How Sodium Acetate Influences Photographic Chemical Reactions?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photochemistry Background
Photochemistry, the study of chemical reactions induced by light, forms the foundation of photographic processes. This field emerged in the early 19th century with the discovery of light-sensitive materials and has since evolved into a complex interplay of chemistry and physics. The fundamental principle of photochemistry involves the absorption of photons by molecules, leading to electronic excitation and subsequent chemical transformations.
In the context of photography, photochemistry primarily focuses on the light-sensitive properties of silver halides, particularly silver chloride, silver bromide, and silver iodide. These compounds, when exposed to light, undergo photolysis, a process where the absorption of photons causes the reduction of silver ions to metallic silver. This reaction forms the basis of latent image formation in traditional film photography.
The photographic process involves several key stages, each relying on specific photochemical reactions. Initially, the exposure of the film to light triggers the formation of a latent image, which is invisible to the naked eye. This latent image consists of small clusters of silver atoms within the silver halide crystals. The subsequent development process amplifies this latent image through a series of redox reactions, converting the exposed silver halide grains into metallic silver.
The role of chemical additives in photographic processes cannot be overstated. Various compounds are introduced to enhance sensitivity, control contrast, and improve image quality. Among these additives, sodium acetate plays a significant role in influencing photographic chemical reactions. Its presence can affect the pH of photographic solutions, impacting the kinetics and equilibrium of key reactions.
Understanding the influence of sodium acetate on photographic chemical reactions requires a deep dive into its chemical properties and interactions within the photographic system. Sodium acetate, being a weak base, can alter the pH of photographic solutions, potentially affecting the rate of silver ion reduction and the stability of developed images. Moreover, its buffering capacity can help maintain optimal conditions for certain photochemical processes, ensuring consistency in image development.
The exploration of sodium acetate's role in photography extends beyond its pH-modifying properties. Its potential to form complexes with silver ions and its influence on the solubility of various photographic chemicals add layers of complexity to its overall impact on photochemical reactions. These interactions can significantly affect the sensitivity, contrast, and resolution of the final photographic image.
In the context of photography, photochemistry primarily focuses on the light-sensitive properties of silver halides, particularly silver chloride, silver bromide, and silver iodide. These compounds, when exposed to light, undergo photolysis, a process where the absorption of photons causes the reduction of silver ions to metallic silver. This reaction forms the basis of latent image formation in traditional film photography.
The photographic process involves several key stages, each relying on specific photochemical reactions. Initially, the exposure of the film to light triggers the formation of a latent image, which is invisible to the naked eye. This latent image consists of small clusters of silver atoms within the silver halide crystals. The subsequent development process amplifies this latent image through a series of redox reactions, converting the exposed silver halide grains into metallic silver.
The role of chemical additives in photographic processes cannot be overstated. Various compounds are introduced to enhance sensitivity, control contrast, and improve image quality. Among these additives, sodium acetate plays a significant role in influencing photographic chemical reactions. Its presence can affect the pH of photographic solutions, impacting the kinetics and equilibrium of key reactions.
Understanding the influence of sodium acetate on photographic chemical reactions requires a deep dive into its chemical properties and interactions within the photographic system. Sodium acetate, being a weak base, can alter the pH of photographic solutions, potentially affecting the rate of silver ion reduction and the stability of developed images. Moreover, its buffering capacity can help maintain optimal conditions for certain photochemical processes, ensuring consistency in image development.
The exploration of sodium acetate's role in photography extends beyond its pH-modifying properties. Its potential to form complexes with silver ions and its influence on the solubility of various photographic chemicals add layers of complexity to its overall impact on photochemical reactions. These interactions can significantly affect the sensitivity, contrast, and resolution of the final photographic image.
Market Analysis
The market for sodium acetate in photographic chemical reactions has experienced significant growth in recent years, driven by the increasing demand for high-quality photographic products and the continuous evolution of photographic technologies. Sodium acetate plays a crucial role in various photographic processes, particularly in film development and printing, where it acts as a pH buffer and stabilizer.
