Sodium Acetate: Transforming Chemical Engineering Practices
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Evolution
Sodium acetate has undergone a significant evolution in chemical engineering practices over the years. Initially discovered in the early 19th century, this compound has transitioned from a laboratory curiosity to a versatile industrial chemical with numerous applications.
In its early stages, sodium acetate was primarily used as a buffering agent in chemical processes. However, as research progressed, scientists began to uncover its unique properties, leading to a diversification of its applications. The mid-20th century saw a surge in sodium acetate's use in the textile industry, where it found applications in dyeing and printing processes.
The 1970s marked a turning point in sodium acetate's evolution. Researchers discovered its potential as a phase change material (PCM), capable of storing and releasing thermal energy. This discovery opened up new avenues in thermal management applications, particularly in the construction and automotive industries.
The 1990s witnessed further advancements in sodium acetate technology. Its role in food preservation expanded, with the compound being recognized as an effective and safe food additive. Simultaneously, its use in de-icing solutions gained traction, offering a more environmentally friendly alternative to traditional salt-based de-icers.
In the early 2000s, sodium acetate's evolution took another leap forward with the development of advanced production methods. These new techniques allowed for higher purity levels and more consistent product quality, further expanding its potential applications in pharmaceuticals and electronics.
The past decade has seen a renewed interest in sodium acetate's PCM properties, driven by the growing demand for sustainable energy solutions. Researchers are exploring its use in solar thermal energy storage systems and building materials for passive temperature control.
Most recently, sodium acetate has found applications in cutting-edge fields such as 3D printing and advanced materials science. Its unique properties are being harnessed to create novel composite materials with enhanced thermal and mechanical characteristics.
Looking ahead, the evolution of sodium acetate in chemical engineering practices shows no signs of slowing down. Ongoing research is focused on optimizing its production processes, enhancing its performance in existing applications, and discovering new potential uses. As sustainability becomes increasingly important in industrial processes, sodium acetate's role is expected to grow, particularly in green chemistry applications and renewable energy technologies.
In its early stages, sodium acetate was primarily used as a buffering agent in chemical processes. However, as research progressed, scientists began to uncover its unique properties, leading to a diversification of its applications. The mid-20th century saw a surge in sodium acetate's use in the textile industry, where it found applications in dyeing and printing processes.
The 1970s marked a turning point in sodium acetate's evolution. Researchers discovered its potential as a phase change material (PCM), capable of storing and releasing thermal energy. This discovery opened up new avenues in thermal management applications, particularly in the construction and automotive industries.
The 1990s witnessed further advancements in sodium acetate technology. Its role in food preservation expanded, with the compound being recognized as an effective and safe food additive. Simultaneously, its use in de-icing solutions gained traction, offering a more environmentally friendly alternative to traditional salt-based de-icers.
In the early 2000s, sodium acetate's evolution took another leap forward with the development of advanced production methods. These new techniques allowed for higher purity levels and more consistent product quality, further expanding its potential applications in pharmaceuticals and electronics.
The past decade has seen a renewed interest in sodium acetate's PCM properties, driven by the growing demand for sustainable energy solutions. Researchers are exploring its use in solar thermal energy storage systems and building materials for passive temperature control.
Most recently, sodium acetate has found applications in cutting-edge fields such as 3D printing and advanced materials science. Its unique properties are being harnessed to create novel composite materials with enhanced thermal and mechanical characteristics.
Looking ahead, the evolution of sodium acetate in chemical engineering practices shows no signs of slowing down. Ongoing research is focused on optimizing its production processes, enhancing its performance in existing applications, and discovering new potential uses. As sustainability becomes increasingly important in industrial processes, sodium acetate's role is expected to grow, particularly in green chemistry applications and renewable energy technologies.
Market Demand Analysis
The market demand for sodium acetate has been steadily growing across various industries, driven by its versatile applications and unique properties. In the chemical engineering sector, sodium acetate plays a crucial role as a buffering agent, pH regulator, and raw material for numerous chemical processes. The food industry represents a significant market for sodium acetate, where it serves as a preservative and flavoring agent. Its ability to extend shelf life and enhance taste profiles has led to increased adoption in processed foods, beverages, and dairy products.
