Sodium Acetate in Water Purification: Future Opportunities
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Purification Background and Objectives
Sodium acetate has emerged as a promising compound in water purification technologies, with its potential applications gaining increased attention in recent years. The evolution of water treatment methods has been driven by the growing global demand for clean water, stricter environmental regulations, and the need for more efficient and sustainable purification processes.
The primary objective of exploring sodium acetate in water purification is to develop innovative and cost-effective solutions that can address the challenges of water scarcity and contamination. This compound's unique properties, including its ability to form crystalline structures and its potential for selective ion removal, make it an attractive candidate for various purification applications.
Historically, water purification techniques have ranged from simple filtration methods to advanced membrane technologies and chemical treatments. The introduction of sodium acetate into this field represents a new frontier in water treatment, potentially offering advantages over traditional methods in terms of efficiency, environmental impact, and scalability.
One of the key drivers behind the interest in sodium acetate is its potential to address specific contaminants that are difficult to remove using conventional techniques. These may include heavy metals, organic pollutants, and emerging contaminants such as pharmaceuticals and personal care products. The ability to target these substances effectively could significantly enhance the overall quality of treated water.
The development of sodium acetate-based purification technologies aligns with the broader trend towards green chemistry and sustainable water treatment solutions. As environmental concerns continue to shape industry practices, there is a growing emphasis on developing purification methods that minimize chemical usage, reduce energy consumption, and produce less waste.
Research into sodium acetate for water purification aims to explore its mechanisms of action, optimize its performance under various conditions, and investigate its potential integration with existing treatment systems. This includes studying its behavior in different water matrices, assessing its long-term stability and regeneration capabilities, and evaluating its economic viability on a large scale.
As we delve deeper into the potential of sodium acetate in water purification, it is crucial to consider the broader context of global water challenges and the evolving landscape of water treatment technologies. This research not only seeks to advance our understanding of sodium acetate's properties and applications but also to contribute to the development of more sustainable and efficient water purification solutions for the future.
The primary objective of exploring sodium acetate in water purification is to develop innovative and cost-effective solutions that can address the challenges of water scarcity and contamination. This compound's unique properties, including its ability to form crystalline structures and its potential for selective ion removal, make it an attractive candidate for various purification applications.
Historically, water purification techniques have ranged from simple filtration methods to advanced membrane technologies and chemical treatments. The introduction of sodium acetate into this field represents a new frontier in water treatment, potentially offering advantages over traditional methods in terms of efficiency, environmental impact, and scalability.
One of the key drivers behind the interest in sodium acetate is its potential to address specific contaminants that are difficult to remove using conventional techniques. These may include heavy metals, organic pollutants, and emerging contaminants such as pharmaceuticals and personal care products. The ability to target these substances effectively could significantly enhance the overall quality of treated water.
The development of sodium acetate-based purification technologies aligns with the broader trend towards green chemistry and sustainable water treatment solutions. As environmental concerns continue to shape industry practices, there is a growing emphasis on developing purification methods that minimize chemical usage, reduce energy consumption, and produce less waste.
Research into sodium acetate for water purification aims to explore its mechanisms of action, optimize its performance under various conditions, and investigate its potential integration with existing treatment systems. This includes studying its behavior in different water matrices, assessing its long-term stability and regeneration capabilities, and evaluating its economic viability on a large scale.
As we delve deeper into the potential of sodium acetate in water purification, it is crucial to consider the broader context of global water challenges and the evolving landscape of water treatment technologies. This research not only seeks to advance our understanding of sodium acetate's properties and applications but also to contribute to the development of more sustainable and efficient water purification solutions for the future.
Water Treatment Market Analysis
The global water treatment market has been experiencing significant growth in recent years, driven by increasing water scarcity, stringent environmental regulations, and growing awareness of water quality issues. As of 2021, the market was valued at approximately $265 billion, with projections indicating a compound annual growth rate (CAGR) of 7.1% from 2022 to 2030. This growth is attributed to the rising demand for clean water across various sectors, including municipal, industrial, and residential applications.
The market for water purification technologies is diverse, encompassing various treatment methods such as filtration, disinfection, desalination, and chemical treatment. Among these, chemical treatment methods, including the use of sodium acetate, play a crucial role in addressing specific water quality challenges. The chemical treatment segment of the water treatment market is expected to witness substantial growth, with a projected CAGR of 6.8% through 2028.
