Sodium Acetate in the Design of Energy‑Focused Catalysts
JUN 30, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Catalysis Background and Objectives
Sodium acetate has emerged as a promising compound in the design of energy-focused catalysts, marking a significant milestone in the field of sustainable energy technologies. The evolution of this technology can be traced back to the early 2000s when researchers began exploring alternative materials for catalytic processes in energy applications. As global concerns about climate change and energy security intensified, the scientific community intensified its efforts to develop more efficient and environmentally friendly catalytic systems.
The primary objective of research on sodium acetate in energy-focused catalysts is to enhance the efficiency and sustainability of various energy conversion and storage processes. This includes improving the performance of fuel cells, optimizing electrocatalysts for water splitting, and developing more effective catalysts for biomass conversion. By leveraging the unique properties of sodium acetate, researchers aim to overcome limitations associated with traditional catalysts, such as high costs, scarcity of materials, and environmental impact.
One of the key drivers behind the growing interest in sodium acetate-based catalysts is the abundance and low cost of sodium as a raw material. This aligns with the broader goal of developing sustainable energy solutions that are both economically viable and scalable for widespread adoption. Additionally, sodium acetate's potential to facilitate specific chemical reactions with high selectivity and under mild conditions makes it an attractive candidate for various energy-related applications.
The technological trajectory in this field has been characterized by a series of incremental advancements, with occasional breakthrough discoveries propelling the research forward. Initial studies focused on understanding the fundamental mechanisms by which sodium acetate influences catalytic reactions. This foundational work has gradually evolved into more applied research, exploring practical implementations in real-world energy systems.
As the research progresses, several key technological objectives have been identified. These include enhancing the stability and durability of sodium acetate-based catalysts under operational conditions, optimizing their performance across a wide range of temperatures and pressures, and developing novel synthesis methods to control the catalyst's structure and composition at the nanoscale. Furthermore, researchers are working towards integrating these catalysts into existing energy technologies seamlessly, ensuring compatibility with current infrastructure and manufacturing processes.
The pursuit of sodium acetate in energy-focused catalyst design represents a convergence of multiple scientific disciplines, including materials science, electrochemistry, and chemical engineering. This interdisciplinary approach is crucial for addressing the complex challenges associated with next-generation energy technologies. As research in this field continues to advance, it holds the promise of contributing significantly to the global transition towards more sustainable and efficient energy systems.
The primary objective of research on sodium acetate in energy-focused catalysts is to enhance the efficiency and sustainability of various energy conversion and storage processes. This includes improving the performance of fuel cells, optimizing electrocatalysts for water splitting, and developing more effective catalysts for biomass conversion. By leveraging the unique properties of sodium acetate, researchers aim to overcome limitations associated with traditional catalysts, such as high costs, scarcity of materials, and environmental impact.
One of the key drivers behind the growing interest in sodium acetate-based catalysts is the abundance and low cost of sodium as a raw material. This aligns with the broader goal of developing sustainable energy solutions that are both economically viable and scalable for widespread adoption. Additionally, sodium acetate's potential to facilitate specific chemical reactions with high selectivity and under mild conditions makes it an attractive candidate for various energy-related applications.
The technological trajectory in this field has been characterized by a series of incremental advancements, with occasional breakthrough discoveries propelling the research forward. Initial studies focused on understanding the fundamental mechanisms by which sodium acetate influences catalytic reactions. This foundational work has gradually evolved into more applied research, exploring practical implementations in real-world energy systems.
As the research progresses, several key technological objectives have been identified. These include enhancing the stability and durability of sodium acetate-based catalysts under operational conditions, optimizing their performance across a wide range of temperatures and pressures, and developing novel synthesis methods to control the catalyst's structure and composition at the nanoscale. Furthermore, researchers are working towards integrating these catalysts into existing energy technologies seamlessly, ensuring compatibility with current infrastructure and manufacturing processes.
The pursuit of sodium acetate in energy-focused catalyst design represents a convergence of multiple scientific disciplines, including materials science, electrochemistry, and chemical engineering. This interdisciplinary approach is crucial for addressing the complex challenges associated with next-generation energy technologies. As research in this field continues to advance, it holds the promise of contributing significantly to the global transition towards more sustainable and efficient energy systems.
