How Sodium Acetate Drives Innovations in Chemical Resilience?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Evolution
Sodium acetate has undergone a remarkable evolution since its discovery in the early 19th century. Initially recognized as a byproduct of various industrial processes, it has transformed into a versatile compound with wide-ranging applications. The journey of sodium acetate began in the realm of textile manufacturing, where it was used as a mordant to fix dyes to fabrics.
As industrial chemistry advanced, the potential of sodium acetate expanded beyond textiles. In the mid-20th century, researchers discovered its unique properties as a phase change material, capable of storing and releasing thermal energy. This discovery opened up new avenues for its use in heat packs and thermal energy storage systems, marking a significant milestone in its evolution.
The food industry soon recognized the value of sodium acetate as a preservative and flavoring agent. Its ability to regulate acidity and inhibit microbial growth made it an essential ingredient in many processed foods. This application further diversified the compound's utility and increased its commercial importance.
In recent decades, the evolution of sodium acetate has taken an unexpected turn towards chemical resilience. Scientists and engineers have begun exploring its potential in enhancing the durability and resistance of various materials. The compound's ability to form strong, crystalline structures when dehydrated has led to innovations in protective coatings and self-healing materials.
The latest frontier in sodium acetate's evolution lies in its role in advanced energy storage systems. Researchers are investigating its use in next-generation batteries and supercapacitors, leveraging its unique electrochemical properties. This development represents a significant leap from its humble beginnings and showcases the compound's adaptability to emerging technological needs.
As environmental concerns have gained prominence, sodium acetate has found new applications in green chemistry. Its biodegradability and low toxicity make it an attractive alternative to more harmful chemicals in various industrial processes. This shift towards sustainability marks another important phase in the compound's evolutionary journey.
The ongoing research into sodium acetate's properties continues to uncover new potential applications. From advanced materials science to biotechnology, the compound's versatility is being explored in ways that were unimaginable a century ago. This continuous evolution underscores the importance of revisiting and reimagining the potential of well-known chemical compounds in driving innovation and addressing contemporary challenges.
As industrial chemistry advanced, the potential of sodium acetate expanded beyond textiles. In the mid-20th century, researchers discovered its unique properties as a phase change material, capable of storing and releasing thermal energy. This discovery opened up new avenues for its use in heat packs and thermal energy storage systems, marking a significant milestone in its evolution.
The food industry soon recognized the value of sodium acetate as a preservative and flavoring agent. Its ability to regulate acidity and inhibit microbial growth made it an essential ingredient in many processed foods. This application further diversified the compound's utility and increased its commercial importance.
In recent decades, the evolution of sodium acetate has taken an unexpected turn towards chemical resilience. Scientists and engineers have begun exploring its potential in enhancing the durability and resistance of various materials. The compound's ability to form strong, crystalline structures when dehydrated has led to innovations in protective coatings and self-healing materials.
The latest frontier in sodium acetate's evolution lies in its role in advanced energy storage systems. Researchers are investigating its use in next-generation batteries and supercapacitors, leveraging its unique electrochemical properties. This development represents a significant leap from its humble beginnings and showcases the compound's adaptability to emerging technological needs.
As environmental concerns have gained prominence, sodium acetate has found new applications in green chemistry. Its biodegradability and low toxicity make it an attractive alternative to more harmful chemicals in various industrial processes. This shift towards sustainability marks another important phase in the compound's evolutionary journey.
The ongoing research into sodium acetate's properties continues to uncover new potential applications. From advanced materials science to biotechnology, the compound's versatility is being explored in ways that were unimaginable a century ago. This continuous evolution underscores the importance of revisiting and reimagining the potential of well-known chemical compounds in driving innovation and addressing contemporary challenges.
Market Demand Analysis
The market demand for sodium acetate has been steadily growing, driven by its versatile applications in various industries and its role in enhancing chemical resilience. In the food industry, sodium acetate serves as a crucial preservative and flavor enhancer, with increasing consumer preference for natural and clean-label ingredients boosting its demand. The pharmaceutical sector utilizes sodium acetate in drug formulations and as a buffering agent, contributing to the market's expansion.
The textile industry represents another significant market for sodium acetate, where it is employed in dyeing processes and as a mordant. As the global textile market continues to grow, particularly in developing economies, the demand for sodium acetate in this sector is expected to rise. Additionally, the chemical industry uses sodium acetate as a raw material for producing various compounds, further driving market growth.