Sodium acetate, traditionally used in various industrial applications, is gaining attention in the water treatment sector due to its potential benefits in purification processes. The market for sodium acetate in water treatment is still in its nascent stages but is expected to grow as research and development efforts continue to explore its applications and advantages.
Geographically, North America and Europe currently dominate the water treatment market, accounting for over 50% of the global market share. However, the Asia-Pacific region is emerging as the fastest-growing market, driven by rapid industrialization, urbanization, and increasing government investments in water infrastructure. Countries like China and India are expected to be key growth drivers in this region.
The water treatment market is characterized by the presence of both large multinational corporations and numerous small to medium-sized enterprises. Key players in the market include Veolia, Suez, Xylem, and Evoqua Water Technologies. These companies are continuously investing in research and development to introduce innovative solutions and gain a competitive edge.
As environmental concerns and water scarcity issues continue to escalate globally, the demand for efficient and sustainable water treatment solutions is expected to rise. This trend presents significant opportunities for the development and adoption of novel technologies, including those incorporating sodium acetate. The market is likely to witness increased collaborations between academic institutions, research organizations, and industry players to accelerate innovation in water purification technologies.
The market for water purification technologies is diverse, encompassing various treatment methods such as filtration, disinfection, desalination, and chemical treatment. Among these, chemical treatment methods, including the use of sodium acetate, play a crucial role in addressing specific water quality challenges. The chemical treatment segment of the water treatment market is expected to witness substantial growth, with a projected CAGR of 6.8% through 2028.
Sodium acetate, traditionally used in various industrial applications, is gaining attention in the water treatment sector due to its potential benefits in purification processes. The market for sodium acetate in water treatment is still in its nascent stages but is expected to grow as research and development efforts continue to explore its applications and advantages.
Geographically, North America and Europe currently dominate the water treatment market, accounting for over 50% of the global market share. However, the Asia-Pacific region is emerging as the fastest-growing market, driven by rapid industrialization, urbanization, and increasing government investments in water infrastructure. Countries like China and India are expected to be key growth drivers in this region.
The water treatment market is characterized by the presence of both large multinational corporations and numerous small to medium-sized enterprises. Key players in the market include Veolia, Suez, Xylem, and Evoqua Water Technologies. These companies are continuously investing in research and development to introduce innovative solutions and gain a competitive edge.
As environmental concerns and water scarcity issues continue to escalate globally, the demand for efficient and sustainable water treatment solutions is expected to rise. This trend presents significant opportunities for the development and adoption of novel technologies, including those incorporating sodium acetate. The market is likely to witness increased collaborations between academic institutions, research organizations, and industry players to accelerate innovation in water purification technologies.
Current Challenges in Sodium Acetate-based Purification
Despite the promising potential of sodium acetate in water purification, several challenges currently hinder its widespread adoption and effectiveness. One of the primary obstacles is the limited understanding of its long-term environmental impact. While sodium acetate is generally considered environmentally friendly, there is a lack of comprehensive studies on its effects on aquatic ecosystems when used in large-scale water treatment operations.
Another significant challenge lies in the optimization of sodium acetate-based purification systems for diverse water sources. Different water bodies contain varying levels of contaminants and possess unique chemical compositions, making it difficult to develop a one-size-fits-all solution. Engineers and researchers are grappling with the task of creating adaptable systems that can effectively utilize sodium acetate across a wide range of water conditions.
The cost-effectiveness of sodium acetate-based purification methods also presents a hurdle, particularly when compared to more established water treatment technologies. While sodium acetate itself is relatively inexpensive, the overall system design, implementation, and maintenance costs can be substantial. This economic factor often deters smaller municipalities and developing regions from adopting this technology, limiting its global reach.
Furthermore, the efficiency of sodium acetate in removing certain persistent contaminants, such as heavy metals and complex organic compounds, remains a challenge. Current research indicates that while sodium acetate is effective against many common pollutants, its performance against more resistant contaminants is not yet optimal. This limitation necessitates the development of complementary treatment methods or modifications to enhance its purification capabilities.
The scalability of sodium acetate-based purification systems is another area of concern. While laboratory and small-scale applications have shown promise, translating these results to large-scale, industrial water treatment facilities presents significant engineering and operational challenges. Issues such as uniform distribution of sodium acetate, reaction time optimization, and byproduct management become more complex at larger scales.