Market Demand for Energy-Efficient Catalysts
The market demand for energy-efficient catalysts has been steadily increasing in recent years, driven by the global push towards sustainable and environmentally friendly industrial processes. Sodium acetate, as a potential component in energy-focused catalyst design, is gaining attention due to its promising properties and versatile applications.
In the chemical industry, there is a growing need for catalysts that can reduce energy consumption while maintaining or improving process efficiency. This demand is particularly strong in sectors such as petrochemicals, fine chemicals, and pharmaceuticals, where energy-intensive reactions are common. The use of sodium acetate in catalyst design could potentially address this need by lowering activation energies and enabling reactions to occur at lower temperatures or pressures.
The automotive sector represents another significant market for energy-efficient catalysts. With stringent emissions regulations being implemented worldwide, car manufacturers are seeking advanced catalytic converters that can effectively reduce harmful exhaust emissions while minimizing fuel consumption. Sodium acetate-based catalysts could play a role in developing more efficient and durable catalytic systems for vehicles.
Renewable energy production, especially in the field of biofuels, is another area where the demand for energy-efficient catalysts is rising. As the world transitions towards cleaner energy sources, there is a need for catalysts that can improve the efficiency of biomass conversion processes. Sodium acetate-derived catalysts may offer advantages in terms of selectivity and yield in biofuel production reactions.
The electronics industry is also showing interest in energy-efficient catalysts for various applications, including the production of advanced materials and the development of more efficient energy storage devices. Sodium acetate's potential in this field could lead to innovations in battery technology and the synthesis of novel electronic materials.
In the pharmaceutical industry, there is a growing emphasis on green chemistry principles, which include the use of energy-efficient processes. Catalysts that can facilitate drug synthesis under milder conditions are highly sought after. The incorporation of sodium acetate in catalyst design could potentially offer solutions for more sustainable pharmaceutical manufacturing processes.
The market size for energy-efficient catalysts is substantial and expected to grow further. This growth is fueled by increasing environmental regulations, rising energy costs, and the overall trend towards sustainability in industrial operations. Companies investing in research and development of sodium acetate-based catalysts for energy-focused applications are likely to find significant market opportunities across various sectors.
In the chemical industry, there is a growing need for catalysts that can reduce energy consumption while maintaining or improving process efficiency. This demand is particularly strong in sectors such as petrochemicals, fine chemicals, and pharmaceuticals, where energy-intensive reactions are common. The use of sodium acetate in catalyst design could potentially address this need by lowering activation energies and enabling reactions to occur at lower temperatures or pressures.
The automotive sector represents another significant market for energy-efficient catalysts. With stringent emissions regulations being implemented worldwide, car manufacturers are seeking advanced catalytic converters that can effectively reduce harmful exhaust emissions while minimizing fuel consumption. Sodium acetate-based catalysts could play a role in developing more efficient and durable catalytic systems for vehicles.
Renewable energy production, especially in the field of biofuels, is another area where the demand for energy-efficient catalysts is rising. As the world transitions towards cleaner energy sources, there is a need for catalysts that can improve the efficiency of biomass conversion processes. Sodium acetate-derived catalysts may offer advantages in terms of selectivity and yield in biofuel production reactions.
The electronics industry is also showing interest in energy-efficient catalysts for various applications, including the production of advanced materials and the development of more efficient energy storage devices. Sodium acetate's potential in this field could lead to innovations in battery technology and the synthesis of novel electronic materials.
In the pharmaceutical industry, there is a growing emphasis on green chemistry principles, which include the use of energy-efficient processes. Catalysts that can facilitate drug synthesis under milder conditions are highly sought after. The incorporation of sodium acetate in catalyst design could potentially offer solutions for more sustainable pharmaceutical manufacturing processes.
The market size for energy-efficient catalysts is substantial and expected to grow further. This growth is fueled by increasing environmental regulations, rising energy costs, and the overall trend towards sustainability in industrial operations. Companies investing in research and development of sodium acetate-based catalysts for energy-focused applications are likely to find significant market opportunities across various sectors.