The pharmaceutical industry has also contributed to the rising demand for sodium acetate. Its use in intravenous fluids and as a component in various medications has expanded its market presence. Additionally, the textile industry utilizes sodium acetate in dyeing processes, further diversifying its applications and market reach.
Environmental concerns and sustainability initiatives have positively impacted the sodium acetate market. Its biodegradability and low toxicity make it an attractive alternative to more harmful chemicals in various applications, including de-icing solutions for roads and runways. This shift towards eco-friendly options has opened new market opportunities and is expected to drive future growth.
The construction industry has emerged as another significant consumer of sodium acetate, particularly in concrete admixtures. Its ability to accelerate setting times and improve concrete properties has led to increased adoption in cold-weather construction projects.
Geographically, North America and Europe have traditionally been the largest markets for sodium acetate, owing to their well-established chemical, pharmaceutical, and food industries. However, rapid industrialization and economic growth in Asia-Pacific regions, particularly China and India, are creating new market opportunities and shifting the demand landscape.
Market analysts project a compound annual growth rate (CAGR) for the sodium acetate market in the mid-single digits over the next five years. This growth is attributed to expanding applications in existing industries and the emergence of new use cases in sectors such as energy storage and advanced materials.
Despite the positive outlook, the market faces challenges such as price volatility of raw materials and increasing competition from alternative chemicals. Manufacturers are focusing on innovation and process optimization to maintain competitiveness and meet evolving customer demands.
The pharmaceutical industry has also contributed to the rising demand for sodium acetate. Its use in intravenous fluids and as a component in various medications has expanded its market presence. Additionally, the textile industry utilizes sodium acetate in dyeing processes, further diversifying its applications and market reach.
Environmental concerns and sustainability initiatives have positively impacted the sodium acetate market. Its biodegradability and low toxicity make it an attractive alternative to more harmful chemicals in various applications, including de-icing solutions for roads and runways. This shift towards eco-friendly options has opened new market opportunities and is expected to drive future growth.
The construction industry has emerged as another significant consumer of sodium acetate, particularly in concrete admixtures. Its ability to accelerate setting times and improve concrete properties has led to increased adoption in cold-weather construction projects.
Geographically, North America and Europe have traditionally been the largest markets for sodium acetate, owing to their well-established chemical, pharmaceutical, and food industries. However, rapid industrialization and economic growth in Asia-Pacific regions, particularly China and India, are creating new market opportunities and shifting the demand landscape.
Market analysts project a compound annual growth rate (CAGR) for the sodium acetate market in the mid-single digits over the next five years. This growth is attributed to expanding applications in existing industries and the emergence of new use cases in sectors such as energy storage and advanced materials.
Despite the positive outlook, the market faces challenges such as price volatility of raw materials and increasing competition from alternative chemicals. Manufacturers are focusing on innovation and process optimization to maintain competitiveness and meet evolving customer demands.
Technical Challenges
The development of sodium acetate technology faces several significant challenges that require innovative solutions. One of the primary obstacles is the energy-intensive nature of its production process. Traditional methods often involve high-temperature reactions and extensive purification steps, leading to substantial energy consumption and increased production costs. This energy inefficiency not only impacts the economic viability of sodium acetate production but also raises environmental concerns due to the associated carbon footprint.
Another critical challenge lies in the purification and quality control of sodium acetate. Achieving high purity levels consistently is essential for many applications, particularly in pharmaceutical and food industries. Impurities can significantly affect the performance and safety of end products. Current purification techniques often struggle to remove trace contaminants efficiently, necessitating the development of more advanced separation and purification methods.
The scalability of sodium acetate production presents another hurdle. As demand grows across various industries, there is a pressing need to develop processes that can be easily scaled up without compromising on quality or efficiency. This challenge is compounded by the variability in raw material quality and the need for precise process control at larger scales.
Environmental sustainability is an increasingly important consideration in sodium acetate production. The chemical industry faces mounting pressure to reduce waste, minimize water usage, and lower emissions. Developing greener production methods for sodium acetate, such as using renewable feedstocks or implementing closed-loop systems, remains a significant technical challenge.
The versatility of sodium acetate applications also presents unique challenges. As its use expands into new areas such as energy storage (in phase change materials) and advanced materials, there is a need to tailor its properties for specific applications. This requires a deep understanding of structure-property relationships and the ability to modify the material at a molecular level.