In recent years, the focus on sustainable and environmentally friendly products has created new opportunities for sodium acetate. Its biodegradability and low toxicity make it an attractive option for eco-conscious consumers and industries seeking greener alternatives. This trend is particularly evident in the personal care and cosmetics sector, where sodium acetate is used in formulations for its pH-balancing properties.
The construction industry has also emerged as a promising market for sodium acetate, particularly in cold regions. Its application in concrete admixtures for cold weather concreting has gained traction, as it helps improve the setting time and strength development of concrete in low-temperature conditions. This niche application is expected to grow as infrastructure development continues in colder climates.
The global sodium acetate market is projected to experience steady growth in the coming years, with Asia-Pacific region leading in terms of consumption and production. Factors such as rapid industrialization, urbanization, and increasing disposable incomes in countries like China and India are driving the demand for sodium acetate across various end-use industries.
However, the market faces challenges such as price volatility of raw materials and competition from alternative products. Manufacturers are focusing on research and development to enhance the properties of sodium acetate and explore new applications to maintain market competitiveness. The ongoing innovations in chemical resilience, particularly in areas such as heat storage materials and phase change materials, are opening up new avenues for sodium acetate applications, potentially expanding its market reach in the near future.
The textile industry represents another significant market for sodium acetate, where it is employed in dyeing processes and as a mordant. As the global textile market continues to grow, particularly in developing economies, the demand for sodium acetate in this sector is expected to rise. Additionally, the chemical industry uses sodium acetate as a raw material for producing various compounds, further driving market growth.
In recent years, the focus on sustainable and environmentally friendly products has created new opportunities for sodium acetate. Its biodegradability and low toxicity make it an attractive option for eco-conscious consumers and industries seeking greener alternatives. This trend is particularly evident in the personal care and cosmetics sector, where sodium acetate is used in formulations for its pH-balancing properties.
The construction industry has also emerged as a promising market for sodium acetate, particularly in cold regions. Its application in concrete admixtures for cold weather concreting has gained traction, as it helps improve the setting time and strength development of concrete in low-temperature conditions. This niche application is expected to grow as infrastructure development continues in colder climates.
The global sodium acetate market is projected to experience steady growth in the coming years, with Asia-Pacific region leading in terms of consumption and production. Factors such as rapid industrialization, urbanization, and increasing disposable incomes in countries like China and India are driving the demand for sodium acetate across various end-use industries.
However, the market faces challenges such as price volatility of raw materials and competition from alternative products. Manufacturers are focusing on research and development to enhance the properties of sodium acetate and explore new applications to maintain market competitiveness. The ongoing innovations in chemical resilience, particularly in areas such as heat storage materials and phase change materials, are opening up new avenues for sodium acetate applications, potentially expanding its market reach in the near future.
Technical Challenges
The development of sodium acetate-based innovations for chemical resilience faces several significant technical challenges. One of the primary obstacles is the material's inherent hygroscopic nature, which can lead to performance degradation in high-humidity environments. This property necessitates the development of advanced encapsulation techniques or moisture-resistant formulations to maintain the compound's effectiveness over extended periods.
Another challenge lies in optimizing the thermal properties of sodium acetate for specific applications. While its phase change characteristics are well-suited for thermal energy storage, fine-tuning the melting point and heat capacity for diverse industrial needs requires sophisticated material engineering. Researchers must explore various additives and processing methods to achieve the desired thermal behavior without compromising other essential properties.
The scalability of sodium acetate-based solutions presents a further hurdle. As demand for chemically resilient materials grows, manufacturers face difficulties in maintaining consistent quality and performance across large-scale production. This challenge is compounded by the need to develop cost-effective synthesis methods that can compete with existing alternatives in the market.
Compatibility issues also arise when integrating sodium acetate into complex chemical systems or composite materials. Ensuring that the compound does not adversely interact with other components or substrates is crucial for its widespread adoption. This requires extensive testing and the development of specialized formulations tailored to specific application environments.
The long-term stability of sodium acetate under various stress conditions remains a concern. Researchers must address potential degradation mechanisms, such as thermal cycling, chemical exposure, and mechanical stress, to ensure the material's reliability in demanding industrial applications. This involves developing accelerated aging protocols and conducting comprehensive life cycle assessments.
