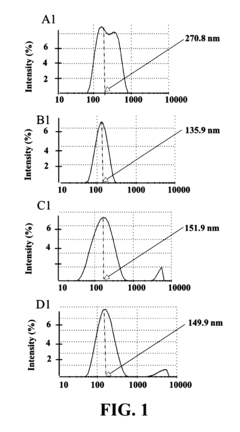
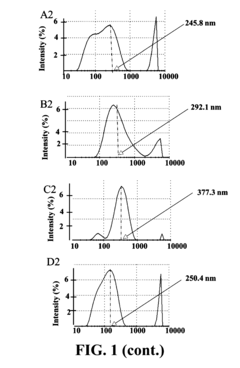
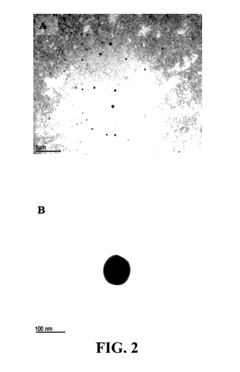
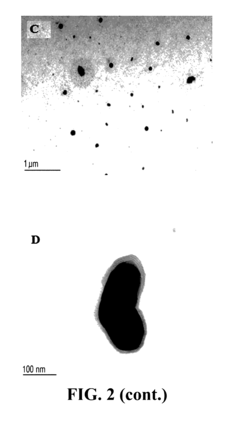
Unlocking the Full Potential of Sodium Acetate in Chemistry
JUN 30, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Evolution
Sodium acetate has undergone a remarkable evolution in the field of chemistry, transforming from a simple compound to a versatile reagent with diverse applications. The journey of sodium acetate began in the early 19th century when it was first synthesized and characterized. Initially, it was primarily used as a food preservative and flavoring agent due to its mild, salty taste and antimicrobial properties.
As chemical knowledge expanded in the late 19th and early 20th centuries, researchers began to explore the broader potential of sodium acetate. Its role in organic synthesis became increasingly apparent, particularly in reactions involving carboxylic acids and their derivatives. The compound's ability to act as a weak base and a source of acetate ions made it valuable in various chemical transformations.
The mid-20th century saw a significant leap in sodium acetate's applications. Its use in buffer solutions became widespread, especially in biochemistry and molecular biology. The compound's ability to maintain a stable pH in aqueous solutions proved crucial in many experimental setups and industrial processes. This period also marked the beginning of sodium acetate's utilization in analytical chemistry, where it found use in titrations and as a standard in various analytical methods.
The latter half of the 20th century brought about a new era for sodium acetate in materials science. Researchers discovered its potential as a phase change material (PCM) for thermal energy storage. This property, based on sodium acetate trihydrate's ability to supercool and release heat upon crystallization, opened up new avenues in sustainable energy applications and temperature regulation systems.
In recent decades, the evolution of sodium acetate has accelerated, driven by advancements in nanotechnology and green chemistry. Its role in the synthesis of nanoparticles and as a precursor for carbon-based materials has gained significant attention. Moreover, the compound's biodegradability and low toxicity have made it increasingly attractive in the development of environmentally friendly chemical processes and products.
The 21st century has seen sodium acetate emerge as a key player in cutting-edge research areas. Its applications in drug delivery systems, smart materials, and even in space technology for life support systems highlight its versatility. The compound's evolution continues as researchers explore its potential in emerging fields such as bioelectronics and sustainable energy storage solutions.
As chemical knowledge expanded in the late 19th and early 20th centuries, researchers began to explore the broader potential of sodium acetate. Its role in organic synthesis became increasingly apparent, particularly in reactions involving carboxylic acids and their derivatives. The compound's ability to act as a weak base and a source of acetate ions made it valuable in various chemical transformations.
The mid-20th century saw a significant leap in sodium acetate's applications. Its use in buffer solutions became widespread, especially in biochemistry and molecular biology. The compound's ability to maintain a stable pH in aqueous solutions proved crucial in many experimental setups and industrial processes. This period also marked the beginning of sodium acetate's utilization in analytical chemistry, where it found use in titrations and as a standard in various analytical methods.
The latter half of the 20th century brought about a new era for sodium acetate in materials science. Researchers discovered its potential as a phase change material (PCM) for thermal energy storage. This property, based on sodium acetate trihydrate's ability to supercool and release heat upon crystallization, opened up new avenues in sustainable energy applications and temperature regulation systems.