Lastly, the integration of sodium acetate production and utilization into existing industrial processes poses technical difficulties. Optimizing its use as a buffer, pH regulator, or preservative in various chemical engineering practices requires careful consideration of compatibility issues, process integration, and potential side reactions. Overcoming these challenges demands interdisciplinary research efforts, combining expertise from chemical engineering, materials science, and process optimization.
Another critical challenge lies in the purification and quality control of sodium acetate. Achieving high purity levels consistently is essential for many applications, particularly in pharmaceutical and food industries. Impurities can significantly affect the performance and safety of end products. Current purification techniques often struggle to remove trace contaminants efficiently, necessitating the development of more advanced separation and purification methods.
The scalability of sodium acetate production presents another hurdle. As demand grows across various industries, there is a pressing need to develop processes that can be easily scaled up without compromising on quality or efficiency. This challenge is compounded by the variability in raw material quality and the need for precise process control at larger scales.
Environmental sustainability is an increasingly important consideration in sodium acetate production. The chemical industry faces mounting pressure to reduce waste, minimize water usage, and lower emissions. Developing greener production methods for sodium acetate, such as using renewable feedstocks or implementing closed-loop systems, remains a significant technical challenge.
The versatility of sodium acetate applications also presents unique challenges. As its use expands into new areas such as energy storage (in phase change materials) and advanced materials, there is a need to tailor its properties for specific applications. This requires a deep understanding of structure-property relationships and the ability to modify the material at a molecular level.
Lastly, the integration of sodium acetate production and utilization into existing industrial processes poses technical difficulties. Optimizing its use as a buffer, pH regulator, or preservative in various chemical engineering practices requires careful consideration of compatibility issues, process integration, and potential side reactions. Overcoming these challenges demands interdisciplinary research efforts, combining expertise from chemical engineering, materials science, and process optimization.
Current Applications
01 Use of sodium acetate in chemical processes
Sodium acetate is widely used in various chemical processes as a reagent, catalyst, or buffer. It plays a role in reactions such as acetylation, esterification, and pH control. Its properties make it valuable in industrial applications, including the production of pharmaceuticals, textiles, and other chemical compounds.- Sodium acetate in chemical processes: Sodium acetate is widely used in various chemical processes, including as a catalyst, pH regulator, and reagent in organic synthesis. It plays a crucial role in industrial applications such as textile manufacturing, food processing, and pharmaceutical production.
- Sodium acetate in heat storage systems: Sodium acetate trihydrate is utilized in phase change materials for thermal energy storage. Its ability to absorb and release heat during phase transitions makes it valuable in heating and cooling applications, including in portable heat packs and building temperature regulation systems.
- Sodium acetate in food preservation: As a food additive, sodium acetate acts as a preservative and acidity regulator. It helps extend the shelf life of various food products by inhibiting microbial growth and maintaining pH levels. It is commonly used in bakery products, snacks, and processed meats.
- Sodium acetate in wastewater treatment: Sodium acetate is employed in wastewater treatment processes, particularly in biological nutrient removal systems. It serves as a carbon source for denitrifying bacteria, aiding in the removal of nitrogen compounds from wastewater and improving overall water quality.
- Sodium acetate in medical applications: In the medical field, sodium acetate is used in various applications, including as a component in dialysis solutions, intravenous fluids, and pharmaceutical formulations. It helps maintain electrolyte balance and serves as a buffering agent in medical treatments.