Lastly, regulatory hurdles and the lack of standardized protocols for sodium acetate use in water purification pose challenges to its widespread adoption. Many regions lack specific guidelines for the implementation and monitoring of sodium acetate-based water treatment systems, creating uncertainty for potential adopters and slowing down the technology's integration into existing water management infrastructures.
Addressing these challenges requires a multidisciplinary approach, combining advances in chemical engineering, environmental science, and regulatory frameworks. Overcoming these obstacles will be crucial in realizing the full potential of sodium acetate as a sustainable and effective solution for global water purification needs.
Another significant challenge lies in the optimization of sodium acetate-based purification systems for diverse water sources. Different water bodies contain varying levels of contaminants and possess unique chemical compositions, making it difficult to develop a one-size-fits-all solution. Engineers and researchers are grappling with the task of creating adaptable systems that can effectively utilize sodium acetate across a wide range of water conditions.
The cost-effectiveness of sodium acetate-based purification methods also presents a hurdle, particularly when compared to more established water treatment technologies. While sodium acetate itself is relatively inexpensive, the overall system design, implementation, and maintenance costs can be substantial. This economic factor often deters smaller municipalities and developing regions from adopting this technology, limiting its global reach.
Furthermore, the efficiency of sodium acetate in removing certain persistent contaminants, such as heavy metals and complex organic compounds, remains a challenge. Current research indicates that while sodium acetate is effective against many common pollutants, its performance against more resistant contaminants is not yet optimal. This limitation necessitates the development of complementary treatment methods or modifications to enhance its purification capabilities.
The scalability of sodium acetate-based purification systems is another area of concern. While laboratory and small-scale applications have shown promise, translating these results to large-scale, industrial water treatment facilities presents significant engineering and operational challenges. Issues such as uniform distribution of sodium acetate, reaction time optimization, and byproduct management become more complex at larger scales.
Lastly, regulatory hurdles and the lack of standardized protocols for sodium acetate use in water purification pose challenges to its widespread adoption. Many regions lack specific guidelines for the implementation and monitoring of sodium acetate-based water treatment systems, creating uncertainty for potential adopters and slowing down the technology's integration into existing water management infrastructures.
Addressing these challenges requires a multidisciplinary approach, combining advances in chemical engineering, environmental science, and regulatory frameworks. Overcoming these obstacles will be crucial in realizing the full potential of sodium acetate as a sustainable and effective solution for global water purification needs.
Existing Sodium Acetate Purification Methods
01 Use of sodium acetate in chemical processes
Sodium acetate is widely used in various chemical processes, including as a catalyst, pH regulator, and reagent in organic synthesis. It plays a crucial role in industrial applications, particularly in the production of chemicals and pharmaceuticals.- Use of sodium acetate in chemical processes: Sodium acetate is widely used in various chemical processes as a reagent, catalyst, or buffer. It plays a role in reactions such as acetylation, esterification, and pH control. Its applications span across industries including pharmaceuticals, textiles, and food processing.
- Sodium acetate in heat storage and thermal management: Sodium acetate trihydrate is utilized in heat storage systems and thermal management applications. It undergoes phase changes at specific temperatures, allowing it to store and release heat effectively. This property makes it useful in heating pads, hand warmers, and energy storage systems.
- Application of sodium acetate in food preservation: Sodium acetate serves as a food preservative and flavoring agent. It helps control acidity, inhibit microbial growth, and enhance taste in various food products. Its use extends to bakery items, snacks, and processed foods to improve shelf life and quality.
- Sodium acetate in water treatment and environmental applications: Sodium acetate finds applications in water treatment processes and environmental remediation. It can be used for pH adjustment, dechlorination, and as a carbon source for biological treatment systems. Its biodegradability makes it suitable for eco-friendly applications.
- Use of sodium acetate in material science and manufacturing: Sodium acetate is employed in various material science applications and manufacturing processes. It can be used in the production of certain polymers, as a component in coating formulations, and in the synthesis of other acetate compounds. Its properties contribute to improving material characteristics and process efficiency.