In recent decades, the evolution of sodium acetate has accelerated, driven by advancements in nanotechnology and green chemistry. Its role in the synthesis of nanoparticles and as a precursor for carbon-based materials has gained significant attention. Moreover, the compound's biodegradability and low toxicity have made it increasingly attractive in the development of environmentally friendly chemical processes and products.
The 21st century has seen sodium acetate emerge as a key player in cutting-edge research areas. Its applications in drug delivery systems, smart materials, and even in space technology for life support systems highlight its versatility. The compound's evolution continues as researchers explore its potential in emerging fields such as bioelectronics and sustainable energy storage solutions.
Market Demand Analysis
The market demand for sodium acetate has been steadily growing across various industries, driven by its versatile applications and unique properties. In the chemical industry, sodium acetate serves as a crucial raw material for the production of numerous compounds, including cellulose acetate, which finds extensive use in textiles, plastics, and cigarette filters. The increasing demand for these end products has consequently boosted the market for sodium acetate.
The food industry represents another significant sector driving the demand for sodium acetate. As a food additive (E262), it functions as a preservative, acidity regulator, and flavoring agent. With the rising consumer preference for processed and convenience foods, the demand for sodium acetate in this sector is expected to witness substantial growth. Additionally, the expanding fast-food industry globally contributes to the increased consumption of sodium acetate in food applications.
In the pharmaceutical sector, sodium acetate plays a vital role in the formulation of various medications and as a buffering agent in intravenous fluids. The growing healthcare industry, coupled with the increasing prevalence of chronic diseases, is anticipated to fuel the demand for sodium acetate in pharmaceutical applications.
The textile industry also contributes significantly to the market demand for sodium acetate. It is used as a dyeing auxiliary and in the production of textile chemicals, enhancing the overall quality and durability of fabrics. As the global textile industry continues to expand, particularly in developing economies, the demand for sodium acetate is expected to rise correspondingly.
Furthermore, the leather industry utilizes sodium acetate in tanning processes, while the oil and gas sector employs it in drilling fluids and as a de-icing agent for offshore platforms. These diverse applications across multiple industries underscore the robust market demand for sodium acetate.
Geographically, Asia-Pacific is emerging as a key market for sodium acetate, driven by rapid industrialization, population growth, and increasing disposable incomes. North America and Europe also maintain significant market shares, primarily due to their well-established chemical and pharmaceutical industries.
The global sodium acetate market is projected to experience steady growth in the coming years, with factors such as technological advancements, increasing research and development activities, and the exploration of new applications contributing to its expansion. As industries continue to unlock the full potential of sodium acetate in chemistry, the market is poised for further development and innovation.
The food industry represents another significant sector driving the demand for sodium acetate. As a food additive (E262), it functions as a preservative, acidity regulator, and flavoring agent. With the rising consumer preference for processed and convenience foods, the demand for sodium acetate in this sector is expected to witness substantial growth. Additionally, the expanding fast-food industry globally contributes to the increased consumption of sodium acetate in food applications.
In the pharmaceutical sector, sodium acetate plays a vital role in the formulation of various medications and as a buffering agent in intravenous fluids. The growing healthcare industry, coupled with the increasing prevalence of chronic diseases, is anticipated to fuel the demand for sodium acetate in pharmaceutical applications.
The textile industry also contributes significantly to the market demand for sodium acetate. It is used as a dyeing auxiliary and in the production of textile chemicals, enhancing the overall quality and durability of fabrics. As the global textile industry continues to expand, particularly in developing economies, the demand for sodium acetate is expected to rise correspondingly.
Furthermore, the leather industry utilizes sodium acetate in tanning processes, while the oil and gas sector employs it in drilling fluids and as a de-icing agent for offshore platforms. These diverse applications across multiple industries underscore the robust market demand for sodium acetate.
Geographically, Asia-Pacific is emerging as a key market for sodium acetate, driven by rapid industrialization, population growth, and increasing disposable incomes. North America and Europe also maintain significant market shares, primarily due to their well-established chemical and pharmaceutical industries.