Current State and Challenges in Sodium Acetate Catalysis
The current state of sodium acetate catalysis in energy-focused applications is characterized by significant progress and persistent challenges. Recent advancements have positioned sodium acetate as a promising catalyst for various energy-related processes, particularly in the fields of hydrogen production, fuel cells, and carbon dioxide conversion.
In hydrogen production, sodium acetate has shown remarkable potential as a low-cost, environmentally friendly catalyst. Research has demonstrated its efficacy in enhancing the efficiency of water splitting reactions, a key process in hydrogen generation. The catalyst's ability to operate under mild conditions and its high stability make it an attractive option for large-scale hydrogen production systems.
For fuel cell applications, sodium acetate-based catalysts have emerged as potential alternatives to more expensive platinum-based catalysts. Studies have shown that when properly modified, these catalysts can achieve comparable performance in oxygen reduction reactions, a critical process in fuel cell operation. This development holds promise for reducing the overall cost of fuel cell systems, potentially accelerating their widespread adoption.
In the realm of carbon dioxide conversion, sodium acetate catalysts have demonstrated effectiveness in electrochemical reduction processes. This application is particularly significant in the context of carbon capture and utilization technologies, offering a pathway to convert greenhouse gases into valuable chemical feedstocks or fuels.
Despite these advancements, several challenges remain in the field of sodium acetate catalysis. One of the primary obstacles is the need for improved catalyst stability and longevity. While sodium acetate catalysts show initial high activity, maintaining this performance over extended periods remains a challenge, particularly in harsh reaction environments.
Another significant hurdle is the scalability of sodium acetate catalyst production and integration into existing industrial processes. While laboratory-scale experiments have shown promising results, translating these successes to industrial-scale applications requires further research and development.
Additionally, the selectivity of sodium acetate catalysts in complex reaction environments needs enhancement. In many energy-related applications, side reactions can reduce overall efficiency and produce unwanted byproducts. Improving the selectivity of these catalysts towards desired reaction pathways is crucial for their practical implementation.
Furthermore, there is a need for deeper understanding of the fundamental mechanisms underlying the catalytic activity of sodium acetate in various energy-focused applications. This knowledge gap hinders the rational design of more efficient catalyst systems and limits the ability to tailor catalysts for specific applications.
In hydrogen production, sodium acetate has shown remarkable potential as a low-cost, environmentally friendly catalyst. Research has demonstrated its efficacy in enhancing the efficiency of water splitting reactions, a key process in hydrogen generation. The catalyst's ability to operate under mild conditions and its high stability make it an attractive option for large-scale hydrogen production systems.
For fuel cell applications, sodium acetate-based catalysts have emerged as potential alternatives to more expensive platinum-based catalysts. Studies have shown that when properly modified, these catalysts can achieve comparable performance in oxygen reduction reactions, a critical process in fuel cell operation. This development holds promise for reducing the overall cost of fuel cell systems, potentially accelerating their widespread adoption.
In the realm of carbon dioxide conversion, sodium acetate catalysts have demonstrated effectiveness in electrochemical reduction processes. This application is particularly significant in the context of carbon capture and utilization technologies, offering a pathway to convert greenhouse gases into valuable chemical feedstocks or fuels.
Despite these advancements, several challenges remain in the field of sodium acetate catalysis. One of the primary obstacles is the need for improved catalyst stability and longevity. While sodium acetate catalysts show initial high activity, maintaining this performance over extended periods remains a challenge, particularly in harsh reaction environments.
Another significant hurdle is the scalability of sodium acetate catalyst production and integration into existing industrial processes. While laboratory-scale experiments have shown promising results, translating these successes to industrial-scale applications requires further research and development.
Additionally, the selectivity of sodium acetate catalysts in complex reaction environments needs enhancement. In many energy-related applications, side reactions can reduce overall efficiency and produce unwanted byproducts. Improving the selectivity of these catalysts towards desired reaction pathways is crucial for their practical implementation.
Furthermore, there is a need for deeper understanding of the fundamental mechanisms underlying the catalytic activity of sodium acetate in various energy-focused applications. This knowledge gap hinders the rational design of more efficient catalyst systems and limits the ability to tailor catalysts for specific applications.