The global photographic chemicals market, which includes sodium acetate as a key component, is projected to expand at a steady rate over the next five years. This growth is primarily attributed to the rising popularity of analog photography among enthusiasts and professionals, as well as the ongoing use of traditional photographic processes in certain industrial and scientific applications.
In the consumer segment, there has been a resurgence of interest in film photography, especially among younger generations seeking a more tactile and authentic photographic experience. This trend has led to increased demand for photographic chemicals, including sodium acetate-based solutions, for home developing and printing.
The professional photography sector continues to be a significant market for sodium acetate-containing photographic chemicals. Despite the widespread adoption of digital photography, many professional photographers still prefer the unique aesthetic qualities of film for certain projects, particularly in fashion, fine art, and documentary photography.
Industrial and scientific applications represent another important market segment for sodium acetate in photographic chemical reactions. These include medical imaging, forensic photography, and archival preservation, where the precise control of chemical processes is essential for achieving accurate and stable results.
Geographically, North America and Europe remain the largest markets for photographic chemicals, including sodium acetate-based products. However, emerging markets in Asia-Pacific, particularly China and India, are showing rapid growth due to increasing disposable incomes and a growing interest in photography as both a hobby and a profession.
The market is characterized by a mix of established chemical manufacturers and specialized photographic chemical suppliers. Key players are focusing on developing environmentally friendly formulations and improving the efficiency of their products to meet the evolving needs of photographers and industry professionals.
Despite the overall positive outlook, the market faces challenges such as the ongoing shift towards digital photography and environmental concerns related to chemical disposal. Manufacturers are responding by investing in research and development to create more sustainable and eco-friendly photographic chemical solutions, which may present new opportunities for sodium acetate applications in the future.
The global photographic chemicals market, which includes sodium acetate as a key component, is projected to expand at a steady rate over the next five years. This growth is primarily attributed to the rising popularity of analog photography among enthusiasts and professionals, as well as the ongoing use of traditional photographic processes in certain industrial and scientific applications.
In the consumer segment, there has been a resurgence of interest in film photography, especially among younger generations seeking a more tactile and authentic photographic experience. This trend has led to increased demand for photographic chemicals, including sodium acetate-based solutions, for home developing and printing.
The professional photography sector continues to be a significant market for sodium acetate-containing photographic chemicals. Despite the widespread adoption of digital photography, many professional photographers still prefer the unique aesthetic qualities of film for certain projects, particularly in fashion, fine art, and documentary photography.
Industrial and scientific applications represent another important market segment for sodium acetate in photographic chemical reactions. These include medical imaging, forensic photography, and archival preservation, where the precise control of chemical processes is essential for achieving accurate and stable results.
Geographically, North America and Europe remain the largest markets for photographic chemicals, including sodium acetate-based products. However, emerging markets in Asia-Pacific, particularly China and India, are showing rapid growth due to increasing disposable incomes and a growing interest in photography as both a hobby and a profession.
The market is characterized by a mix of established chemical manufacturers and specialized photographic chemical suppliers. Key players are focusing on developing environmentally friendly formulations and improving the efficiency of their products to meet the evolving needs of photographers and industry professionals.
Despite the overall positive outlook, the market faces challenges such as the ongoing shift towards digital photography and environmental concerns related to chemical disposal. Manufacturers are responding by investing in research and development to create more sustainable and eco-friendly photographic chemical solutions, which may present new opportunities for sodium acetate applications in the future.
Sodium Acetate Challenges
The use of sodium acetate in photographic chemical reactions presents several challenges that researchers and industry professionals must address. One of the primary issues is the potential for pH fluctuations in photographic solutions. Sodium acetate, being a weak base, can alter the acidity or alkalinity of the chemical environment, which may lead to unexpected changes in the development process or the final image quality.