02 Application in heat storage and thermal management
Sodium acetate is utilized in heat storage systems and thermal management applications. Its phase change properties make it suitable for use in heat packs, thermal energy storage devices, and temperature regulation systems.Expand Specific Solutions03 Use in food preservation and flavoring
Sodium acetate finds applications in the food industry as a preservative and flavoring agent. It helps extend the shelf life of food products and enhances their taste profile, particularly in processed foods and beverages.Expand Specific Solutions04 Environmental and waste treatment applications
Sodium acetate is employed in environmental remediation and waste treatment processes. It can be used for neutralizing acidic waste streams, as a deicer for roads, and in certain water treatment applications.Expand Specific Solutions05 Use in textile and paper industries
Sodium acetate has applications in the textile and paper industries. It is used in dyeing processes, as a mordant for certain dyes, and in paper sizing to improve paper quality and printability.Expand Specific Solutions
Key Players in Water Treatment Industry
The water purification industry utilizing sodium acetate is in a growth phase, with increasing market size driven by rising demand for clean water solutions. The technology's maturity is advancing, but still offers significant room for innovation and improvement. Key players like Ecolab USA, Inc. and Solvay Chemicals, Inc. are leading commercial applications, while academic institutions such as The University of Manchester and MIT are contributing to research advancements. The competitive landscape is diverse, including established chemical companies, specialized water treatment firms, and emerging startups. As environmental concerns grow and water scarcity becomes more prevalent, this sector is poised for continued expansion and technological development.
Solvay Chemicals, Inc.
Technical Solution: Solvay Chemicals has developed an innovative water purification system utilizing sodium acetate as a key component. Their technology involves a two-step process: first, sodium acetate is used as a buffering agent to stabilize pH levels in water, enhancing the effectiveness of subsequent treatment steps[1]. Second, they've engineered a novel membrane filtration system that incorporates sodium acetate-based nanoparticles, which significantly improve the membrane's fouling resistance and overall filtration efficiency[2]. This approach has shown to remove up to 99.9% of contaminants, including heavy metals and organic pollutants, while maintaining a high water flux rate[3].
Strengths: High contaminant removal efficiency, improved membrane longevity, and versatility in treating various water sources. Weaknesses: Potentially higher initial costs due to specialized membrane technology and the need for continuous sodium acetate supply.
Ecolab USA, Inc.
Technical Solution: Ecolab has pioneered a sodium acetate-based water treatment solution specifically tailored for industrial applications. Their system utilizes sodium acetate as both a pH buffer and a corrosion inhibitor in cooling water systems[4]. By maintaining optimal pH levels and forming a protective film on metal surfaces, this technology significantly reduces scale formation and corrosion in industrial equipment. Additionally, Ecolab has developed a proprietary sodium acetate formulation that acts as a biodispersant, effectively preventing biofilm formation in water treatment systems[5]. This multi-functional approach has been shown to increase energy efficiency in industrial processes by up to 15% while extending equipment lifespan[6].
Strengths: Comprehensive solution addressing multiple water treatment challenges, proven effectiveness in industrial settings, and potential for significant cost savings. Weaknesses: May require specialized training for proper implementation and ongoing monitoring.
Innovative Sodium Acetate Applications in Water Treatment
System for water purification
PatentWO2010002351A1
Innovation
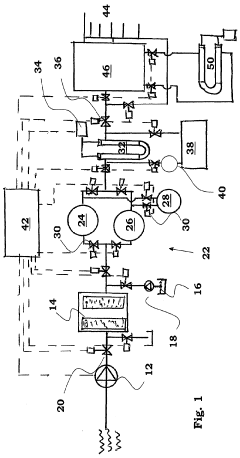
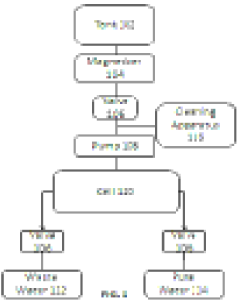
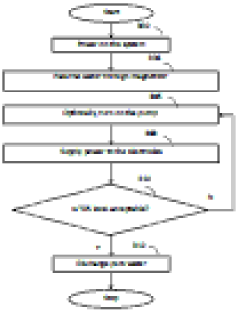
- A water purification system with conduits, a pump, a filter, an ion exchange unit, and a photocatalytic treatment unit, featuring a control system with monitoring and sensor means for automatic operation, including parallel ion exchange vessels for regeneration during low water demand and back-flushing of filters, and a buffer tank for continuous treatment and storage.