Environmental considerations pose additional challenges. As industries strive for sustainability, there is a growing need to develop eco-friendly production methods for sodium acetate and explore its potential for biodegradability or recyclability. This aligns with the broader trend towards green chemistry but requires significant research and development efforts.
Lastly, the regulatory landscape surrounding novel chemical compounds and their applications can present obstacles to innovation. Navigating safety assessments, obtaining necessary certifications, and ensuring compliance with evolving environmental regulations are critical steps in bringing sodium acetate-based solutions to market. This requires close collaboration between researchers, industry partners, and regulatory bodies to establish appropriate standards and guidelines for the safe and effective use of these materials in chemical resilience applications.
Another challenge lies in optimizing the thermal properties of sodium acetate for specific applications. While its phase change characteristics are well-suited for thermal energy storage, fine-tuning the melting point and heat capacity for diverse industrial needs requires sophisticated material engineering. Researchers must explore various additives and processing methods to achieve the desired thermal behavior without compromising other essential properties.
The scalability of sodium acetate-based solutions presents a further hurdle. As demand for chemically resilient materials grows, manufacturers face difficulties in maintaining consistent quality and performance across large-scale production. This challenge is compounded by the need to develop cost-effective synthesis methods that can compete with existing alternatives in the market.
Compatibility issues also arise when integrating sodium acetate into complex chemical systems or composite materials. Ensuring that the compound does not adversely interact with other components or substrates is crucial for its widespread adoption. This requires extensive testing and the development of specialized formulations tailored to specific application environments.
The long-term stability of sodium acetate under various stress conditions remains a concern. Researchers must address potential degradation mechanisms, such as thermal cycling, chemical exposure, and mechanical stress, to ensure the material's reliability in demanding industrial applications. This involves developing accelerated aging protocols and conducting comprehensive life cycle assessments.
Environmental considerations pose additional challenges. As industries strive for sustainability, there is a growing need to develop eco-friendly production methods for sodium acetate and explore its potential for biodegradability or recyclability. This aligns with the broader trend towards green chemistry but requires significant research and development efforts.
Lastly, the regulatory landscape surrounding novel chemical compounds and their applications can present obstacles to innovation. Navigating safety assessments, obtaining necessary certifications, and ensuring compliance with evolving environmental regulations are critical steps in bringing sodium acetate-based solutions to market. This requires close collaboration between researchers, industry partners, and regulatory bodies to establish appropriate standards and guidelines for the safe and effective use of these materials in chemical resilience applications.
Current Solutions
01 Chemical stability of sodium acetate
Sodium acetate exhibits chemical resilience due to its stable molecular structure. It maintains its properties under various conditions, making it suitable for use in diverse applications. The compound's stability contributes to its effectiveness in industrial processes and chemical reactions.- Chemical stability of sodium acetate: Sodium acetate exhibits chemical resilience due to its stable molecular structure. It maintains its properties under various conditions, making it suitable for use in diverse applications. The compound's stability contributes to its effectiveness in industrial processes and chemical reactions.
- Sodium acetate in heat storage applications: The chemical resilience of sodium acetate is utilized in heat storage systems. Its ability to undergo phase changes while maintaining chemical integrity makes it an effective material for thermal energy storage. This property is particularly useful in developing energy-efficient heating and cooling solutions.
- Corrosion resistance properties: Sodium acetate demonstrates resilience against corrosion, making it valuable in protective coatings and anti-corrosion formulations. Its ability to form stable complexes with metal ions contributes to its effectiveness in preventing or reducing corrosion in various materials and environments.
- Use in chemical synthesis and catalysis: The chemical resilience of sodium acetate makes it a versatile reagent in organic synthesis and catalysis. It serves as a stable source of acetate ions and can withstand various reaction conditions, making it useful in a wide range of chemical transformations and industrial processes.
- Environmental and biological applications: Sodium acetate's chemical resilience extends to environmental and biological applications. Its stability and low toxicity make it suitable for use in wastewater treatment, biodegradable materials, and as a buffering agent in biological systems. These properties contribute to its effectiveness in sustainable and eco-friendly solutions.