The global sodium acetate market is projected to experience steady growth in the coming years, with factors such as technological advancements, increasing research and development activities, and the exploration of new applications contributing to its expansion. As industries continue to unlock the full potential of sodium acetate in chemistry, the market is poised for further development and innovation.
Technical Challenges
Despite the widespread use of sodium acetate in various chemical applications, several technical challenges persist in fully harnessing its potential. One of the primary obstacles is the limited solubility of sodium acetate in certain organic solvents, which restricts its use in some organic synthesis reactions. This solubility issue often necessitates the development of alternative reaction conditions or the use of phase-transfer catalysts, adding complexity to chemical processes.
Another significant challenge lies in the hygroscopic nature of sodium acetate. Its tendency to absorb moisture from the air can lead to clumping and reduced effectiveness in storage and handling. This property also complicates its use in moisture-sensitive reactions, requiring stringent control of environmental conditions during storage and application.
The thermal stability of sodium acetate presents both opportunities and challenges. While its ability to form supersaturated solutions and release heat upon crystallization makes it useful for heat packs, controlling this process precisely for other applications can be difficult. Achieving consistent and predictable crystallization rates for various industrial uses remains a technical hurdle.
In the field of electrochemistry, sodium acetate's potential as an electrolyte is limited by its relatively low conductivity compared to other salts. Enhancing its ionic conductivity without compromising other desirable properties is an ongoing area of research, particularly for applications in energy storage devices.
The pH buffering capacity of sodium acetate solutions, while beneficial in many contexts, can also pose challenges in reactions where precise pH control is critical. Developing methods to fine-tune the buffering effect for specific applications without altering other reaction parameters is a complex task facing chemists.
Furthermore, the production of high-purity sodium acetate at an industrial scale presents its own set of challenges. Removing trace impurities, especially in applications requiring ultra-high purity such as in the pharmaceutical industry, demands sophisticated purification techniques that can be both energy-intensive and costly.
Lastly, the environmental impact of sodium acetate production and disposal is an emerging concern. While generally considered less harmful than many other chemicals, developing more sustainable production methods and exploring biodegradable alternatives for certain applications are becoming increasingly important in the face of growing environmental awareness.
Another significant challenge lies in the hygroscopic nature of sodium acetate. Its tendency to absorb moisture from the air can lead to clumping and reduced effectiveness in storage and handling. This property also complicates its use in moisture-sensitive reactions, requiring stringent control of environmental conditions during storage and application.
The thermal stability of sodium acetate presents both opportunities and challenges. While its ability to form supersaturated solutions and release heat upon crystallization makes it useful for heat packs, controlling this process precisely for other applications can be difficult. Achieving consistent and predictable crystallization rates for various industrial uses remains a technical hurdle.
In the field of electrochemistry, sodium acetate's potential as an electrolyte is limited by its relatively low conductivity compared to other salts. Enhancing its ionic conductivity without compromising other desirable properties is an ongoing area of research, particularly for applications in energy storage devices.
The pH buffering capacity of sodium acetate solutions, while beneficial in many contexts, can also pose challenges in reactions where precise pH control is critical. Developing methods to fine-tune the buffering effect for specific applications without altering other reaction parameters is a complex task facing chemists.
Furthermore, the production of high-purity sodium acetate at an industrial scale presents its own set of challenges. Removing trace impurities, especially in applications requiring ultra-high purity such as in the pharmaceutical industry, demands sophisticated purification techniques that can be both energy-intensive and costly.
Lastly, the environmental impact of sodium acetate production and disposal is an emerging concern. While generally considered less harmful than many other chemicals, developing more sustainable production methods and exploring biodegradable alternatives for certain applications are becoming increasingly important in the face of growing environmental awareness.
Current Applications
01 Use of sodium acetate in chemical processes
Sodium acetate is widely used in various chemical processes as a reagent, catalyst, or buffer. It plays a role in reactions such as acetylation, esterification, and pH control. Its properties make it valuable in industrial applications and laboratory settings.- Use of sodium acetate in chemical processes: Sodium acetate is utilized in various chemical processes, including as a catalyst, pH regulator, or reagent. It plays a role in reactions such as esterification, saponification, and neutralization. The compound's properties make it valuable in industrial applications and laboratory settings.