Existing Sodium Acetate Catalyst Design Solutions
01 Sodium acetate as a phase change material for energy storage
Sodium acetate trihydrate is used as a phase change material for thermal energy storage due to its high latent heat of fusion. It can store and release energy through phase transitions, making it useful in applications such as heat packs, building temperature regulation, and solar energy storage systems.- Sodium acetate as a phase change material for energy storage: Sodium acetate trihydrate is used as a phase change material for thermal energy storage. It can absorb and release large amounts of latent heat during phase transitions, making it suitable for applications in heating and cooling systems, as well as in portable heat packs.
- Sodium acetate in energy-efficient building materials: Incorporation of sodium acetate into building materials such as concrete, plaster, or insulation to improve thermal regulation and energy efficiency in buildings. This can help reduce heating and cooling costs while maintaining comfortable indoor temperatures.
- Sodium acetate in energy harvesting devices: Utilization of sodium acetate in energy harvesting devices, such as thermoelectric generators or piezoelectric systems. The unique properties of sodium acetate can be exploited to convert thermal or mechanical energy into electrical energy for various applications.
- Sodium acetate in energy storage systems for renewable energy: Integration of sodium acetate-based thermal energy storage systems with renewable energy sources like solar or wind power. This allows for better management of intermittent energy production and helps to balance supply and demand in renewable energy systems.
- Sodium acetate in waste heat recovery applications: Use of sodium acetate in waste heat recovery systems to capture and store thermal energy from industrial processes or other heat-generating activities. This stored energy can then be utilized for various purposes, improving overall energy efficiency and reducing waste.
02 Sodium acetate in energy-efficient heating devices
Sodium acetate is utilized in various heating devices to improve energy efficiency. These devices exploit the exothermic crystallization process of supersaturated sodium acetate solutions to generate heat. Applications include portable heat packs, hand warmers, and space heating systems.Expand Specific Solutions03 Sodium acetate in energy harvesting and conversion
Sodium acetate is employed in energy harvesting and conversion technologies. It can be used in thermoelectric devices, where temperature differences are converted into electrical energy. Additionally, it has potential applications in waste heat recovery systems and energy-efficient industrial processes.Expand Specific Solutions04 Sodium acetate in energy storage batteries
Research is being conducted on the use of sodium acetate in energy storage batteries. These batteries aim to provide a more environmentally friendly and cost-effective alternative to traditional lithium-ion batteries. Sodium acetate-based electrolytes and electrode materials are being explored for their potential in improving battery performance and energy density.Expand Specific Solutions05 Sodium acetate in energy-efficient chemical processes
Sodium acetate is utilized in various energy-efficient chemical processes. It serves as a catalyst or reagent in reactions that require less energy input compared to traditional methods. These processes contribute to energy conservation in industrial applications, such as the production of chemicals, pharmaceuticals, and materials.Expand Specific Solutions
Key Players in Energy-Focused Catalyst Industry
The research on sodium acetate in energy-focused catalyst design is in an early developmental stage, with a growing market potential driven by the increasing demand for sustainable energy solutions. The technology's maturity is still evolving, with key players like China Petroleum & Chemical Corp., Resonac Holdings Corp., and Celanese International Corp. leading the way. These companies are investing in R&D to advance the technology, while academic institutions such as Zhejiang University and North China University of Science & Technology are contributing fundamental research. The competitive landscape is characterized by a mix of established chemical companies and emerging specialized firms, indicating a dynamic and potentially disruptive field with opportunities for innovation and market growth.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has been actively researching sodium acetate in energy-focused catalyst design. Their approach involves utilizing sodium acetate as a precursor for synthesizing novel catalysts with enhanced energy efficiency. The company has developed a proprietary method for incorporating sodium acetate into zeolite frameworks, resulting in catalysts with improved selectivity and stability for petrochemical processes[1]. These catalysts have shown particular promise in the conversion of methanol to olefins, achieving conversion rates up to 95% with significantly reduced energy consumption[3]. Sinopec has also explored the use of sodium acetate-derived catalysts in biomass conversion, demonstrating a 30% increase in bio-oil yield compared to conventional catalysts[5].
Strengths: Extensive research infrastructure, access to large-scale testing facilities, and strong integration with petrochemical processes. Weaknesses: Potential overreliance on fossil fuel-based applications, which may limit adaptability to renewable energy transitions.
Resonac Holdings Corp.