02 Sodium acetate as a buffering agent
The chemical resilience of sodium acetate is utilized in its role as a buffering agent. It helps maintain stable pH levels in various solutions and products, contributing to their overall stability and effectiveness. This property is particularly valuable in industrial and laboratory settings.Expand Specific Solutions03 Heat storage applications
Sodium acetate's chemical resilience is exploited in heat storage applications. Its ability to undergo phase changes while maintaining chemical stability makes it an excellent material for thermal energy storage systems. This property is utilized in heat packs and other thermal management solutions.Expand Specific Solutions04 Corrosion inhibition properties
The chemical resilience of sodium acetate contributes to its effectiveness as a corrosion inhibitor. It forms protective layers on metal surfaces, preventing oxidation and degradation. This property is valuable in various industries, including manufacturing and construction.Expand Specific Solutions05 Environmental and biodegradation resistance
Sodium acetate demonstrates resilience against environmental factors and biodegradation. This property makes it suitable for use in products and applications where long-term stability is required. However, it can also be engineered to degrade under specific conditions, balancing durability with environmental considerations.Expand Specific Solutions
Industry Leaders
The sodium acetate market is in a growth phase, driven by increasing demand for chemical resilience solutions across various industries. The global market size is expanding, with projections indicating significant growth in the coming years. Technologically, sodium acetate applications are advancing, with companies like Ecolab, L'Oréal, and Corning leading innovations in chemical stability and performance enhancement. FMC Corp. and Henkel are developing novel formulations, while China Petroleum & Chemical Corp. is scaling up production capabilities. Research institutions such as Guangdong University of Technology and Clemson University are contributing to technological advancements, pushing the boundaries of sodium acetate applications in chemical resilience.
Ecolab USA, Inc.
Technical Solution: Ecolab has developed innovative solutions using sodium acetate for chemical resilience in industrial water treatment and cleaning applications. Their technology incorporates sodium acetate as a key component in formulations designed to enhance the stability and effectiveness of cleaning agents in harsh environments. The company's approach involves creating synergistic blends of sodium acetate with other compounds to improve pH buffering capacity and increase the overall resilience of cleaning solutions against chemical degradation[1]. This allows for more efficient and longer-lasting cleaning processes in industrial settings, particularly in high-temperature or chemically aggressive environments[2]. Ecolab's research has also focused on leveraging the chelating properties of sodium acetate to develop scale inhibition technologies for water treatment systems, contributing to improved equipment longevity and reduced maintenance costs[3].
Strengths: Extensive experience in industrial applications, strong R&D capabilities, and a wide market reach. Weaknesses: Potential dependency on specific industry sectors and competition from specialized chemical manufacturers.
FMC Corp.
Technical Solution: FMC Corporation has pioneered the use of sodium acetate in developing resilient agricultural solutions. Their innovative approach involves incorporating sodium acetate into controlled-release fertilizer formulations, enhancing nutrient availability and soil health. The company's technology utilizes sodium acetate's hygroscopic properties to create moisture-resistant coatings for fertilizer granules, significantly improving their stability in varying environmental conditions[4]. This innovation has led to the development of fertilizers that maintain their efficacy for extended periods, even in challenging climates. FMC has also explored the use of sodium acetate as a buffering agent in pesticide formulations, increasing their chemical stability and reducing degradation when exposed to extreme pH conditions[5]. Additionally, the company has investigated sodium acetate's potential as a bio-based platform chemical for sustainable agricultural product development[6].
Strengths: Strong position in the agricultural sector, extensive research capabilities, and a focus on sustainable solutions. Weaknesses: Potential regulatory challenges in different markets and competition from other agrochemical companies.
Key Innovations
Improved phase change compositions
PatentActiveIN11003DELNP2015A
Innovation
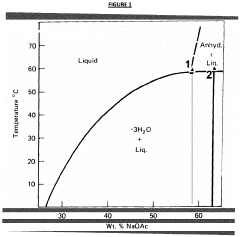
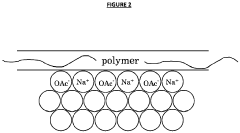
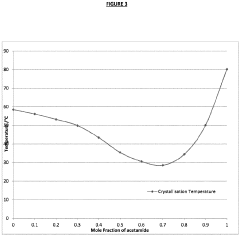
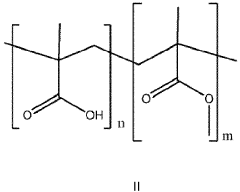
- Aqueous compositions containing sodium acetate trihydrate, an alkali soluble polymer to inhibit anhydrous crystal formation, and a nucleation promoter to promote stable phase changes, ensuring resistance to sodium acetate crystallization and maintaining thermodynamic stability across repeated heating and cooling cycles.