- Application in heat storage and thermal management: Sodium acetate is employed in heat storage systems and thermal management solutions. Its phase change properties allow it to absorb and release heat effectively, making it useful in heating pads, hand warmers, and energy storage applications. The compound's ability to supercool and crystallize on demand is particularly advantageous in these contexts.
- Use in food and beverage industry: Sodium acetate finds applications in the food and beverage industry as a preservative, flavoring agent, and acidity regulator. It helps extend shelf life, enhance taste, and maintain pH balance in various food products. The compound's generally recognized as safe (GRAS) status makes it suitable for use in food applications.
- Application in textile and paper industries: Sodium acetate is used in textile and paper industries for various purposes. In textiles, it can serve as a mordant in dyeing processes or as a neutralizing agent. In paper production, it may be used for pH control or as an additive to improve paper properties. The compound's solubility and chemical properties make it suitable for these industrial applications.
- Use in pharmaceutical and medical applications: Sodium acetate has applications in the pharmaceutical and medical fields. It can be used as a buffering agent in intravenous fluids, as an excipient in drug formulations, or as a component in dialysis solutions. The compound's biocompatibility and pH-regulating properties make it valuable in these healthcare-related applications.
02 Application in heat storage and thermal management
Sodium acetate trihydrate is utilized in heat storage systems and thermal management applications. It undergoes phase changes that allow it to store and release heat effectively, making it useful in heating pads, hand warmers, and energy storage solutions.Expand Specific Solutions03 Use in food and beverage industry
Sodium acetate serves as a food additive and preservative in the food and beverage industry. It acts as a flavoring agent, acidity regulator, and helps extend the shelf life of various products. Its use is regulated and approved by food safety authorities.Expand Specific Solutions04 Application in textile and leather processing
In the textile and leather industries, sodium acetate is used in dyeing processes, as a mordant, and for pH adjustment. It helps improve color fastness and overall quality of the treated materials. Its properties make it suitable for various fabric and leather treatments.Expand Specific Solutions05 Use in environmental and waste treatment
Sodium acetate finds applications in environmental and waste treatment processes. It is used in wastewater treatment, as a deicer for roads and runways, and in certain pollution control methods. Its biodegradability and low toxicity make it suitable for these purposes.Expand Specific Solutions
Key Industry Players
The sodium acetate market is in a growth phase, driven by increasing applications in various industries. The global market size is expanding, with projections indicating continued growth due to rising demand in sectors like pharmaceuticals, textiles, and food preservation. Technologically, sodium acetate production is mature, but innovations in applications and production methods are ongoing. Key players like BASF, Celanese, and Nantong Alchemy Biotech are investing in R&D to enhance product quality and explore new uses. Emerging companies such as Zhejiang Yishu Environmental Protection Technology are also contributing to market dynamics, focusing on eco-friendly production processes. The competitive landscape is characterized by a mix of established chemical giants and specialized manufacturers, indicating a diverse and evolving market.
Resonac Holdings Corp.
Technical Solution: Resonac Holdings Corp. has focused on unlocking the potential of sodium acetate in specialty chemical applications. They have developed a high-purity grade of sodium acetate for use in electronic materials, particularly in the production of advanced ceramic capacitors[1]. Resonac has also incorporated sodium acetate into their portfolio of functional materials, utilizing it as a key component in heat storage compounds for thermal management solutions in electronics and automotive applications[2]. Additionally, the company has explored the use of sodium acetate in their life science division, leveraging its properties as a pH buffer and preservative in biotechnology and pharmaceutical formulations[3].
Strengths: Specialization in high-value applications, strong presence in Asian markets, and focus on technological innovation. Weaknesses: Potential limitations in scale compared to larger chemical conglomerates and exposure to cyclical demand in electronics industry.
Saudi Basic Industries Corp.
Technical Solution: Saudi Basic Industries Corp. (SABIC) has made significant advancements in utilizing sodium acetate across various chemical applications. They have developed a proprietary process for large-scale sodium acetate production from petrochemical feedstocks, ensuring a stable supply for their downstream operations[1]. SABIC has integrated sodium acetate into their portfolio of performance chemicals, using it as a key ingredient in their range of eco-friendly de-icing solutions for industrial and commercial applications[2]. Furthermore, the company has explored the use of sodium acetate in their innovative materials science division, incorporating it into novel polymer blends to enhance thermal stability and processing characteristics[3].