02 Application in heat storage and thermal management
Sodium acetate trihydrate is utilized in heat storage systems and thermal management applications. It undergoes a phase change at specific temperatures, allowing it to store and release latent heat. This property is exploited in heat packs, building materials for temperature regulation, and energy storage systems.Expand Specific Solutions03 Use in food and beverage industry
Sodium acetate finds applications in the food and beverage industry as a preservative, flavoring agent, and acidity regulator. It helps extend shelf life, enhance taste, and maintain pH levels in various food products. Its use is regulated and approved by food safety authorities in many countries.Expand Specific Solutions04 Application in water treatment and purification
Sodium acetate is used in water treatment processes for pH adjustment, as a dechlorinating agent, and in the removal of heavy metals. It can also serve as a nutrient source for certain microorganisms in biological water treatment systems. Its effectiveness and relatively low toxicity make it suitable for various water purification applications.Expand Specific Solutions05 Use in material science and nanotechnology
Sodium acetate is employed in material science and nanotechnology for the synthesis of various nanostructures and advanced materials. It can act as a precursor, reducing agent, or stabilizer in the preparation of nanoparticles, thin films, and other nanomaterials. These applications have potential uses in electronics, catalysis, and biomedical fields.Expand Specific Solutions
Industry Leaders
The research on sodium acetate is currently in a developing stage, with growing interest from both academic institutions and industrial players. The market for sodium acetate is expanding, driven by its diverse applications in chemical engineering, pharmaceuticals, and sustainable technologies. While the market size is moderate, it shows potential for significant growth. Technologically, the field is advancing rapidly, with companies like China Petroleum & Chemical Corp., Celanese International Corp., and Eastman Chemical Co. leading industrial applications. Academic institutions such as Rice University and South China University of Technology are contributing to fundamental research, pushing the boundaries of sodium acetate's potential uses. The collaboration between industry and academia is accelerating the maturation of this technology, promising innovative solutions for various sectors.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has been at the forefront of research on sodium acetate, focusing on its applications in chemical engineering practices. The company has developed an innovative process for the production of high-purity sodium acetate using a combination of acetic acid and sodium hydroxide [1]. This method involves a carefully controlled neutralization reaction followed by crystallization and purification steps. Sinopec has also explored the use of sodium acetate as a phase change material (PCM) for thermal energy storage applications in industrial processes [2]. Their research has led to the development of encapsulated sodium acetate trihydrate with enhanced thermal properties and stability, potentially revolutionizing heat management in petrochemical plants [3].
Strengths: Vast resources for large-scale production and implementation, extensive experience in chemical engineering. Weaknesses: May face challenges in adapting to rapidly changing environmental regulations.
Sunamp Ltd.
Technical Solution: Sunamp Ltd. has made significant strides in the application of sodium acetate for thermal energy storage. Their patented technology utilizes sodium acetate trihydrate as a phase change material in compact heat batteries [4]. These batteries can store and release large amounts of thermal energy during the phase change process. Sunamp has developed a proprietary nucleation technique that ensures reliable crystallization of the supercooled sodium acetate solution, addressing one of the key challenges in PCM technology [5]. The company has also focused on enhancing the thermal conductivity of sodium acetate-based PCMs by incorporating graphene and other nanomaterials, resulting in faster charging and discharging rates [6].
Strengths: Specialized expertise in thermal energy storage, innovative nucleation technology. Weaknesses: Limited scope beyond thermal storage applications, potential scalability challenges.
Key Innovations
Improved phase change compositions
PatentActiveIN11003DELNP2015A
Innovation
- Aqueous compositions containing sodium acetate trihydrate, an alkali soluble polymer to inhibit anhydrous crystal formation, and a nucleation promoter to promote stable phase changes, ensuring resistance to sodium acetate crystallization and maintaining thermodynamic stability across repeated heating and cooling cycles.
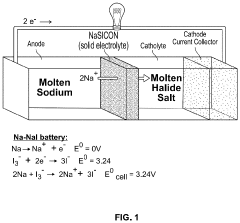
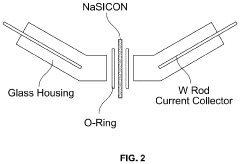
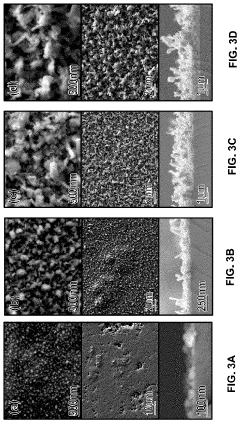
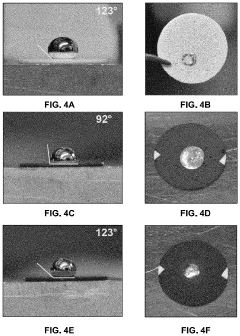
Method to Improve Sodium Electrochemical Interfaces of Sodium Ion-Conducting Ceramics
PatentPendingUS20230207787A1
Innovation
- Coating sodium ion-conducting ceramics with materials such as tin, bismuth, lead, antimony, germanium, silicon, or gold to form a sodium intermetallic phase, which enhances sodium ion conduction and reduces interfacial resistance, allowing for improved charge transfer and wetting at lower temperatures.
