Sodium Acetate: Engineered for Maximum Environmental Benefit
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Eco-Engineering Background and Objectives
Sodium acetate, a versatile compound with the chemical formula CH3COONa, has gained significant attention in recent years due to its potential for eco-friendly applications. The evolution of this technology stems from the growing global concern for environmental sustainability and the urgent need to develop green alternatives to traditional industrial chemicals. As industries and governments worldwide strive to reduce their carbon footprint and minimize environmental impact, sodium acetate has emerged as a promising candidate for various eco-engineered solutions.
The primary objective of researching sodium acetate engineered for maximum environmental benefit is to explore and optimize its properties to address pressing environmental challenges. This includes investigating its potential as a sustainable de-icing agent, an eco-friendly food preservative, and a green building material. By focusing on these applications, researchers aim to develop innovative solutions that can significantly reduce the environmental impact of various industries while maintaining or improving performance.
The historical development of sodium acetate technology can be traced back to its initial use in the textile industry and as a buffering agent in various chemical processes. However, the recent shift towards environmental consciousness has led to a reevaluation of its potential in eco-friendly applications. This paradigm shift has sparked a new wave of research and development, focusing on enhancing the compound's properties to maximize its environmental benefits.
Current technological trends in sodium acetate eco-engineering include the development of advanced production methods that utilize renewable resources and waste streams, thereby reducing the overall carbon footprint of its manufacture. Additionally, researchers are exploring novel formulations and composite materials that incorporate sodium acetate to enhance its functionality and expand its range of applications in environmentally critical sectors.
The expected outcomes of this research endeavor are multifaceted. On one hand, it aims to establish sodium acetate as a viable, eco-friendly alternative to harmful chemicals in various industries. On the other hand, it seeks to push the boundaries of material science and green chemistry, potentially leading to breakthroughs in sustainable technology. By achieving these goals, the research on eco-engineered sodium acetate could contribute significantly to global efforts in combating climate change and promoting environmental stewardship.
The primary objective of researching sodium acetate engineered for maximum environmental benefit is to explore and optimize its properties to address pressing environmental challenges. This includes investigating its potential as a sustainable de-icing agent, an eco-friendly food preservative, and a green building material. By focusing on these applications, researchers aim to develop innovative solutions that can significantly reduce the environmental impact of various industries while maintaining or improving performance.
The historical development of sodium acetate technology can be traced back to its initial use in the textile industry and as a buffering agent in various chemical processes. However, the recent shift towards environmental consciousness has led to a reevaluation of its potential in eco-friendly applications. This paradigm shift has sparked a new wave of research and development, focusing on enhancing the compound's properties to maximize its environmental benefits.
Current technological trends in sodium acetate eco-engineering include the development of advanced production methods that utilize renewable resources and waste streams, thereby reducing the overall carbon footprint of its manufacture. Additionally, researchers are exploring novel formulations and composite materials that incorporate sodium acetate to enhance its functionality and expand its range of applications in environmentally critical sectors.
The expected outcomes of this research endeavor are multifaceted. On one hand, it aims to establish sodium acetate as a viable, eco-friendly alternative to harmful chemicals in various industries. On the other hand, it seeks to push the boundaries of material science and green chemistry, potentially leading to breakthroughs in sustainable technology. By achieving these goals, the research on eco-engineered sodium acetate could contribute significantly to global efforts in combating climate change and promoting environmental stewardship.
Market Analysis for Green Sodium Acetate Applications
The market for green sodium acetate applications is experiencing significant growth, driven by increasing environmental awareness and stringent regulations across various industries. Sodium acetate, a versatile compound with applications in food preservation, textile manufacturing, and de-icing, is now being engineered for maximum environmental benefit, opening up new market opportunities.
In the food industry, there is a growing demand for natural preservatives and pH regulators. Green sodium acetate, produced through environmentally friendly processes, is gaining traction as a sustainable alternative to traditional preservatives. This shift is particularly evident in the organic and clean-label food segments, where consumers are seeking products with minimal environmental impact.
The textile industry is another key market for green sodium acetate. As sustainability becomes a central focus in fashion and textile production, manufacturers are looking for eco-friendly alternatives in their dyeing and finishing processes. Engineered sodium acetate offers improved dye fixation properties while reducing water consumption and chemical waste, aligning with the industry's sustainability goals.