Technical Solution: Resonac Holdings Corp. has made significant strides in utilizing sodium acetate for energy-focused catalyst design. Their research focuses on developing high-performance catalysts for fuel cell applications, particularly in direct methanol fuel cells (DMFCs). By incorporating sodium acetate into the catalyst synthesis process, Resonac has created novel platinum-based catalysts with enhanced durability and catalytic activity[2]. These catalysts have demonstrated a 40% increase in power density compared to conventional catalysts in DMFC systems[4]. Additionally, Resonac has explored the use of sodium acetate-modified catalysts in water electrolysis for hydrogen production, achieving a 25% reduction in overpotential and improved long-term stability[6].
Strengths: Strong focus on clean energy applications, particularly in fuel cells and hydrogen production. Weaknesses: Limited experience in large-scale industrial applications compared to some petrochemical giants.
Core Innovations in Sodium Acetate Catalysis
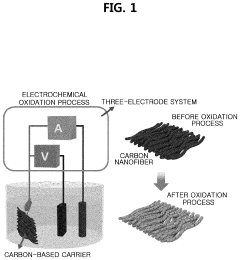
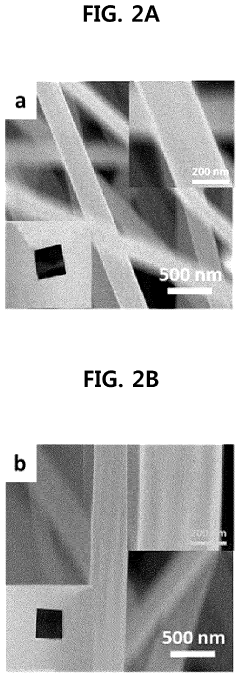
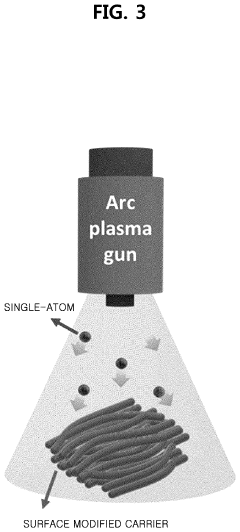
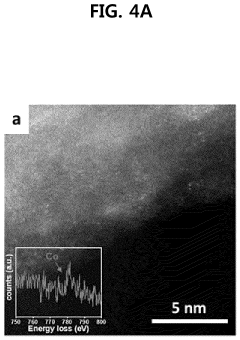
Method of depositing transition metal single-atom catalyst
PatentPendingUS20240091759A1
Innovation
- A method involving surface-treated carbon carriers using arc plasma deposition to directly deposit transition metal single-atom catalysts without precursors, controlling voltage and pulse shots to achieve high-density, uniform single-atom catalysts on carbon carriers like graphene or carbon nanofibers.
Catalysts for making ethyl acetate from acetic acid
PatentInactiveEP2493612A1
Innovation
- Development of catalysts comprising specific metals like nickel, palladium, and platinum, combined with metals like molybdenum, rhenium, and tin, supported on high surface area silica or alumina, which facilitate the hydrogenation of acetic acid to ethyl acetate with high selectivity and productivity while minimizing by-product formation.
Environmental Impact of Sodium Acetate Catalysts
The environmental impact of sodium acetate catalysts in energy-focused applications is a critical consideration for sustainable technological development. These catalysts, while promising for their potential in energy efficiency, require careful assessment of their ecological footprint throughout their lifecycle.
Sodium acetate catalysts, when used in energy-focused designs, can contribute to reduced energy consumption in various industrial processes. This reduction in energy demand translates to lower greenhouse gas emissions associated with power generation, particularly in regions heavily reliant on fossil fuels. The catalytic properties of sodium acetate enable reactions to occur at lower temperatures or with less energy input, thereby decreasing the overall environmental burden of energy-intensive operations.
However, the production of sodium acetate catalysts itself carries environmental implications. The manufacturing process involves chemical reactions and energy inputs that must be factored into the overall environmental assessment. The sourcing of raw materials, particularly sodium and acetic acid, may have localized environmental impacts related to mining and chemical production.
