonization Strategies
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Decarbonization Overview
Sodium acetate, a versatile compound with the chemical formula CH3COONa, is emerging as a potential player in decarbonization strategies. This overview explores its role in reducing carbon emissions and promoting sustainable practices across various industries. As global efforts to combat climate change intensify, innovative solutions are sought to decrease reliance on fossil fuels and minimize greenhouse gas emissions.
Sodium acetate's unique properties make it an attractive option for several decarbonization applications. Its ability to store and release thermal energy efficiently positions it as a promising candidate for thermal energy storage systems. These systems are crucial for enhancing the integration of renewable energy sources into the power grid, addressing the intermittency issues associated with solar and wind power.
In the construction industry, sodium acetate is being investigated for its potential in developing phase change materials (PCMs). PCMs can significantly improve building energy efficiency by absorbing excess heat during the day and releasing it at night, reducing the need for artificial heating and cooling. This application could lead to substantial energy savings and decreased carbon footprints in both residential and commercial buildings.
The transportation sector, a major contributor to global carbon emissions, is also exploring sodium acetate's potential. Research is underway to develop sodium acetate-based de-icing solutions for roads and aircraft, which could replace traditional salt-based methods. These eco-friendly alternatives could reduce the environmental impact of winter road maintenance while also minimizing corrosion damage to vehicles and infrastructure.
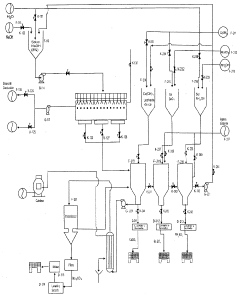
In the realm of industrial processes, sodium acetate is being studied for its role in carbon capture and utilization technologies. Its ability to react with carbon dioxide under specific conditions presents opportunities for developing novel CO2 sequestration methods. This could contribute to reducing emissions from heavy industries and power plants, which are significant sources of greenhouse gases.
Furthermore, sodium acetate's biodegradability and non-toxicity make it an environmentally friendly option for various applications. Its use in sustainable packaging materials and as a food preservative aligns with the growing demand for eco-conscious consumer products. By replacing less sustainable alternatives, sodium acetate contributes to reducing the overall carbon footprint of product lifecycles.
As research and development in this field progress, the full potential of sodium acetate in decarbonization strategies continues to unfold. Its versatility and environmentally friendly characteristics position it as a promising component in the transition towards a low-carbon economy. However, further studies and practical implementations are necessary to fully realize its benefits and overcome any potential limitations in large-scale applications.
Sodium acetate's unique properties make it an attractive option for several decarbonization applications. Its ability to store and release thermal energy efficiently positions it as a promising candidate for thermal energy storage systems. These systems are crucial for enhancing the integration of renewable energy sources into the power grid, addressing the intermittency issues associated with solar and wind power.
In the construction industry, sodium acetate is being investigated for its potential in developing phase change materials (PCMs). PCMs can significantly improve building energy efficiency by absorbing excess heat during the day and releasing it at night, reducing the need for artificial heating and cooling. This application could lead to substantial energy savings and decreased carbon footprints in both residential and commercial buildings.
The transportation sector, a major contributor to global carbon emissions, is also exploring sodium acetate's potential. Research is underway to develop sodium acetate-based de-icing solutions for roads and aircraft, which could replace traditional salt-based methods. These eco-friendly alternatives could reduce the environmental impact of winter road maintenance while also minimizing corrosion damage to vehicles and infrastructure.
In the realm of industrial processes, sodium acetate is being studied for its role in carbon capture and utilization technologies. Its ability to react with carbon dioxide under specific conditions presents opportunities for developing novel CO2 sequestration methods. This could contribute to reducing emissions from heavy industries and power plants, which are significant sources of greenhouse gases.
Furthermore, sodium acetate's biodegradability and non-toxicity make it an environmentally friendly option for various applications. Its use in sustainable packaging materials and as a food preservative aligns with the growing demand for eco-conscious consumer products. By replacing less sustainable alternatives, sodium acetate contributes to reducing the overall carbon footprint of product lifecycles.
As research and development in this field progress, the full potential of sodium acetate in decarbonization strategies continues to unfold. Its versatility and environmentally friendly characteristics position it as a promising component in the transition towards a low-carbon economy. However, further studies and practical implementations are necessary to fully realize its benefits and overcome any potential limitations in large-scale applications.