De-icing applications present a substantial market opportunity for environmentally engineered sodium acetate. Traditional de-icing agents, such as rock salt, have been linked to environmental degradation and infrastructure damage. Green sodium acetate formulations provide effective ice melting capabilities with reduced environmental impact, making them attractive for use in airports, roads, and urban areas concerned with ecological preservation.
The water treatment sector is emerging as a promising market for green sodium acetate. Its ability to act as a pH buffer and sequestering agent makes it valuable in wastewater treatment processes. As water scarcity and pollution become global concerns, the demand for environmentally friendly water treatment solutions is expected to drive the adoption of green sodium acetate in this sector.
In the pharmaceutical and personal care industries, there is a growing trend towards green chemistry and sustainable ingredient sourcing. Engineered sodium acetate with enhanced environmental profiles is finding applications in drug formulations, cosmetics, and personal care products, catering to the increasing consumer preference for eco-friendly options.
The global push towards circular economy models is creating new market opportunities for green sodium acetate. Its potential for biodegradability and recyclability makes it an attractive option for industries seeking to reduce their environmental footprint and comply with evolving regulations on waste management and product lifecycle.
As research continues to improve the environmental benefits of sodium acetate, its market potential is expected to expand into new applications and industries. The ongoing development of more efficient and sustainable production methods is likely to further enhance its market appeal, positioning green sodium acetate as a key component in the transition towards more environmentally responsible industrial practices.
In the food industry, there is a growing demand for natural preservatives and pH regulators. Green sodium acetate, produced through environmentally friendly processes, is gaining traction as a sustainable alternative to traditional preservatives. This shift is particularly evident in the organic and clean-label food segments, where consumers are seeking products with minimal environmental impact.
The textile industry is another key market for green sodium acetate. As sustainability becomes a central focus in fashion and textile production, manufacturers are looking for eco-friendly alternatives in their dyeing and finishing processes. Engineered sodium acetate offers improved dye fixation properties while reducing water consumption and chemical waste, aligning with the industry's sustainability goals.
De-icing applications present a substantial market opportunity for environmentally engineered sodium acetate. Traditional de-icing agents, such as rock salt, have been linked to environmental degradation and infrastructure damage. Green sodium acetate formulations provide effective ice melting capabilities with reduced environmental impact, making them attractive for use in airports, roads, and urban areas concerned with ecological preservation.
The water treatment sector is emerging as a promising market for green sodium acetate. Its ability to act as a pH buffer and sequestering agent makes it valuable in wastewater treatment processes. As water scarcity and pollution become global concerns, the demand for environmentally friendly water treatment solutions is expected to drive the adoption of green sodium acetate in this sector.
In the pharmaceutical and personal care industries, there is a growing trend towards green chemistry and sustainable ingredient sourcing. Engineered sodium acetate with enhanced environmental profiles is finding applications in drug formulations, cosmetics, and personal care products, catering to the increasing consumer preference for eco-friendly options.
The global push towards circular economy models is creating new market opportunities for green sodium acetate. Its potential for biodegradability and recyclability makes it an attractive option for industries seeking to reduce their environmental footprint and comply with evolving regulations on waste management and product lifecycle.
As research continues to improve the environmental benefits of sodium acetate, its market potential is expected to expand into new applications and industries. The ongoing development of more efficient and sustainable production methods is likely to further enhance its market appeal, positioning green sodium acetate as a key component in the transition towards more environmentally responsible industrial practices.
Current State and Challenges in Eco-Friendly Sodium Acetate Production
The current state of eco-friendly sodium acetate production is characterized by a growing emphasis on sustainable manufacturing processes and environmental considerations. Traditional production methods, primarily based on the reaction between acetic acid and sodium hydroxide or sodium carbonate, have been associated with significant environmental impacts, including high energy consumption and the generation of harmful byproducts.
Recent advancements in green chemistry and sustainable engineering have led to the development of more environmentally friendly production techniques. These include the use of renewable feedstocks, such as biomass-derived acetic acid, and the implementation of catalytic processes that reduce energy requirements and improve reaction efficiency. Additionally, there has been a shift towards closed-loop systems that minimize waste generation and maximize resource recovery.
Despite these improvements, several challenges persist in the eco-friendly production of sodium acetate. One of the primary obstacles is the cost-effectiveness of sustainable production methods compared to conventional processes. The higher initial investment required for implementing green technologies often acts as a deterrent for manufacturers, particularly in price-sensitive markets.