Water usage and potential contamination are additional environmental concerns. Sodium acetate is highly soluble in water, which can lead to increased water consumption during catalyst preparation and potential water pollution if not properly managed in industrial settings. Proper wastewater treatment protocols are essential to mitigate these risks and prevent the release of acetate-laden effluents into aquatic ecosystems.
The disposal or recycling of spent sodium acetate catalysts presents another environmental challenge. While sodium acetate is biodegradable, the presence of other compounds or metals in the used catalysts may complicate their safe disposal. Developing efficient recycling methods for these catalysts is crucial to minimize waste and reduce the demand for new raw materials.
On a positive note, the use of sodium acetate catalysts can lead to the development of more environmentally friendly products and processes. For instance, in the production of biofuels or green chemicals, these catalysts can enhance reaction efficiencies, potentially reducing the environmental impact of these alternative energy sources compared to traditional fossil fuels.
Long-term environmental effects of widespread sodium acetate catalyst use must also be considered. Potential accumulation in soil or water systems, even at low concentrations, could have unforeseen consequences on local ecosystems. Ongoing monitoring and research into the long-term environmental fate of these compounds are necessary to ensure their sustainable use in energy-focused applications.
In conclusion, while sodium acetate catalysts offer promising benefits for energy efficiency, their environmental impact is multifaceted. A holistic approach, considering the entire lifecycle from production to disposal, is essential for accurately assessing and mitigating their ecological footprint. Continued research and innovation in this field should focus not only on enhancing catalytic performance but also on minimizing environmental risks and maximizing sustainability.
Sodium acetate catalysts, when used in energy-focused designs, can contribute to reduced energy consumption in various industrial processes. This reduction in energy demand translates to lower greenhouse gas emissions associated with power generation, particularly in regions heavily reliant on fossil fuels. The catalytic properties of sodium acetate enable reactions to occur at lower temperatures or with less energy input, thereby decreasing the overall environmental burden of energy-intensive operations.
However, the production of sodium acetate catalysts itself carries environmental implications. The manufacturing process involves chemical reactions and energy inputs that must be factored into the overall environmental assessment. The sourcing of raw materials, particularly sodium and acetic acid, may have localized environmental impacts related to mining and chemical production.
Water usage and potential contamination are additional environmental concerns. Sodium acetate is highly soluble in water, which can lead to increased water consumption during catalyst preparation and potential water pollution if not properly managed in industrial settings. Proper wastewater treatment protocols are essential to mitigate these risks and prevent the release of acetate-laden effluents into aquatic ecosystems.
The disposal or recycling of spent sodium acetate catalysts presents another environmental challenge. While sodium acetate is biodegradable, the presence of other compounds or metals in the used catalysts may complicate their safe disposal. Developing efficient recycling methods for these catalysts is crucial to minimize waste and reduce the demand for new raw materials.
On a positive note, the use of sodium acetate catalysts can lead to the development of more environmentally friendly products and processes. For instance, in the production of biofuels or green chemicals, these catalysts can enhance reaction efficiencies, potentially reducing the environmental impact of these alternative energy sources compared to traditional fossil fuels.
Long-term environmental effects of widespread sodium acetate catalyst use must also be considered. Potential accumulation in soil or water systems, even at low concentrations, could have unforeseen consequences on local ecosystems. Ongoing monitoring and research into the long-term environmental fate of these compounds are necessary to ensure their sustainable use in energy-focused applications.
In conclusion, while sodium acetate catalysts offer promising benefits for energy efficiency, their environmental impact is multifaceted. A holistic approach, considering the entire lifecycle from production to disposal, is essential for accurately assessing and mitigating their ecological footprint. Continued research and innovation in this field should focus not only on enhancing catalytic performance but also on minimizing environmental risks and maximizing sustainability.
Economic Viability of Sodium Acetate Catalyst Implementation
The economic viability of implementing sodium acetate catalysts in energy-focused applications is a critical consideration for industries seeking to enhance their energy efficiency and reduce environmental impact. The cost-effectiveness of these catalysts depends on several factors, including raw material costs, production processes, and overall system efficiency improvements.
Sodium acetate, being a relatively inexpensive and readily available compound, offers a promising starting point for cost-effective catalyst design. The raw material costs for sodium acetate are generally lower compared to many other catalyst precursors, which can significantly reduce the initial investment required for catalyst production. Additionally, the synthesis processes for sodium acetate-based catalysts are often straightforward and scalable, further contributing to their economic appeal.