Strengths: Vertically integrated production, strong R&D capabilities, and global market presence. Weaknesses: Potential dependency on oil prices and challenges in diversifying beyond petrochemicals.
Innovative Research
Formulations for pharmaceutical agents
PatentActiveUS20160361327A1
Innovation
- In situ formation of sodium acetate is used to coat chitosan nanoparticles during the freeze-drying process, enhancing encapsulation efficiency, preventing aggregation, and achieving sustained release by forming a stable core-shell structure.
Process for preparing an unsaturated carboxylic acid salt using an aryloxide
PatentWO2015173277A1
Innovation
- A catalytic process involving a transition metal complex, such as nickel or palladium, with an aryloxide that deprotonates metallalactones formed from ethylene and carbon dioxide, facilitating the formation of unsaturated carboxylic acid salts by controlling the reaction conditions and using specific ligands and bases that coordinate effectively with the transition metal.
Environmental Impact
The environmental impact of sodium acetate in chemistry is a crucial aspect to consider as we explore its full potential. This compound, while versatile and widely used, has both positive and negative implications for the environment. One of the primary benefits of sodium acetate is its biodegradability, which makes it an environmentally friendly alternative to many synthetic chemicals. When released into the environment, it naturally breaks down into harmless components, reducing long-term ecological risks.
In terms of waste management, sodium acetate offers significant advantages. Its use in certain industrial processes can lead to a reduction in harmful byproducts, thereby minimizing the environmental footprint of chemical manufacturing. Additionally, its role in wastewater treatment helps in removing contaminants, contributing to cleaner water systems and reduced pollution levels in aquatic ecosystems.
However, the production of sodium acetate does have some environmental considerations. The manufacturing process, particularly when done on a large scale, can be energy-intensive, contributing to carbon emissions if not managed sustainably. There's also the potential for localized environmental impacts near production facilities, such as increased salt concentrations in soil or water bodies if proper disposal methods are not employed.
In agriculture, sodium acetate's use as a de-icing agent presents a double-edged sword. While it's less corrosive and more environmentally friendly than traditional rock salt, excessive use can still lead to soil salinization and affect plant growth in surrounding areas. This necessitates careful application and monitoring to balance its benefits with potential ecological impacts.
The compound's role in green chemistry initiatives is noteworthy. As a safer alternative to more hazardous chemicals in various applications, sodium acetate contributes to reducing the overall environmental impact of chemical processes. Its use in sustainable packaging materials, for instance, aligns with global efforts to reduce plastic waste and promote biodegradable alternatives.
Looking ahead, further research into optimizing the production and application of sodium acetate could enhance its environmental profile. Developing more energy-efficient manufacturing processes, exploring renewable energy sources for production, and finding innovative applications that leverage its biodegradable properties could significantly boost its eco-friendly credentials. As industries continue to seek sustainable solutions, the environmental impact of sodium acetate will likely remain a key focus area, driving innovations that balance its chemical utility with ecological responsibility.
In terms of waste management, sodium acetate offers significant advantages. Its use in certain industrial processes can lead to a reduction in harmful byproducts, thereby minimizing the environmental footprint of chemical manufacturing. Additionally, its role in wastewater treatment helps in removing contaminants, contributing to cleaner water systems and reduced pollution levels in aquatic ecosystems.
However, the production of sodium acetate does have some environmental considerations. The manufacturing process, particularly when done on a large scale, can be energy-intensive, contributing to carbon emissions if not managed sustainably. There's also the potential for localized environmental impacts near production facilities, such as increased salt concentrations in soil or water bodies if proper disposal methods are not employed.
In agriculture, sodium acetate's use as a de-icing agent presents a double-edged sword. While it's less corrosive and more environmentally friendly than traditional rock salt, excessive use can still lead to soil salinization and affect plant growth in surrounding areas. This necessitates careful application and monitoring to balance its benefits with potential ecological impacts.
The compound's role in green chemistry initiatives is noteworthy. As a safer alternative to more hazardous chemicals in various applications, sodium acetate contributes to reducing the overall environmental impact of chemical processes. Its use in sustainable packaging materials, for instance, aligns with global efforts to reduce plastic waste and promote biodegradable alternatives.