Another significant challenge is the scalability of environmentally benign production techniques. While many innovative approaches have shown promise at laboratory or pilot scales, translating these methods to industrial-scale production without compromising efficiency or environmental benefits remains a complex task. This scaling issue is particularly evident in the optimization of reaction conditions and the design of large-scale, energy-efficient reactors.
The sourcing of sustainable raw materials presents an additional hurdle. While the use of bio-based acetic acid is gaining traction, ensuring a consistent and sufficient supply of renewable feedstocks can be challenging, especially as demand for eco-friendly sodium acetate increases.
Furthermore, the industry faces regulatory challenges in defining and standardizing what constitutes "eco-friendly" sodium acetate production. The lack of universally accepted criteria for environmental performance makes it difficult for consumers and industries to make informed choices and for manufacturers to effectively communicate their sustainability efforts.
Addressing these challenges requires a multifaceted approach involving continued research and development, policy support, and industry collaboration. Innovations in process intensification, such as the use of advanced catalysts and novel reactor designs, are being explored to enhance the efficiency and reduce the environmental footprint of sodium acetate production. Additionally, the integration of renewable energy sources and the development of more efficient waste recovery systems are areas of active research aimed at further improving the sustainability profile of sodium acetate manufacturing.
Recent advancements in green chemistry and sustainable engineering have led to the development of more environmentally friendly production techniques. These include the use of renewable feedstocks, such as biomass-derived acetic acid, and the implementation of catalytic processes that reduce energy requirements and improve reaction efficiency. Additionally, there has been a shift towards closed-loop systems that minimize waste generation and maximize resource recovery.
Despite these improvements, several challenges persist in the eco-friendly production of sodium acetate. One of the primary obstacles is the cost-effectiveness of sustainable production methods compared to conventional processes. The higher initial investment required for implementing green technologies often acts as a deterrent for manufacturers, particularly in price-sensitive markets.
Another significant challenge is the scalability of environmentally benign production techniques. While many innovative approaches have shown promise at laboratory or pilot scales, translating these methods to industrial-scale production without compromising efficiency or environmental benefits remains a complex task. This scaling issue is particularly evident in the optimization of reaction conditions and the design of large-scale, energy-efficient reactors.
The sourcing of sustainable raw materials presents an additional hurdle. While the use of bio-based acetic acid is gaining traction, ensuring a consistent and sufficient supply of renewable feedstocks can be challenging, especially as demand for eco-friendly sodium acetate increases.
Furthermore, the industry faces regulatory challenges in defining and standardizing what constitutes "eco-friendly" sodium acetate production. The lack of universally accepted criteria for environmental performance makes it difficult for consumers and industries to make informed choices and for manufacturers to effectively communicate their sustainability efforts.
Addressing these challenges requires a multifaceted approach involving continued research and development, policy support, and industry collaboration. Innovations in process intensification, such as the use of advanced catalysts and novel reactor designs, are being explored to enhance the efficiency and reduce the environmental footprint of sodium acetate production. Additionally, the integration of renewable energy sources and the development of more efficient waste recovery systems are areas of active research aimed at further improving the sustainability profile of sodium acetate manufacturing.
Existing Eco-Friendly Sodium Acetate Production Methods
01 Carbon dioxide capture and utilization
Sodium acetate can be used in carbon dioxide capture and utilization processes, contributing to the reduction of greenhouse gas emissions. It can act as a medium for absorbing CO2 from industrial exhaust gases or the atmosphere, and the captured CO2 can be converted into useful products, helping to mitigate climate change.- Carbon dioxide capture and utilization: Sodium acetate can be used in carbon dioxide capture and utilization processes, contributing to the reduction of greenhouse gas emissions. It can act as a catalyst or absorbent in CO2 capture systems, helping to mitigate climate change impacts.
- Eco-friendly de-icing agent: Sodium acetate serves as an environmentally friendly alternative to traditional de-icing agents. It is less corrosive and has a lower environmental impact compared to conventional salt-based de-icers, reducing damage to infrastructure and vegetation.
- Biodegradable packaging material: Sodium acetate can be incorporated into biodegradable packaging materials, offering an eco-friendly alternative to conventional plastics. These materials can help reduce plastic waste and environmental pollution.