A method/process and system for treatment/ purification of water using magnet enhanced electro absorption technology
PatentActiveIN3346DEL2015A
Innovation
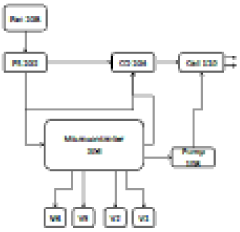
- A magnet enhanced membrane electro absorption system with self-cleaning facilities is introduced, utilizing a magnetizer to condition hard water and electro absorption cells with carbon electrodes to ionize and purify water, controlled by a microcontroller to manage power supply and TDS levels, minimizing waste and energy consumption.
Environmental Impact of Sodium Acetate in Water Treatment
The environmental impact of sodium acetate in water treatment is a crucial consideration as we explore its future opportunities in water purification. Sodium acetate, while effective in certain water treatment processes, can have both positive and negative effects on the environment.
One of the primary environmental benefits of using sodium acetate in water treatment is its ability to neutralize acidic water. This is particularly important in areas where acid rain or industrial runoff has lowered the pH of water bodies. By raising the pH to more neutral levels, sodium acetate can help restore aquatic ecosystems and protect aquatic life from the harmful effects of acidification.
However, the introduction of sodium acetate into water systems also raises concerns about potential ecological imbalances. The increased sodium content in treated water can affect soil structure and plant growth when used for irrigation. This is especially problematic in areas with already high soil salinity, as it may exacerbate existing issues and impact agricultural productivity.
The biodegradability of sodium acetate is another important factor to consider. While it is generally considered biodegradable, the rate of decomposition can vary depending on environmental conditions. In some cases, the accumulation of sodium acetate in water bodies may lead to temporary increases in biological oxygen demand (BOD), potentially affecting aquatic organisms.
From a broader perspective, the production and transportation of sodium acetate for water treatment purposes also contribute to its environmental footprint. The manufacturing process requires energy and resources, and the transportation of the chemical to treatment facilities generates carbon emissions. As we look towards future opportunities, it is essential to consider more sustainable production methods and localized sourcing to minimize these impacts.
The disposal of sodium acetate-containing waste from water treatment plants is another environmental concern. Proper management and treatment of this waste are necessary to prevent contamination of soil and groundwater. Advanced waste treatment technologies and circular economy approaches could potentially mitigate these issues in the future.
As we explore future opportunities for sodium acetate in water purification, it is crucial to balance its effectiveness with its environmental impact. Research into optimizing dosage levels, developing more environmentally friendly formulations, and improving removal techniques for residual sodium acetate will be key areas of focus. Additionally, integrating sodium acetate treatment with other complementary purification methods may help maximize its benefits while minimizing negative environmental effects.
One of the primary environmental benefits of using sodium acetate in water treatment is its ability to neutralize acidic water. This is particularly important in areas where acid rain or industrial runoff has lowered the pH of water bodies. By raising the pH to more neutral levels, sodium acetate can help restore aquatic ecosystems and protect aquatic life from the harmful effects of acidification.
However, the introduction of sodium acetate into water systems also raises concerns about potential ecological imbalances. The increased sodium content in treated water can affect soil structure and plant growth when used for irrigation. This is especially problematic in areas with already high soil salinity, as it may exacerbate existing issues and impact agricultural productivity.
The biodegradability of sodium acetate is another important factor to consider. While it is generally considered biodegradable, the rate of decomposition can vary depending on environmental conditions. In some cases, the accumulation of sodium acetate in water bodies may lead to temporary increases in biological oxygen demand (BOD), potentially affecting aquatic organisms.
From a broader perspective, the production and transportation of sodium acetate for water treatment purposes also contribute to its environmental footprint. The manufacturing process requires energy and resources, and the transportation of the chemical to treatment facilities generates carbon emissions. As we look towards future opportunities, it is essential to consider more sustainable production methods and localized sourcing to minimize these impacts.
The disposal of sodium acetate-containing waste from water treatment plants is another environmental concern. Proper management and treatment of this waste are necessary to prevent contamination of soil and groundwater. Advanced waste treatment technologies and circular economy approaches could potentially mitigate these issues in the future.