Another significant challenge is the impact of sodium acetate on the stability of photographic emulsions. The presence of this compound can affect the delicate balance of silver halides and other light-sensitive materials, potentially causing premature development or uneven distribution of chemicals across the photographic surface. This instability can result in inconsistent image formation and reduced overall quality of the photographs.
The solubility of sodium acetate in various photographic solutions also poses a challenge. While it is generally soluble in water, its behavior in complex chemical mixtures used in photography can be unpredictable. This variability may lead to the formation of precipitates or the creation of localized areas with higher concentrations of sodium acetate, both of which can interfere with the uniform development of photographic images.
Temperature sensitivity is another concern when working with sodium acetate in photographic processes. The compound's solubility and chemical reactivity can change significantly with temperature fluctuations, making it difficult to maintain consistent results across different environmental conditions. This sensitivity requires careful control of temperature during all stages of the photographic process, from chemical preparation to development and fixing.
Furthermore, the interaction between sodium acetate and other common photographic chemicals, such as silver nitrate or potassium bromide, can lead to unexpected side reactions. These interactions may produce unwanted byproducts that could potentially damage photographic materials or alter the intended chemical processes. Understanding and controlling these complex chemical relationships is crucial for achieving reliable and high-quality photographic results.
The long-term effects of sodium acetate on the archival properties of photographs also present a challenge. There are concerns about its potential to contribute to the degradation of photographic materials over time, possibly affecting the longevity and preservation of important images. This necessitates extensive research into the compound's impact on different types of photographic papers and films under various storage conditions.
Lastly, the environmental impact of using sodium acetate in photographic processes is an emerging challenge. As the photography industry seeks more sustainable practices, there is a need to evaluate the ecological footprint of this chemical and explore potential alternatives that may offer similar benefits with reduced environmental consequences. This challenge encompasses not only the direct effects of sodium acetate use but also its production, transportation, and disposal within the photographic industry.
Another significant challenge is the impact of sodium acetate on the stability of photographic emulsions. The presence of this compound can affect the delicate balance of silver halides and other light-sensitive materials, potentially causing premature development or uneven distribution of chemicals across the photographic surface. This instability can result in inconsistent image formation and reduced overall quality of the photographs.
The solubility of sodium acetate in various photographic solutions also poses a challenge. While it is generally soluble in water, its behavior in complex chemical mixtures used in photography can be unpredictable. This variability may lead to the formation of precipitates or the creation of localized areas with higher concentrations of sodium acetate, both of which can interfere with the uniform development of photographic images.
Temperature sensitivity is another concern when working with sodium acetate in photographic processes. The compound's solubility and chemical reactivity can change significantly with temperature fluctuations, making it difficult to maintain consistent results across different environmental conditions. This sensitivity requires careful control of temperature during all stages of the photographic process, from chemical preparation to development and fixing.
Furthermore, the interaction between sodium acetate and other common photographic chemicals, such as silver nitrate or potassium bromide, can lead to unexpected side reactions. These interactions may produce unwanted byproducts that could potentially damage photographic materials or alter the intended chemical processes. Understanding and controlling these complex chemical relationships is crucial for achieving reliable and high-quality photographic results.
The long-term effects of sodium acetate on the archival properties of photographs also present a challenge. There are concerns about its potential to contribute to the degradation of photographic materials over time, possibly affecting the longevity and preservation of important images. This necessitates extensive research into the compound's impact on different types of photographic papers and films under various storage conditions.
Lastly, the environmental impact of using sodium acetate in photographic processes is an emerging challenge. As the photography industry seeks more sustainable practices, there is a need to evaluate the ecological footprint of this chemical and explore potential alternatives that may offer similar benefits with reduced environmental consequences. This challenge encompasses not only the direct effects of sodium acetate use but also its production, transportation, and disposal within the photographic industry.