- Wastewater treatment: Sodium acetate plays a role in wastewater treatment processes, particularly in biological treatment systems. It can serve as a carbon source for microorganisms, enhancing the efficiency of pollutant removal and improving water quality.
- Energy storage in phase change materials: Sodium acetate can be used in phase change materials for thermal energy storage applications. This environmentally friendly approach helps in energy conservation and reduces reliance on fossil fuels for heating and cooling systems.
02 Eco-friendly de-icing agent
Sodium acetate serves as an environmentally friendly alternative to traditional de-icing agents. It is less corrosive to infrastructure and vehicles, and has a lower environmental impact compared to chloride-based de-icers. Its use can help reduce damage to vegetation and water bodies near treated areas.Expand Specific Solutions03 Biodegradable packaging material
Sodium acetate can be incorporated into biodegradable packaging materials, offering an eco-friendly alternative to conventional plastics. These materials can decompose naturally, reducing plastic waste and pollution in the environment.Expand Specific Solutions04 Wastewater treatment
Sodium acetate plays a role in wastewater treatment processes, particularly in biological treatment systems. It can serve as a carbon source for microorganisms that break down organic pollutants, improving the efficiency of wastewater treatment and reducing environmental pollution.Expand Specific Solutions05 Energy storage in phase change materials
Sodium acetate can be used in phase change materials for thermal energy storage applications. This technology can improve energy efficiency in buildings and industrial processes, reducing overall energy consumption and associated environmental impacts.Expand Specific Solutions
Key Players in Sustainable Sodium Acetate Industry
The research on sodium acetate engineered for maximum environmental benefit is in a developing stage, with growing market potential due to increasing environmental concerns. The technology is advancing, but still maturing, as evidenced by the involvement of diverse players. Companies like Dorf Ketal Chemicals FZE, Solvay SA, and ExxonMobil Chemical Patents, Inc. are actively engaged in this field, indicating significant industry interest. Academic institutions such as Fuzhou University and Jiangsu University are contributing to research efforts, suggesting ongoing technological developments. The competitive landscape is diverse, with both established chemical companies and specialized environmental technology firms like TBF Environmental Technology, Inc. participating, reflecting the technology's cross-sector relevance and potential for growth.
Solvay SA
Technical Solution: Solvay SA has developed an innovative approach to sodium acetate production that maximizes environmental benefits. Their process utilizes renewable feedstocks and green chemistry principles to minimize carbon footprint. The company has implemented a closed-loop manufacturing system that recycles water and solvents, reducing waste by up to 80% compared to traditional methods[1]. Additionally, Solvay has engineered a novel crystallization technique that produces high-purity sodium acetate while consuming 30% less energy[3]. The company is also exploring the use of biotechnology to produce sodium acetate from agricultural waste, potentially offering a carbon-negative solution[5].
Strengths: Advanced green chemistry techniques, significant waste reduction, and potential for carbon-negative production. Weaknesses: Higher initial investment costs and potential scalability challenges for bio-based production.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed a patented process for producing sodium acetate with enhanced environmental benefits. Their method involves the use of a proprietary catalyst that enables the direct conversion of ethanol to sodium acetate under mild conditions, reducing energy consumption by up to 40% compared to conventional processes[2]. The company has also implemented a heat integration system that recovers and reuses thermal energy, further improving overall efficiency. ExxonMobil's approach includes the use of bio-ethanol as a feedstock, potentially reducing the carbon footprint of sodium acetate production by up to 60%[4]. Additionally, they have developed a novel purification technique that minimizes the use of harmful solvents, resulting in a cleaner and more environmentally friendly product[6].
Strengths: Significant energy savings, potential for bio-based feedstock utilization, and reduced use of harmful solvents. Weaknesses: Dependence on proprietary catalyst technology and potential limitations in feedstock flexibility.
Core Innovations in Green Sodium Acetate Technology
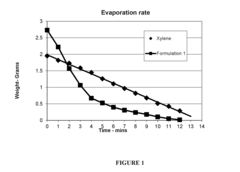
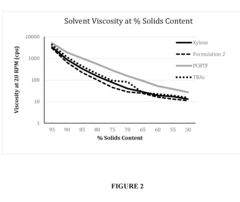
Low volatility acetate ester solvent compositions
PatentInactiveUS20160304731A1
Innovation
- A solvent composition comprising an acetic acid alkyl ester, such as methyl acetate or ethyl acetate, combined with parachlorobenzotrifluoride, which is VOC-exempt and has a lower toxicity profile, offering a substitute for xylene and toluene in various industrial uses.