Looking ahead, further research into optimizing the production and application of sodium acetate could enhance its environmental profile. Developing more energy-efficient manufacturing processes, exploring renewable energy sources for production, and finding innovative applications that leverage its biodegradable properties could significantly boost its eco-friendly credentials. As industries continue to seek sustainable solutions, the environmental impact of sodium acetate will likely remain a key focus area, driving innovations that balance its chemical utility with ecological responsibility.
Safety Considerations
When considering the full potential of sodium acetate in chemistry, safety considerations are paramount. Sodium acetate is generally regarded as a relatively safe compound, but proper handling and storage procedures are essential to prevent potential hazards.
Firstly, sodium acetate can cause mild irritation to the eyes, skin, and respiratory system upon direct contact or inhalation. Therefore, appropriate personal protective equipment (PPE) should be worn when handling the compound, including safety goggles, gloves, and a lab coat. In cases where fine particles may become airborne, the use of a dust mask or respirator is recommended.
Storage of sodium acetate requires attention to environmental conditions. The compound is hygroscopic, meaning it readily absorbs moisture from the air. This property can lead to caking or clumping of the material, potentially affecting its quality and usability. To mitigate this issue, sodium acetate should be stored in airtight containers in a cool, dry place.
In laboratory settings, it is crucial to be aware of sodium acetate's compatibility with other chemicals. While generally stable, it can react vigorously with strong oxidizing agents, potentially leading to the release of heat and toxic fumes. Therefore, it should be kept separate from such substances to prevent accidental mixing.
When sodium acetate is heated to decomposition, it can produce toxic fumes including carbon monoxide and sodium oxide. Adequate ventilation is necessary when working with the compound at elevated temperatures, and any thermal decomposition should be conducted in a fume hood.
In industrial applications, where larger quantities of sodium acetate are used, additional safety measures may be required. This includes proper labeling of containers, implementing spill containment strategies, and ensuring that employees are trained in the safe handling and emergency procedures related to the compound.
It is worth noting that sodium acetate has a relatively low toxicity profile compared to many other chemicals used in industry and research. However, ingestion of large quantities can lead to gastrointestinal distress and electrolyte imbalances. As with all chemicals, it should never be consumed intentionally, and accidental ingestion should be addressed immediately by seeking medical attention.
Environmental considerations are also important when working with sodium acetate. While it is biodegradable and not considered highly toxic to aquatic life, large releases into the environment should be avoided. Proper disposal methods should be followed, typically involving dilution and treatment in accordance with local regulations.
Firstly, sodium acetate can cause mild irritation to the eyes, skin, and respiratory system upon direct contact or inhalation. Therefore, appropriate personal protective equipment (PPE) should be worn when handling the compound, including safety goggles, gloves, and a lab coat. In cases where fine particles may become airborne, the use of a dust mask or respirator is recommended.
Storage of sodium acetate requires attention to environmental conditions. The compound is hygroscopic, meaning it readily absorbs moisture from the air. This property can lead to caking or clumping of the material, potentially affecting its quality and usability. To mitigate this issue, sodium acetate should be stored in airtight containers in a cool, dry place.
In laboratory settings, it is crucial to be aware of sodium acetate's compatibility with other chemicals. While generally stable, it can react vigorously with strong oxidizing agents, potentially leading to the release of heat and toxic fumes. Therefore, it should be kept separate from such substances to prevent accidental mixing.
When sodium acetate is heated to decomposition, it can produce toxic fumes including carbon monoxide and sodium oxide. Adequate ventilation is necessary when working with the compound at elevated temperatures, and any thermal decomposition should be conducted in a fume hood.
In industrial applications, where larger quantities of sodium acetate are used, additional safety measures may be required. This includes proper labeling of containers, implementing spill containment strategies, and ensuring that employees are trained in the safe handling and emergency procedures related to the compound.
It is worth noting that sodium acetate has a relatively low toxicity profile compared to many other chemicals used in industry and research. However, ingestion of large quantities can lead to gastrointestinal distress and electrolyte imbalances. As with all chemicals, it should never be consumed intentionally, and accidental ingestion should be addressed immediately by seeking medical attention.
Environmental considerations are also important when working with sodium acetate. While it is biodegradable and not considered highly toxic to aquatic life, large releases into the environment should be avoided. Proper disposal methods should be followed, typically involving dilution and treatment in accordance with local regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!