Current Solutions
01 Neutralization reactions
Sodium acetate is commonly involved in neutralization reactions, particularly with acids. These reactions are fundamental in various chemical processes and can be used for pH adjustment or salt formation. The product of such reactions is typically water and the corresponding salt.- Neutralization reactions: Sodium acetate is commonly involved in neutralization reactions, particularly with acids. These reactions are fundamental in various chemical processes and industrial applications. The product of such reactions often depends on the specific acid used and the reaction conditions.
- Thermal decomposition: When subjected to high temperatures, sodium acetate undergoes thermal decomposition. This process can result in the formation of various products, including sodium carbonate and acetone. The specific products and reaction pathways depend on the temperature and other conditions of the decomposition process.
- Hydrolysis reactions: Sodium acetate can participate in hydrolysis reactions, particularly in aqueous solutions. These reactions involve the breaking of chemical bonds by the addition of water molecules. The hydrolysis of sodium acetate can lead to the formation of acetic acid and sodium hydroxide under certain conditions.
- Esterification processes: Sodium acetate can be used in esterification reactions, often serving as a catalyst or reagent. These reactions typically involve the formation of esters from carboxylic acids and alcohols. The presence of sodium acetate can influence the reaction kinetics and yield of these processes.
- Redox reactions: In certain conditions, sodium acetate can participate in redox (reduction-oxidation) reactions. These reactions involve the transfer of electrons between species, potentially leading to changes in oxidation states. The specific nature of these reactions depends on the other reactants involved and the reaction environment.
02 Thermal decomposition
When subjected to high temperatures, sodium acetate undergoes thermal decomposition. This process can result in the formation of various products, including sodium carbonate and acetone. The thermal behavior of sodium acetate is important in certain industrial applications and material processing.Expand Specific Solutions03 Hydrolysis reactions
Sodium acetate can participate in hydrolysis reactions, particularly in aqueous solutions. These reactions involve the breaking of chemical bonds by water molecules, potentially leading to the formation of acetic acid and sodium hydroxide. Hydrolysis of sodium acetate is relevant in various chemical and biological processes.Expand Specific Solutions04 Crystallization and phase transitions
Sodium acetate exhibits interesting crystallization behavior, particularly in supersaturated solutions. It can form crystalline hydrates and undergo phase transitions, which are utilized in applications such as heat packs and thermal energy storage systems. The crystallization properties of sodium acetate are also relevant in purification processes.Expand Specific Solutions05 Catalytic reactions
Sodium acetate can act as a catalyst or promoter in various organic reactions. It is particularly useful in certain condensation reactions, esterifications, and as a base in organic syntheses. The catalytic properties of sodium acetate make it valuable in the production of various organic compounds and materials.Expand Specific Solutions
Key Industry Players
The photographic chemical reaction industry, influenced by sodium acetate, is in a mature stage with established players and technologies. The global market size for photographic chemicals is estimated to be in the billions of dollars, with steady growth projected. Technologically, the field is well-developed but continues to evolve, particularly in digital imaging applications. Key players like Eastman Kodak, FUJIFILM, and Agfa NV have long-standing expertise in this area, while companies such as Sumitomo Seika Chemicals and Nissan Chemical Corp. contribute to the broader chemical landscape supporting photographic processes. These firms continue to innovate, focusing on improving efficiency, environmental sustainability, and adapting to digital trends in photography.
Eastman Kodak Co.
Technical Solution: Eastman Kodak has developed a novel approach to utilizing sodium acetate in photographic chemical reactions. Their method involves incorporating sodium acetate as a pH buffer in developer solutions, which helps maintain optimal alkalinity during the development process[1]. This stabilizes the chemical environment, leading to more consistent and predictable results. Additionally, Kodak has found that sodium acetate can act as a preservative in certain emulsions, extending the shelf life of photographic materials[3]. The company has also explored the use of sodium acetate in fixing baths, where it aids in the removal of unreacted silver halides from the film or paper[5].