Market Demand Analysis
The market demand for sodium acetate in decarbonization strategies has been steadily increasing as industries and governments worldwide seek sustainable solutions to reduce carbon emissions. This compound, traditionally used in various industrial applications, is now gaining attention for its potential role in carbon capture and utilization (CCU) technologies.
In the energy sector, sodium acetate is being explored as a phase change material (PCM) for thermal energy storage systems. These systems are crucial for enhancing the efficiency of renewable energy sources, particularly in solar thermal power plants. The global thermal energy storage market is projected to grow significantly, driven by the increasing adoption of renewable energy and the need for grid stability.
The construction industry is another key market for sodium acetate in decarbonization efforts. As a concrete admixture, it can potentially reduce the carbon footprint of cement production, which accounts for approximately 8% of global CO2 emissions. With the growing emphasis on green building practices and stringent environmental regulations, the demand for low-carbon construction materials is expected to rise substantially in the coming years.
In the transportation sector, sodium acetate is being investigated for its use in electric vehicle battery technologies. As the automotive industry shifts towards electrification to meet emission reduction targets, the demand for advanced battery materials is soaring. Sodium-based batteries are emerging as a potential alternative to lithium-ion batteries, offering advantages in cost and resource availability.
The chemical industry is also exploring sodium acetate as a feedstock for producing bio-based chemicals and materials. This aligns with the growing trend of circular economy principles and the shift away from fossil-based raw materials. The bio-based chemicals market is expected to expand rapidly, driven by consumer preferences for sustainable products and regulatory pressures to reduce carbon emissions.
Furthermore, the agriculture sector is showing interest in sodium acetate for its potential in carbon sequestration and soil amendment applications. As governments implement policies to promote carbon farming and sustainable agricultural practices, the demand for innovative soil additives is likely to increase.
The market potential for sodium acetate in decarbonization strategies is closely tied to global climate policies and commitments. As countries strive to meet their Paris Agreement targets and corporate entities set ambitious net-zero goals, the demand for effective carbon reduction technologies is expected to surge. This creates a favorable environment for the adoption of sodium acetate-based solutions across multiple industries.
In the energy sector, sodium acetate is being explored as a phase change material (PCM) for thermal energy storage systems. These systems are crucial for enhancing the efficiency of renewable energy sources, particularly in solar thermal power plants. The global thermal energy storage market is projected to grow significantly, driven by the increasing adoption of renewable energy and the need for grid stability.
The construction industry is another key market for sodium acetate in decarbonization efforts. As a concrete admixture, it can potentially reduce the carbon footprint of cement production, which accounts for approximately 8% of global CO2 emissions. With the growing emphasis on green building practices and stringent environmental regulations, the demand for low-carbon construction materials is expected to rise substantially in the coming years.
In the transportation sector, sodium acetate is being investigated for its use in electric vehicle battery technologies. As the automotive industry shifts towards electrification to meet emission reduction targets, the demand for advanced battery materials is soaring. Sodium-based batteries are emerging as a potential alternative to lithium-ion batteries, offering advantages in cost and resource availability.
The chemical industry is also exploring sodium acetate as a feedstock for producing bio-based chemicals and materials. This aligns with the growing trend of circular economy principles and the shift away from fossil-based raw materials. The bio-based chemicals market is expected to expand rapidly, driven by consumer preferences for sustainable products and regulatory pressures to reduce carbon emissions.
Furthermore, the agriculture sector is showing interest in sodium acetate for its potential in carbon sequestration and soil amendment applications. As governments implement policies to promote carbon farming and sustainable agricultural practices, the demand for innovative soil additives is likely to increase.
The market potential for sodium acetate in decarbonization strategies is closely tied to global climate policies and commitments. As countries strive to meet their Paris Agreement targets and corporate entities set ambitious net-zero goals, the demand for effective carbon reduction technologies is expected to surge. This creates a favorable environment for the adoption of sodium acetate-based solutions across multiple industries.
Technical Challenges and Limitations
While sodium acetate shows promise in decarbonization strategies, several technical challenges and limitations must be addressed for its widespread adoption. One significant hurdle is the energy-intensive production process of sodium acetate, which currently relies heavily on fossil fuels. This dependency contradicts the ultimate goal of reducing carbon emissions, necessitating the development of more sustainable manufacturing methods.