Environmental Impact
The environmental impact of sodium acetate production and usage is a critical consideration in the transformation of chemical engineering practices. This compound, widely used in various industries, has both positive and negative effects on the environment that warrant careful examination.
Sodium acetate production primarily involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. This process is relatively straightforward and can be conducted using renewable resources, potentially reducing the carbon footprint compared to more complex chemical syntheses. However, the sourcing of raw materials, particularly acetic acid, may still contribute to environmental concerns if derived from fossil fuel-based processes.
One of the most significant environmental benefits of sodium acetate lies in its potential as a phase change material for thermal energy storage. This application can contribute to energy efficiency in buildings and industrial processes, indirectly reducing greenhouse gas emissions associated with heating and cooling systems. Additionally, sodium acetate's use in de-icing solutions offers a more environmentally friendly alternative to traditional rock salt, potentially reducing chloride contamination in soil and water bodies.
In the textile industry, sodium acetate serves as a dyeing auxiliary, improving color fastness and reducing the need for harsh chemicals. This can lead to decreased water pollution from textile effluents. Similarly, its use in the food industry as a preservative and acidity regulator may reduce food waste, indirectly mitigating environmental impacts associated with food production and disposal.
However, the widespread use of sodium acetate also presents environmental challenges. Its production and transportation contribute to carbon emissions, albeit at lower levels compared to many other industrial chemicals. The disposal of sodium acetate-containing products can lead to increased sodium levels in water bodies, potentially affecting aquatic ecosystems. This is particularly concerning in areas where large quantities are used for de-icing purposes.
Furthermore, the increasing demand for sodium acetate in various applications may lead to expanded production facilities, potentially impacting local ecosystems through land use changes and industrial emissions. Proper regulation and sustainable production practices are essential to mitigate these risks.
To address these environmental concerns, research efforts are focusing on developing more sustainable production methods for sodium acetate, such as utilizing bio-based acetic acid sources. Additionally, closed-loop recycling systems for sodium acetate in industrial applications are being explored to minimize waste and environmental discharge.
In conclusion, while sodium acetate offers several environmental benefits in its applications, its production and use still pose challenges that require ongoing research and innovation to ensure sustainable practices in chemical engineering.
Sodium acetate production primarily involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. This process is relatively straightforward and can be conducted using renewable resources, potentially reducing the carbon footprint compared to more complex chemical syntheses. However, the sourcing of raw materials, particularly acetic acid, may still contribute to environmental concerns if derived from fossil fuel-based processes.
One of the most significant environmental benefits of sodium acetate lies in its potential as a phase change material for thermal energy storage. This application can contribute to energy efficiency in buildings and industrial processes, indirectly reducing greenhouse gas emissions associated with heating and cooling systems. Additionally, sodium acetate's use in de-icing solutions offers a more environmentally friendly alternative to traditional rock salt, potentially reducing chloride contamination in soil and water bodies.
In the textile industry, sodium acetate serves as a dyeing auxiliary, improving color fastness and reducing the need for harsh chemicals. This can lead to decreased water pollution from textile effluents. Similarly, its use in the food industry as a preservative and acidity regulator may reduce food waste, indirectly mitigating environmental impacts associated with food production and disposal.
However, the widespread use of sodium acetate also presents environmental challenges. Its production and transportation contribute to carbon emissions, albeit at lower levels compared to many other industrial chemicals. The disposal of sodium acetate-containing products can lead to increased sodium levels in water bodies, potentially affecting aquatic ecosystems. This is particularly concerning in areas where large quantities are used for de-icing purposes.
Furthermore, the increasing demand for sodium acetate in various applications may lead to expanded production facilities, potentially impacting local ecosystems through land use changes and industrial emissions. Proper regulation and sustainable production practices are essential to mitigate these risks.
To address these environmental concerns, research efforts are focusing on developing more sustainable production methods for sodium acetate, such as utilizing bio-based acetic acid sources. Additionally, closed-loop recycling systems for sodium acetate in industrial applications are being explored to minimize waste and environmental discharge.
In conclusion, while sodium acetate offers several environmental benefits in its applications, its production and use still pose challenges that require ongoing research and innovation to ensure sustainable practices in chemical engineering.