Strengths: Improved consistency in development process, extended shelf life of materials, and enhanced fixing capabilities. Weaknesses: May require adjustments to existing workflows and potential increased cost of materials.
FUJIFILM Corp.
Technical Solution: FUJIFILM has innovated in the use of sodium acetate for photographic chemical reactions, particularly in their digital imaging technologies. They have developed a proprietary process that utilizes sodium acetate as a key component in their instant film formulations[2]. This approach allows for rapid development of images while maintaining color accuracy and stability. FUJIFILM has also incorporated sodium acetate into their photographic paper coatings, where it acts as a hardening agent, improving the durability and water resistance of prints[4]. In their research, they have found that sodium acetate can enhance the light sensitivity of certain emulsions when used in conjunction with specific silver halide crystals[6].
Strengths: Rapid image development, improved print durability, and enhanced light sensitivity. Weaknesses: Potential limitations in extreme temperature conditions and compatibility issues with some older photographic systems.
Sodium Acetate Innovations
Photoacid generator and photoreactive composition
PatentInactiveUS20100233621A1
Innovation
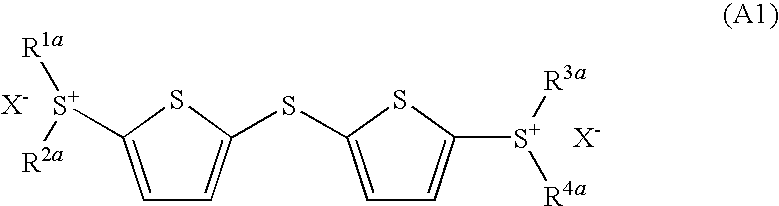
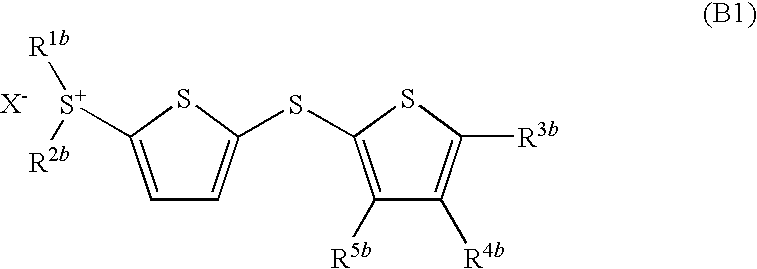
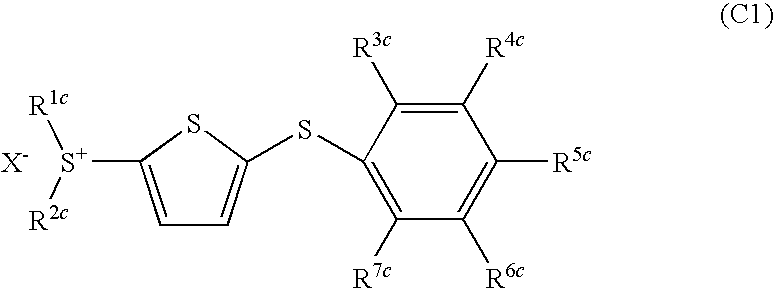
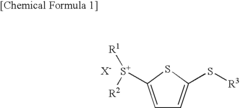
- Development of (5-arylthio-thiophen-2-yl)-diaryl sulfonium salts, specifically dithienyl sulfide disulfonium, dithienyl sulfide sulfonium, and phenylthiothiophene sulfonium salts, which serve as photoacid generators, enhancing sensitivity and reaction rates in the near ultraviolet range by incorporating these salts into photoreactive compositions.
Developer for processing black and white photographs and method of manufacturing of a working solution thereof
PatentInactiveEP2624052A1
Innovation
- A photographic developer comprising disodium salt of ethylenediaminetetraacetic acid, diatomic phenol, phenidon, sodium sulfite, alkali metal hydroxide, potassium bromide, boric acid, benzotriazole, and polyatomic alcohol, which collectively enhance light sensitivity, stability, and shelf life while minimizing graininess and fogging.