As we explore future opportunities for sodium acetate in water purification, it is crucial to balance its effectiveness with its environmental impact. Research into optimizing dosage levels, developing more environmentally friendly formulations, and improving removal techniques for residual sodium acetate will be key areas of focus. Additionally, integrating sodium acetate treatment with other complementary purification methods may help maximize its benefits while minimizing negative environmental effects.
Regulatory Framework for Water Purification Technologies
The regulatory framework for water purification technologies plays a crucial role in ensuring the safety and efficacy of water treatment processes, including those involving sodium acetate. In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing water quality standards and treatment technologies. The Safe Drinking Water Act (SDWA) provides the legal basis for these regulations, setting maximum contaminant levels (MCLs) for various pollutants and mandating specific treatment techniques.
For sodium acetate and similar compounds used in water purification, the EPA's National Primary Drinking Water Regulations (NPDWRs) and National Secondary Drinking Water Regulations (NSDWRs) are particularly relevant. These regulations establish both enforceable standards and non-enforceable guidelines for drinking water quality. The use of sodium acetate in water treatment must comply with these standards, particularly concerning its potential impact on water pH, taste, and odor.
Internationally, the World Health Organization (WHO) provides guidelines for drinking water quality, which many countries use as a basis for their national standards. These guidelines address chemical and microbial contaminants, treatment processes, and monitoring requirements. While sodium acetate is not specifically regulated under these guidelines, its use must not compromise the overall water quality standards set by the WHO.
In the European Union, the Drinking Water Directive (98/83/EC) sets quality standards for drinking water. This directive is currently undergoing revision to incorporate the latest scientific knowledge and address emerging contaminants. The revised directive may have implications for the use of sodium acetate and similar compounds in water treatment processes within EU member states.
Regulatory bodies also require extensive testing and validation of new water treatment technologies before they can be implemented at scale. For sodium acetate-based purification methods, this would involve demonstrating its effectiveness in removing targeted contaminants, assessing any potential by-products or residuals, and evaluating its impact on overall water quality. The NSF International and the American National Standards Institute (ANSI) provide certification programs for water treatment chemicals and processes, which can be crucial for gaining regulatory approval and market acceptance.
As water scarcity and contamination issues become more prevalent globally, regulatory frameworks are likely to evolve to address new challenges and technologies. Future regulations may focus more on emerging contaminants, such as pharmaceuticals and microplastics, which could influence the development and application of sodium acetate-based purification methods. Additionally, there is a growing trend towards risk-based approaches in water quality management, which may provide more flexibility in the application of innovative treatment technologies while maintaining stringent safety standards.
For sodium acetate and similar compounds used in water purification, the EPA's National Primary Drinking Water Regulations (NPDWRs) and National Secondary Drinking Water Regulations (NSDWRs) are particularly relevant. These regulations establish both enforceable standards and non-enforceable guidelines for drinking water quality. The use of sodium acetate in water treatment must comply with these standards, particularly concerning its potential impact on water pH, taste, and odor.
Internationally, the World Health Organization (WHO) provides guidelines for drinking water quality, which many countries use as a basis for their national standards. These guidelines address chemical and microbial contaminants, treatment processes, and monitoring requirements. While sodium acetate is not specifically regulated under these guidelines, its use must not compromise the overall water quality standards set by the WHO.
In the European Union, the Drinking Water Directive (98/83/EC) sets quality standards for drinking water. This directive is currently undergoing revision to incorporate the latest scientific knowledge and address emerging contaminants. The revised directive may have implications for the use of sodium acetate and similar compounds in water treatment processes within EU member states.
Regulatory bodies also require extensive testing and validation of new water treatment technologies before they can be implemented at scale. For sodium acetate-based purification methods, this would involve demonstrating its effectiveness in removing targeted contaminants, assessing any potential by-products or residuals, and evaluating its impact on overall water quality. The NSF International and the American National Standards Institute (ANSI) provide certification programs for water treatment chemicals and processes, which can be crucial for gaining regulatory approval and market acceptance.
As water scarcity and contamination issues become more prevalent globally, regulatory frameworks are likely to evolve to address new challenges and technologies. Future regulations may focus more on emerging contaminants, such as pharmaceuticals and microplastics, which could influence the development and application of sodium acetate-based purification methods. Additionally, there is a growing trend towards risk-based approaches in water quality management, which may provide more flexibility in the application of innovative treatment technologies while maintaining stringent safety standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!