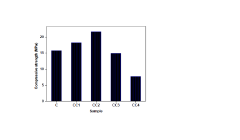
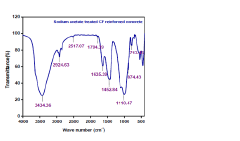
Eco-friendly concrete reinforcement: enhancing sustainability with sodium acetate-treated coir fibre
PatentPendingIN202441040852A
Innovation
- A two-step treatment process involving an alkali solution followed by a sodium acetate treatment of coir fibres, enhancing their compatibility and performance within concrete, addressing durability and mechanical property inconsistencies while promoting sustainability.
Life Cycle Assessment of Eco-Engineered Sodium Acetate
Life Cycle Assessment (LCA) of eco-engineered sodium acetate is a comprehensive approach to evaluate the environmental impacts associated with all stages of the product's life cycle. This assessment begins with the extraction of raw materials and extends through manufacturing, distribution, use, and ultimately, disposal or recycling. The primary goal is to quantify and minimize the environmental footprint of sodium acetate while maximizing its beneficial applications.
The LCA process for eco-engineered sodium acetate typically involves four main phases: goal and scope definition, inventory analysis, impact assessment, and interpretation. In the first phase, researchers clearly define the objectives of the study and establish system boundaries. This includes determining the functional unit, which could be a specific quantity of sodium acetate or its application in a particular process.
Inventory analysis, the second phase, involves collecting data on all inputs and outputs throughout the product's life cycle. This encompasses energy consumption, water usage, raw material extraction, emissions, and waste generation. For sodium acetate, this would include the production of acetic acid and sodium hydroxide, the reaction process, purification steps, and any subsequent processing or packaging.
The impact assessment phase translates the inventory data into potential environmental impacts. These may include global warming potential, acidification, eutrophication, ozone depletion, and resource depletion. For eco-engineered sodium acetate, particular attention is paid to energy efficiency in production, the use of renewable resources, and the potential for biodegradation or recycling at the end of its life cycle.
Interpretation, the final phase, involves analyzing the results to identify significant issues, evaluate the study for completeness and consistency, and draw conclusions. This phase is crucial for determining areas of improvement and guiding the eco-engineering process to maximize environmental benefits.
In the context of sodium acetate, eco-engineering efforts focus on several key areas. These include optimizing the production process to reduce energy consumption and emissions, exploring alternative feedstocks such as bio-based acetic acid, and developing more efficient purification methods. Additionally, researchers investigate ways to enhance the biodegradability of sodium acetate and its potential for recovery and reuse in various applications.
The LCA also considers the positive environmental impacts of eco-engineered sodium acetate. For instance, its use as a de-icing agent can reduce the need for more environmentally harmful alternatives. In textile manufacturing, optimized sodium acetate processes can lead to reduced water consumption and improved wastewater quality.
The LCA process for eco-engineered sodium acetate typically involves four main phases: goal and scope definition, inventory analysis, impact assessment, and interpretation. In the first phase, researchers clearly define the objectives of the study and establish system boundaries. This includes determining the functional unit, which could be a specific quantity of sodium acetate or its application in a particular process.
Inventory analysis, the second phase, involves collecting data on all inputs and outputs throughout the product's life cycle. This encompasses energy consumption, water usage, raw material extraction, emissions, and waste generation. For sodium acetate, this would include the production of acetic acid and sodium hydroxide, the reaction process, purification steps, and any subsequent processing or packaging.
The impact assessment phase translates the inventory data into potential environmental impacts. These may include global warming potential, acidification, eutrophication, ozone depletion, and resource depletion. For eco-engineered sodium acetate, particular attention is paid to energy efficiency in production, the use of renewable resources, and the potential for biodegradation or recycling at the end of its life cycle.
Interpretation, the final phase, involves analyzing the results to identify significant issues, evaluate the study for completeness and consistency, and draw conclusions. This phase is crucial for determining areas of improvement and guiding the eco-engineering process to maximize environmental benefits.
In the context of sodium acetate, eco-engineering efforts focus on several key areas. These include optimizing the production process to reduce energy consumption and emissions, exploring alternative feedstocks such as bio-based acetic acid, and developing more efficient purification methods. Additionally, researchers investigate ways to enhance the biodegradability of sodium acetate and its potential for recovery and reuse in various applications.