The storage and transportation of sodium acetate present another set of challenges. As a hygroscopic substance, it readily absorbs moisture from the air, potentially compromising its effectiveness and stability during long-term storage. This characteristic requires specialized packaging and handling procedures, adding complexity and cost to its implementation in various applications.
Scalability remains a critical issue for sodium acetate-based decarbonization solutions. While effective on a small scale, the transition to industrial-level applications faces obstacles in terms of infrastructure requirements and process optimization. The need for large-scale production facilities and distribution networks poses significant logistical and economic challenges.
The thermal properties of sodium acetate, particularly its phase change behavior, present both opportunities and limitations. While its ability to store and release latent heat is advantageous for thermal energy storage, the precise control of crystallization and melting processes in large-scale systems can be technically demanding. Ensuring uniform heat distribution and preventing supercooling in extensive applications requires sophisticated engineering solutions.
Compatibility with existing infrastructure is another limitation. Many current industrial processes and energy systems are not designed to integrate sodium acetate-based technologies seamlessly. Retrofitting or replacing established systems to accommodate sodium acetate solutions can be costly and time-consuming, potentially slowing down adoption rates.
The environmental impact of increased sodium acetate production and use must also be carefully evaluated. While it offers benefits in terms of carbon reduction, the potential effects on local ecosystems, water resources, and soil chemistry need thorough assessment to ensure that the solution does not create new environmental challenges while addressing carbon emissions.
Lastly, the economic viability of sodium acetate in decarbonization strategies remains a significant hurdle. The cost of production, implementation, and maintenance of sodium acetate-based systems must be competitive with alternative low-carbon technologies to encourage widespread adoption. Achieving cost-effectiveness while maintaining performance and reliability is a complex challenge that requires ongoing research and development efforts.
The storage and transportation of sodium acetate present another set of challenges. As a hygroscopic substance, it readily absorbs moisture from the air, potentially compromising its effectiveness and stability during long-term storage. This characteristic requires specialized packaging and handling procedures, adding complexity and cost to its implementation in various applications.
Scalability remains a critical issue for sodium acetate-based decarbonization solutions. While effective on a small scale, the transition to industrial-level applications faces obstacles in terms of infrastructure requirements and process optimization. The need for large-scale production facilities and distribution networks poses significant logistical and economic challenges.
The thermal properties of sodium acetate, particularly its phase change behavior, present both opportunities and limitations. While its ability to store and release latent heat is advantageous for thermal energy storage, the precise control of crystallization and melting processes in large-scale systems can be technically demanding. Ensuring uniform heat distribution and preventing supercooling in extensive applications requires sophisticated engineering solutions.
Compatibility with existing infrastructure is another limitation. Many current industrial processes and energy systems are not designed to integrate sodium acetate-based technologies seamlessly. Retrofitting or replacing established systems to accommodate sodium acetate solutions can be costly and time-consuming, potentially slowing down adoption rates.
The environmental impact of increased sodium acetate production and use must also be carefully evaluated. While it offers benefits in terms of carbon reduction, the potential effects on local ecosystems, water resources, and soil chemistry need thorough assessment to ensure that the solution does not create new environmental challenges while addressing carbon emissions.
Lastly, the economic viability of sodium acetate in decarbonization strategies remains a significant hurdle. The cost of production, implementation, and maintenance of sodium acetate-based systems must be competitive with alternative low-carbon technologies to encourage widespread adoption. Achieving cost-effectiveness while maintaining performance and reliability is a complex challenge that requires ongoing research and development efforts.
Current Decarbonization Solutions
01 Use of sodium acetate in heat storage materials
Sodium acetate is utilized in heat storage materials due to its phase change properties. It can absorb and release heat during phase transitions, making it suitable for thermal energy storage applications. These materials can be used in various heating and cooling systems to improve energy efficiency.- Sodium acetate in chemical processes: Sodium acetate is widely used in various chemical processes as a reagent, buffer, or catalyst. It plays a role in reactions such as acetylation, esterification, and pH control. Its properties make it valuable in industrial applications, including the production of pharmaceuticals, textiles, and other chemical compounds.
- Sodium acetate in heat storage applications: Sodium acetate trihydrate is utilized in heat storage systems due to its phase change properties. It can absorb and release heat during phase transitions, making it suitable for thermal energy storage in various applications, including heating and cooling systems, temperature-controlled packaging, and thermal management in electronics.