Regulatory Framework
The regulatory framework surrounding sodium acetate plays a crucial role in shaping its use and applications in chemical engineering practices. As a widely used compound in various industries, sodium acetate is subject to a complex web of regulations that govern its production, handling, storage, and disposal.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of sodium acetate under the Toxic Substances Control Act (TSCA). The compound is listed on the TSCA Inventory, which means it has been assessed for potential risks to human health and the environment. Manufacturers and importers of sodium acetate must comply with reporting requirements and adhere to specific guidelines for its use in commercial applications.
The Food and Drug Administration (FDA) also regulates sodium acetate, particularly in its use as a food additive and in pharmaceutical applications. It is classified as Generally Recognized as Safe (GRAS) for use in food products, subject to certain limitations and specifications. In the pharmaceutical industry, sodium acetate is used in various formulations, and its production and use must comply with Good Manufacturing Practices (GMP) as outlined by the FDA.
Internationally, the regulation of sodium acetate varies by country and region. In the European Union, it falls under the purview of the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Companies manufacturing or importing sodium acetate in quantities exceeding one tonne per year must register the substance with the European Chemicals Agency (ECHA) and provide detailed safety information.
Occupational safety regulations also apply to the handling of sodium acetate in industrial settings. The Occupational Safety and Health Administration (OSHA) in the United States sets standards for workplace exposure limits and safety protocols. Similarly, other countries have their own occupational health and safety regulations that must be adhered to when working with sodium acetate.
Transportation of sodium acetate is regulated by various agencies, including the Department of Transportation (DOT) in the United States and the International Air Transport Association (IATA) for global air transport. These regulations specify packaging, labeling, and documentation requirements to ensure safe transport of the compound.
As research on sodium acetate continues to advance chemical engineering practices, it is essential for researchers and industry professionals to stay abreast of evolving regulatory requirements. This includes monitoring changes in classification, labeling, and safety data sheet requirements, as well as any new restrictions or authorizations that may impact its use in specific applications.
The regulatory landscape for sodium acetate is dynamic, with ongoing assessments of its environmental impact and potential health effects. As new data emerges, regulatory bodies may update their guidelines, potentially affecting the compound's use in various industries. Therefore, continuous monitoring and compliance with regulatory frameworks are crucial for the sustainable and responsible use of sodium acetate in transforming chemical engineering practices.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of sodium acetate under the Toxic Substances Control Act (TSCA). The compound is listed on the TSCA Inventory, which means it has been assessed for potential risks to human health and the environment. Manufacturers and importers of sodium acetate must comply with reporting requirements and adhere to specific guidelines for its use in commercial applications.
The Food and Drug Administration (FDA) also regulates sodium acetate, particularly in its use as a food additive and in pharmaceutical applications. It is classified as Generally Recognized as Safe (GRAS) for use in food products, subject to certain limitations and specifications. In the pharmaceutical industry, sodium acetate is used in various formulations, and its production and use must comply with Good Manufacturing Practices (GMP) as outlined by the FDA.
Internationally, the regulation of sodium acetate varies by country and region. In the European Union, it falls under the purview of the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Companies manufacturing or importing sodium acetate in quantities exceeding one tonne per year must register the substance with the European Chemicals Agency (ECHA) and provide detailed safety information.
Occupational safety regulations also apply to the handling of sodium acetate in industrial settings. The Occupational Safety and Health Administration (OSHA) in the United States sets standards for workplace exposure limits and safety protocols. Similarly, other countries have their own occupational health and safety regulations that must be adhered to when working with sodium acetate.
Transportation of sodium acetate is regulated by various agencies, including the Department of Transportation (DOT) in the United States and the International Air Transport Association (IATA) for global air transport. These regulations specify packaging, labeling, and documentation requirements to ensure safe transport of the compound.
As research on sodium acetate continues to advance chemical engineering practices, it is essential for researchers and industry professionals to stay abreast of evolving regulatory requirements. This includes monitoring changes in classification, labeling, and safety data sheet requirements, as well as any new restrictions or authorizations that may impact its use in specific applications.
The regulatory landscape for sodium acetate is dynamic, with ongoing assessments of its environmental impact and potential health effects. As new data emerges, regulatory bodies may update their guidelines, potentially affecting the compound's use in various industries. Therefore, continuous monitoring and compliance with regulatory frameworks are crucial for the sustainable and responsible use of sodium acetate in transforming chemical engineering practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!