The LCA also considers the positive environmental impacts of eco-engineered sodium acetate. For instance, its use as a de-icing agent can reduce the need for more environmentally harmful alternatives. In textile manufacturing, optimized sodium acetate processes can lead to reduced water consumption and improved wastewater quality.
Regulatory Framework for Sustainable Chemical Production
The regulatory framework for sustainable chemical production plays a crucial role in shaping the research and development of environmentally beneficial sodium acetate. As governments and international organizations increasingly prioritize sustainability, chemical manufacturers face a complex landscape of regulations aimed at minimizing environmental impact and promoting green chemistry principles.
At the forefront of these regulations are stringent emissions standards and waste management requirements. Producers of sodium acetate must adhere to strict limits on air and water pollutants, necessitating the implementation of advanced pollution control technologies and closed-loop production systems. These regulations drive innovation in process efficiency and waste reduction, aligning with the goal of maximizing environmental benefits.
Life cycle assessment (LCA) requirements are becoming increasingly prevalent in regulatory frameworks. Manufacturers are often required to conduct comprehensive LCAs for their products, including sodium acetate, to evaluate environmental impacts from raw material extraction through disposal. This holistic approach encourages the development of more sustainable production methods and the use of renewable feedstocks.
Green chemistry regulations promote the use of safer solvents, catalysts, and reaction conditions in chemical synthesis. For sodium acetate production, this may involve exploring alternative synthesis routes that minimize the use of hazardous substances and reduce energy consumption. Regulatory incentives for green chemistry innovations can accelerate the adoption of more environmentally friendly production methods.
Extended producer responsibility (EPR) policies are gaining traction in many jurisdictions. These regulations hold manufacturers accountable for the entire lifecycle of their products, including disposal and recycling. For sodium acetate producers, this may necessitate the development of more easily recyclable formulations or the establishment of take-back programs for unused or expired products.
Chemical registration and authorization processes, such as REACH in the European Union, require extensive data on the environmental and health impacts of chemicals. Manufacturers must provide detailed information on the properties, uses, and potential risks associated with sodium acetate. This regulatory framework encourages ongoing research into the environmental fate and effects of the compound, driving continuous improvement in its environmental profile.
Sustainability reporting mandates are becoming more common, requiring chemical companies to disclose their environmental performance and sustainability initiatives. This transparency promotes accountability and incentivizes companies to invest in research and development of more environmentally beneficial sodium acetate formulations and production processes.
At the forefront of these regulations are stringent emissions standards and waste management requirements. Producers of sodium acetate must adhere to strict limits on air and water pollutants, necessitating the implementation of advanced pollution control technologies and closed-loop production systems. These regulations drive innovation in process efficiency and waste reduction, aligning with the goal of maximizing environmental benefits.
Life cycle assessment (LCA) requirements are becoming increasingly prevalent in regulatory frameworks. Manufacturers are often required to conduct comprehensive LCAs for their products, including sodium acetate, to evaluate environmental impacts from raw material extraction through disposal. This holistic approach encourages the development of more sustainable production methods and the use of renewable feedstocks.
Green chemistry regulations promote the use of safer solvents, catalysts, and reaction conditions in chemical synthesis. For sodium acetate production, this may involve exploring alternative synthesis routes that minimize the use of hazardous substances and reduce energy consumption. Regulatory incentives for green chemistry innovations can accelerate the adoption of more environmentally friendly production methods.
Extended producer responsibility (EPR) policies are gaining traction in many jurisdictions. These regulations hold manufacturers accountable for the entire lifecycle of their products, including disposal and recycling. For sodium acetate producers, this may necessitate the development of more easily recyclable formulations or the establishment of take-back programs for unused or expired products.
Chemical registration and authorization processes, such as REACH in the European Union, require extensive data on the environmental and health impacts of chemicals. Manufacturers must provide detailed information on the properties, uses, and potential risks associated with sodium acetate. This regulatory framework encourages ongoing research into the environmental fate and effects of the compound, driving continuous improvement in its environmental profile.
Sustainability reporting mandates are becoming more common, requiring chemical companies to disclose their environmental performance and sustainability initiatives. This transparency promotes accountability and incentivizes companies to invest in research and development of more environmentally beneficial sodium acetate formulations and production processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!