Environmental Impact
The use of sodium acetate in photographic chemical reactions has significant environmental implications that warrant careful consideration. As a common ingredient in photographic developers and fixers, sodium acetate plays a crucial role in the processing of photographic materials. However, its widespread use also raises concerns about its potential impact on the environment.
One of the primary environmental considerations is the disposal of photographic chemicals containing sodium acetate. When improperly discarded, these chemicals can contaminate water sources and soil, potentially harming aquatic ecosystems and terrestrial flora and fauna. The high solubility of sodium acetate in water means that it can easily leach into groundwater or surface water, altering the pH balance and potentially affecting the survival of sensitive aquatic organisms.
Furthermore, the production of sodium acetate for photographic purposes contributes to industrial emissions and resource consumption. The manufacturing process involves the reaction of acetic acid with sodium carbonate or sodium hydroxide, which requires energy and raw materials. This production chain has its own environmental footprint, including greenhouse gas emissions and potential chemical waste generation.
On a positive note, sodium acetate is biodegradable and less toxic compared to some other photographic chemicals. This characteristic makes it a relatively environmentally friendly option within the realm of photographic processing. However, its biodegradability does not negate the need for proper handling and disposal, as high concentrations can still disrupt local ecosystems.
The photography industry has been making efforts to reduce the environmental impact of chemical processes, including those involving sodium acetate. These efforts include the development of more efficient processing techniques that use smaller quantities of chemicals, as well as the implementation of closed-loop systems that recycle and reuse photographic solutions. Such innovations help minimize the release of sodium acetate and other chemicals into the environment.
Additionally, there is a growing trend towards digital photography, which significantly reduces the need for chemical processing and, consequently, the use of sodium acetate in photographic applications. This shift has led to a decrease in the overall environmental impact of photography as an industry. However, for specialized applications and in certain sectors where traditional film photography remains prevalent, the environmental considerations surrounding sodium acetate use continue to be relevant.
In conclusion, while sodium acetate plays an important role in photographic chemical reactions, its environmental impact must be carefully managed. The photography industry's ongoing efforts to develop more sustainable practices and the gradual transition to digital technologies are positive steps towards mitigating these environmental concerns. However, continued research into eco-friendly alternatives and improved disposal methods remains crucial for minimizing the long-term environmental effects of sodium acetate use in photography.
One of the primary environmental considerations is the disposal of photographic chemicals containing sodium acetate. When improperly discarded, these chemicals can contaminate water sources and soil, potentially harming aquatic ecosystems and terrestrial flora and fauna. The high solubility of sodium acetate in water means that it can easily leach into groundwater or surface water, altering the pH balance and potentially affecting the survival of sensitive aquatic organisms.
Furthermore, the production of sodium acetate for photographic purposes contributes to industrial emissions and resource consumption. The manufacturing process involves the reaction of acetic acid with sodium carbonate or sodium hydroxide, which requires energy and raw materials. This production chain has its own environmental footprint, including greenhouse gas emissions and potential chemical waste generation.
On a positive note, sodium acetate is biodegradable and less toxic compared to some other photographic chemicals. This characteristic makes it a relatively environmentally friendly option within the realm of photographic processing. However, its biodegradability does not negate the need for proper handling and disposal, as high concentrations can still disrupt local ecosystems.
The photography industry has been making efforts to reduce the environmental impact of chemical processes, including those involving sodium acetate. These efforts include the development of more efficient processing techniques that use smaller quantities of chemicals, as well as the implementation of closed-loop systems that recycle and reuse photographic solutions. Such innovations help minimize the release of sodium acetate and other chemicals into the environment.
Additionally, there is a growing trend towards digital photography, which significantly reduces the need for chemical processing and, consequently, the use of sodium acetate in photographic applications. This shift has led to a decrease in the overall environmental impact of photography as an industry. However, for specialized applications and in certain sectors where traditional film photography remains prevalent, the environmental considerations surrounding sodium acetate use continue to be relevant.