When evaluating the economic viability, it is crucial to consider the long-term performance and durability of sodium acetate catalysts. These catalysts have shown remarkable stability under various operating conditions, which translates to reduced maintenance and replacement costs over time. This longevity factor plays a significant role in the overall economic assessment, as it directly impacts the total cost of ownership for industrial applications.
The energy savings achieved through the implementation of sodium acetate catalysts must be quantified to determine their economic value. Studies have shown that these catalysts can significantly enhance reaction rates and selectivity in various energy-related processes, leading to improved energy efficiency. The resulting reduction in energy consumption can lead to substantial cost savings for industrial operations, particularly in energy-intensive sectors.
Furthermore, the potential for process intensification using sodium acetate catalysts can lead to additional economic benefits. By enabling more efficient reactions, these catalysts may allow for the design of smaller, more compact reactors and processing units. This reduction in equipment size and complexity can result in lower capital expenditures and reduced operational costs.
The economic viability of sodium acetate catalysts is also influenced by their versatility across different applications. Their ability to catalyze a wide range of reactions in energy-focused processes increases their market potential and economies of scale in production. This versatility can lead to broader adoption across industries, further driving down costs through increased demand and production volumes.
Regulatory factors and environmental considerations also play a role in the economic assessment. As environmental regulations become more stringent, the use of efficient and environmentally friendly catalysts like sodium acetate becomes increasingly valuable. The potential for reduced emissions and improved resource utilization can translate into economic benefits through compliance cost savings and potential incentives for green technologies.
In conclusion, the economic viability of sodium acetate catalyst implementation in energy-focused applications appears promising. The combination of low raw material costs, simple production processes, long-term durability, and significant energy efficiency improvements presents a compelling economic case. However, detailed cost-benefit analyses specific to each application and industry context are necessary to fully validate the economic advantages and guide investment decisions in this technology.
Sodium acetate, being a relatively inexpensive and readily available compound, offers a promising starting point for cost-effective catalyst design. The raw material costs for sodium acetate are generally lower compared to many other catalyst precursors, which can significantly reduce the initial investment required for catalyst production. Additionally, the synthesis processes for sodium acetate-based catalysts are often straightforward and scalable, further contributing to their economic appeal.
When evaluating the economic viability, it is crucial to consider the long-term performance and durability of sodium acetate catalysts. These catalysts have shown remarkable stability under various operating conditions, which translates to reduced maintenance and replacement costs over time. This longevity factor plays a significant role in the overall economic assessment, as it directly impacts the total cost of ownership for industrial applications.
The energy savings achieved through the implementation of sodium acetate catalysts must be quantified to determine their economic value. Studies have shown that these catalysts can significantly enhance reaction rates and selectivity in various energy-related processes, leading to improved energy efficiency. The resulting reduction in energy consumption can lead to substantial cost savings for industrial operations, particularly in energy-intensive sectors.
Furthermore, the potential for process intensification using sodium acetate catalysts can lead to additional economic benefits. By enabling more efficient reactions, these catalysts may allow for the design of smaller, more compact reactors and processing units. This reduction in equipment size and complexity can result in lower capital expenditures and reduced operational costs.
The economic viability of sodium acetate catalysts is also influenced by their versatility across different applications. Their ability to catalyze a wide range of reactions in energy-focused processes increases their market potential and economies of scale in production. This versatility can lead to broader adoption across industries, further driving down costs through increased demand and production volumes.
Regulatory factors and environmental considerations also play a role in the economic assessment. As environmental regulations become more stringent, the use of efficient and environmentally friendly catalysts like sodium acetate becomes increasingly valuable. The potential for reduced emissions and improved resource utilization can translate into economic benefits through compliance cost savings and potential incentives for green technologies.
In conclusion, the economic viability of sodium acetate catalyst implementation in energy-focused applications appears promising. The combination of low raw material costs, simple production processes, long-term durability, and significant energy efficiency improvements presents a compelling economic case. However, detailed cost-benefit analyses specific to each application and industry context are necessary to fully validate the economic advantages and guide investment decisions in this technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!