- Sodium acetate in food and beverage industry: Sodium acetate is used as a food additive and preservative in the food and beverage industry. It acts as a acidity regulator, flavoring agent, and antimicrobial preservative. Its applications include enhancing flavors, extending shelf life, and maintaining the quality of various food products.
- Sodium acetate in wastewater treatment: Sodium acetate is employed in wastewater treatment processes, particularly in biological treatment systems. It serves as a carbon source for microorganisms involved in denitrification and other biological processes, helping to improve the efficiency of nutrient removal and overall water treatment.
- Sodium acetate in material science and manufacturing: Sodium acetate finds applications in material science and manufacturing processes. It is used in the production of certain polymers, as a component in adhesives and sealants, and in the treatment of textiles and leather. Its properties contribute to improving material characteristics and enhancing product performance in various industries.
02 Application of sodium acetate in food preservation
Sodium acetate is employed as a food preservative and pH regulator in the food industry. It helps extend the shelf life of various food products by inhibiting microbial growth and maintaining optimal acidity levels. This compound is particularly useful in preserving processed foods and beverages.Expand Specific Solutions03 Sodium acetate in chemical synthesis processes
Sodium acetate serves as a reagent or catalyst in various chemical synthesis processes. It is used in the production of organic compounds, pharmaceuticals, and other industrial chemicals. The compound's properties make it valuable in reactions such as acetylation and as a buffer in chemical processes.Expand Specific Solutions04 Use of sodium acetate in textile and leather industries
Sodium acetate finds applications in textile and leather processing. It is used as a dyeing auxiliary, helping to improve color fastness and dye penetration. In the leather industry, it can be employed in tanning processes to enhance leather quality and durability.Expand Specific Solutions05 Sodium acetate in environmental applications
Sodium acetate is utilized in various environmental applications, including wastewater treatment and air pollution control. It can be used as a deicer for roads and runways, offering a more environmentally friendly alternative to traditional deicing agents. Additionally, it plays a role in certain bioremediation processes for contaminated soils and water.Expand Specific Solutions
Key Industry Players
The competition landscape for evaluating sodium acetate's role in decarbonization strategies is in its early stages, with the market still developing. Major players like China Petroleum & Chemical Corp., Saudi Arabian Oil Co., and ExxonMobil are exploring this technology alongside innovative startups such as Climeworks AG. The market size is expected to grow as decarbonization efforts intensify globally. Technical maturity varies, with established petrochemical companies leveraging their expertise in chemical processes, while newer entrants focus on novel carbon capture and utilization methods. Research institutions like IFP Energies Nouvelles and the University of Leeds are contributing to advancing the technology, indicating a collaborative approach to development in this emerging field.
Climeworks AG
Technical Solution: Climeworks AG has developed a direct air capture (DAC) technology that can remove CO2 directly from the atmosphere. While not directly related to sodium acetate, their approach complements decarbonization strategies. Their DAC plants use a two-step process: first, air is drawn into the collector with a fan and CO2 is captured on a highly selective filter material. Once the filter is saturated, the collector is closed and the temperature is increased to around 100°C, which releases high-purity CO2. This CO2 can then be utilized in various applications or permanently stored underground[1][2]. The company has successfully scaled up its technology, with the world's largest DAC plant, Orca, operational in Iceland since 2021[3].
Strengths: Proven technology for direct CO2 removal from air, scalable solution, potential for negative emissions. Weaknesses: High energy requirements, currently limited scale compared to global emissions, relatively high cost per ton of CO2 removed.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has been exploring various decarbonization strategies, including the use of carbon capture, utilization, and storage (CCUS) technologies. While not specifically focused on sodium acetate, their approach to CCUS could potentially incorporate sodium acetate-based solutions. Sinopec has implemented CCUS projects at several of its facilities, including the Qilu-Shengli Oilfield CCUS project, which can reduce carbon emissions by 1 million tons per year[4]. The company has also invested in research and development of new materials and catalysts for CO2 capture and conversion, which could potentially include sodium acetate-based technologies in the future[5].
Strengths: Large-scale implementation of CCUS technologies, significant R&D capabilities, potential for integration with existing petrochemical infrastructure. Weaknesses: Primary focus on traditional fossil fuel business, which may slow transition to low-carbon technologies.
Innovative Sodium Acetate Applications
Acetate of sodium and alkaline earth metal, process of preparation and use
PatentInactiveEP0603548A1
Innovation
- Alkaline earth sodium acetate with specific composition and production processes that enhance its defrosting performance, speed, and environmental friendliness, including mixing with up to 50% alkaline earth or alkali metal carboxylates, and using various reaction methods to achieve granulation and drying.