In conclusion, while sodium acetate plays an important role in photographic chemical reactions, its environmental impact must be carefully managed. The photography industry's ongoing efforts to develop more sustainable practices and the gradual transition to digital technologies are positive steps towards mitigating these environmental concerns. However, continued research into eco-friendly alternatives and improved disposal methods remains crucial for minimizing the long-term environmental effects of sodium acetate use in photography.
Regulatory Compliance
The use of sodium acetate in photographic chemical reactions is subject to various regulatory considerations. Manufacturers and users of photographic chemicals containing sodium acetate must comply with safety regulations set by agencies such as the Occupational Safety and Health Administration (OSHA) in the United States. These regulations typically cover aspects such as proper handling, storage, and disposal of the chemical.
Environmental protection agencies also play a crucial role in regulating the use of sodium acetate in photographic processes. The Environmental Protection Agency (EPA) in the U.S., for instance, has guidelines on the disposal of photographic chemicals to prevent environmental contamination. Companies must adhere to these guidelines to ensure that waste containing sodium acetate is properly treated before release into the environment.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation applies to the use of sodium acetate in photographic applications. This regulation requires manufacturers and importers to register chemicals and provide safety information, which may impact the use of sodium acetate in photographic processes.
Product safety regulations are another important aspect of compliance. Manufacturers of photographic chemicals containing sodium acetate must ensure their products meet safety standards and provide appropriate labeling and safety data sheets. This includes information on potential hazards, proper handling procedures, and emergency measures in case of accidents.
Transportation of sodium acetate and related photographic chemicals is regulated by agencies such as the Department of Transportation (DOT) in the U.S. and the International Air Transport Association (IATA) for international shipments. These regulations dictate proper packaging, labeling, and documentation requirements for the safe transport of these materials.
Workplace safety regulations also apply to facilities using sodium acetate in photographic processes. This includes requirements for personal protective equipment, proper ventilation, and emergency response procedures. Regular safety training and audits may be necessary to ensure ongoing compliance with these regulations.
As the photographic industry evolves, regulatory bodies may update their guidelines to address new concerns or technological advancements. Companies working with sodium acetate in photographic applications must stay informed about these changes and adapt their practices accordingly to maintain compliance and ensure the safety of workers and the environment.
Environmental protection agencies also play a crucial role in regulating the use of sodium acetate in photographic processes. The Environmental Protection Agency (EPA) in the U.S., for instance, has guidelines on the disposal of photographic chemicals to prevent environmental contamination. Companies must adhere to these guidelines to ensure that waste containing sodium acetate is properly treated before release into the environment.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation applies to the use of sodium acetate in photographic applications. This regulation requires manufacturers and importers to register chemicals and provide safety information, which may impact the use of sodium acetate in photographic processes.
Product safety regulations are another important aspect of compliance. Manufacturers of photographic chemicals containing sodium acetate must ensure their products meet safety standards and provide appropriate labeling and safety data sheets. This includes information on potential hazards, proper handling procedures, and emergency measures in case of accidents.
Transportation of sodium acetate and related photographic chemicals is regulated by agencies such as the Department of Transportation (DOT) in the U.S. and the International Air Transport Association (IATA) for international shipments. These regulations dictate proper packaging, labeling, and documentation requirements for the safe transport of these materials.
Workplace safety regulations also apply to facilities using sodium acetate in photographic processes. This includes requirements for personal protective equipment, proper ventilation, and emergency response procedures. Regular safety training and audits may be necessary to ensure ongoing compliance with these regulations.
As the photographic industry evolves, regulatory bodies may update their guidelines to address new concerns or technological advancements. Companies working with sodium acetate in photographic applications must stay informed about these changes and adapt their practices accordingly to maintain compliance and ensure the safety of workers and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!