Process for the removal of acid gases from the air and from combustion gases from burners and internal combustion engines by means of absorption with sodium hydroxide solution and process for obtaining sodium carbonate in order to acquire carbon cred
PatentWO2011122925A1
Innovation
- A chemical process using a 2N sodium hydroxide solution to absorb acid gases in a horizontal absorber, converting CO2 into sodium carbonate, which can be further processed to produce sodium carbonate, thereby accrediting carbon credits through the capture and commercialization of CO2.
Environmental Impact Assessment
The environmental impact assessment of sodium acetate's role in decarbonization strategies reveals both promising benefits and potential concerns. Sodium acetate, a salt formed by the combination of sodium and acetic acid, has gained attention for its potential applications in carbon capture and storage (CCS) technologies.
One of the primary environmental advantages of sodium acetate in decarbonization efforts is its ability to absorb carbon dioxide efficiently. When used in CCS systems, sodium acetate can capture CO2 from industrial emissions, potentially reducing greenhouse gas releases into the atmosphere. This property makes it a valuable tool in the fight against climate change, particularly in hard-to-abate sectors such as cement and steel production.
Furthermore, sodium acetate is considered a relatively safe and non-toxic compound, which minimizes the risk of environmental contamination during its production, transportation, and use. Its biodegradability also ensures that any accidental releases would have limited long-term impacts on ecosystems.
However, the environmental impact assessment also highlights some potential drawbacks. The production of sodium acetate requires energy and resources, which could offset some of its carbon reduction benefits if not carefully managed. The manufacturing process may involve the use of fossil fuels or energy-intensive methods, potentially contributing to carbon emissions in the short term.
Water usage is another environmental consideration. Some CCS technologies utilizing sodium acetate may require significant amounts of water, which could strain local water resources, particularly in water-scarce regions. This aspect necessitates careful planning and implementation to ensure sustainable water management practices.
The disposal or regeneration of spent sodium acetate solutions also presents environmental challenges. While the compound itself is biodegradable, the captured carbon and any impurities accumulated during the CCS process must be properly managed to prevent unintended releases back into the environment.
Land use changes associated with large-scale sodium acetate production and CCS facilities must also be considered. The development of new industrial sites for these purposes could potentially impact local ecosystems and biodiversity, requiring thorough environmental impact studies and mitigation measures.
In conclusion, while sodium acetate shows promise in decarbonization strategies, its environmental impact is multifaceted. The positive effects on carbon capture must be weighed against the potential negative impacts of its production and use. Sustainable implementation will require careful lifecycle assessments, optimization of production processes, and integration with renewable energy sources to maximize its net positive environmental impact in the fight against climate change.
One of the primary environmental advantages of sodium acetate in decarbonization efforts is its ability to absorb carbon dioxide efficiently. When used in CCS systems, sodium acetate can capture CO2 from industrial emissions, potentially reducing greenhouse gas releases into the atmosphere. This property makes it a valuable tool in the fight against climate change, particularly in hard-to-abate sectors such as cement and steel production.
Furthermore, sodium acetate is considered a relatively safe and non-toxic compound, which minimizes the risk of environmental contamination during its production, transportation, and use. Its biodegradability also ensures that any accidental releases would have limited long-term impacts on ecosystems.
However, the environmental impact assessment also highlights some potential drawbacks. The production of sodium acetate requires energy and resources, which could offset some of its carbon reduction benefits if not carefully managed. The manufacturing process may involve the use of fossil fuels or energy-intensive methods, potentially contributing to carbon emissions in the short term.
Water usage is another environmental consideration. Some CCS technologies utilizing sodium acetate may require significant amounts of water, which could strain local water resources, particularly in water-scarce regions. This aspect necessitates careful planning and implementation to ensure sustainable water management practices.
The disposal or regeneration of spent sodium acetate solutions also presents environmental challenges. While the compound itself is biodegradable, the captured carbon and any impurities accumulated during the CCS process must be properly managed to prevent unintended releases back into the environment.
Land use changes associated with large-scale sodium acetate production and CCS facilities must also be considered. The development of new industrial sites for these purposes could potentially impact local ecosystems and biodiversity, requiring thorough environmental impact studies and mitigation measures.
In conclusion, while sodium acetate shows promise in decarbonization strategies, its environmental impact is multifaceted. The positive effects on carbon capture must be weighed against the potential negative impacts of its production and use. Sustainable implementation will require careful lifecycle assessments, optimization of production processes, and integration with renewable energy sources to maximize its net positive environmental impact in the fight against climate change.
Regulatory Framework Analysis
The regulatory framework surrounding sodium acetate's role in decarbonization strategies is complex and evolving, reflecting the growing emphasis on sustainable practices and carbon reduction across industries. At the international level, the Paris Agreement serves as a cornerstone for climate action, setting the stage for national and regional policies that impact the use of sodium acetate in decarbonization efforts.
In the European Union, the European Green Deal provides a comprehensive framework for climate and environmental regulations. The EU Emissions Trading System (ETS) and the Carbon Border Adjustment Mechanism (CBAM) are key instruments that indirectly influence the adoption of sodium acetate-based solutions. These mechanisms create economic incentives for industries to reduce their carbon footprint, potentially driving the demand for innovative decarbonization technologies.
The United States has a more fragmented regulatory approach, with a mix of federal and state-level policies. The Environmental Protection Agency (EPA) plays a crucial role in setting standards and regulations that may affect the use of sodium acetate in industrial processes. The Clean Air Act and its amendments provide the legal basis for many of these regulations, while recent initiatives like the Inflation Reduction Act offer incentives for clean energy and carbon capture technologies.
In Asia, countries like China and Japan have implemented their own regulatory frameworks to address climate change. China's national emissions trading scheme, the largest in the world, creates a market-based approach to reducing carbon emissions. This system could potentially influence the adoption of sodium acetate-based technologies in various industries.
Specific regulations governing the use of sodium acetate in decarbonization strategies are still emerging. However, broader chemical safety regulations, such as REACH in the EU and TSCA in the US, impact the production, handling, and application of sodium acetate. These regulations ensure that the environmental and health impacts of the compound are thoroughly assessed.
As decarbonization efforts intensify, it is likely that more targeted regulations will emerge. These may include standards for carbon capture efficiency, guidelines for the integration of sodium acetate in thermal energy storage systems, or requirements for lifecycle assessments of sodium acetate-based solutions. Industry stakeholders should closely monitor regulatory developments to ensure compliance and capitalize on potential opportunities.
The regulatory landscape also includes voluntary standards and certification schemes that can influence the adoption of sodium acetate in decarbonization strategies. These include ISO standards related to environmental management and energy efficiency, as well as industry-specific initiatives that promote sustainable practices.
In the European Union, the European Green Deal provides a comprehensive framework for climate and environmental regulations. The EU Emissions Trading System (ETS) and the Carbon Border Adjustment Mechanism (CBAM) are key instruments that indirectly influence the adoption of sodium acetate-based solutions. These mechanisms create economic incentives for industries to reduce their carbon footprint, potentially driving the demand for innovative decarbonization technologies.
The United States has a more fragmented regulatory approach, with a mix of federal and state-level policies. The Environmental Protection Agency (EPA) plays a crucial role in setting standards and regulations that may affect the use of sodium acetate in industrial processes. The Clean Air Act and its amendments provide the legal basis for many of these regulations, while recent initiatives like the Inflation Reduction Act offer incentives for clean energy and carbon capture technologies.
In Asia, countries like China and Japan have implemented their own regulatory frameworks to address climate change. China's national emissions trading scheme, the largest in the world, creates a market-based approach to reducing carbon emissions. This system could potentially influence the adoption of sodium acetate-based technologies in various industries.
Specific regulations governing the use of sodium acetate in decarbonization strategies are still emerging. However, broader chemical safety regulations, such as REACH in the EU and TSCA in the US, impact the production, handling, and application of sodium acetate. These regulations ensure that the environmental and health impacts of the compound are thoroughly assessed.
As decarbonization efforts intensify, it is likely that more targeted regulations will emerge. These may include standards for carbon capture efficiency, guidelines for the integration of sodium acetate in thermal energy storage systems, or requirements for lifecycle assessments of sodium acetate-based solutions. Industry stakeholders should closely monitor regulatory developments to ensure compliance and capitalize on potential opportunities.
The regulatory landscape also includes voluntary standards and certification schemes that can influence the adoption of sodium acetate in decarbonization strategies. These include ISO standards related to environmental management and energy efficiency, as well as industry-specific initiatives that promote sustainable practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!