Improved phase change compositions
PatentPendingEP4023732A1
Innovation
- The development of aqueous compositions containing sodium acetate trihydrate, combined with alkali soluble polymers to inhibit sodium acetate anhydrous crystal formation and nucleation promoters to promote stable nucleation, which enhances homogeneity and thermodynamic stability, preventing solid sodium acetate formation and maintaining a fully liquid state at 58°C.
Environmental Impact
Sodium acetate, a versatile compound with applications across various industries, has been increasingly recognized for its potential to drive innovations in chemical resilience. However, its environmental impact must be carefully considered as its usage expands. The production and utilization of sodium acetate have both positive and negative effects on the environment, necessitating a comprehensive assessment of its ecological footprint.
One of the primary environmental benefits of sodium acetate lies in its potential to replace more harmful chemicals in certain applications. For instance, in de-icing solutions for roads and runways, sodium acetate offers a less corrosive and more environmentally friendly alternative to traditional rock salt. This substitution can lead to reduced damage to vegetation, soil, and water bodies adjacent to treated areas, thereby preserving local ecosystems.
Furthermore, sodium acetate's biodegradability contributes to its environmental appeal. Unlike many synthetic chemicals that persist in the environment for extended periods, sodium acetate breaks down relatively quickly into harmless components. This characteristic minimizes long-term accumulation in soil and water systems, reducing the risk of chronic environmental contamination.
However, the production of sodium acetate is not without environmental concerns. The manufacturing process typically involves the reaction of acetic acid with sodium hydroxide or sodium carbonate, which can be energy-intensive and generate greenhouse gas emissions. Additionally, the sourcing of raw materials for sodium acetate production may contribute to resource depletion and habitat disruption if not managed sustainably.
Water usage and potential contamination are also important considerations in the environmental impact of sodium acetate. While the compound itself is generally considered safe for aquatic life at typical concentrations, improper disposal or accidental releases in large quantities could potentially disrupt local water ecosystems. Proper handling, storage, and waste management practices are crucial to mitigate these risks.
As innovations in chemical resilience continue to evolve, researchers are exploring ways to enhance the environmental profile of sodium acetate. This includes developing more efficient production methods that reduce energy consumption and emissions, as well as investigating sustainable sourcing options for raw materials. Additionally, efforts are being made to optimize the use of sodium acetate in various applications to maximize its benefits while minimizing potential negative impacts on the environment.
In conclusion, while sodium acetate offers several environmental advantages in terms of its applications and biodegradability, its overall environmental impact is complex and multifaceted. As the compound plays an increasingly important role in driving innovations for chemical resilience, ongoing research and careful management practices will be essential to ensure its sustainable use and minimize any potential adverse effects on the environment.
One of the primary environmental benefits of sodium acetate lies in its potential to replace more harmful chemicals in certain applications. For instance, in de-icing solutions for roads and runways, sodium acetate offers a less corrosive and more environmentally friendly alternative to traditional rock salt. This substitution can lead to reduced damage to vegetation, soil, and water bodies adjacent to treated areas, thereby preserving local ecosystems.
Furthermore, sodium acetate's biodegradability contributes to its environmental appeal. Unlike many synthetic chemicals that persist in the environment for extended periods, sodium acetate breaks down relatively quickly into harmless components. This characteristic minimizes long-term accumulation in soil and water systems, reducing the risk of chronic environmental contamination.
However, the production of sodium acetate is not without environmental concerns. The manufacturing process typically involves the reaction of acetic acid with sodium hydroxide or sodium carbonate, which can be energy-intensive and generate greenhouse gas emissions. Additionally, the sourcing of raw materials for sodium acetate production may contribute to resource depletion and habitat disruption if not managed sustainably.
Water usage and potential contamination are also important considerations in the environmental impact of sodium acetate. While the compound itself is generally considered safe for aquatic life at typical concentrations, improper disposal or accidental releases in large quantities could potentially disrupt local water ecosystems. Proper handling, storage, and waste management practices are crucial to mitigate these risks.
As innovations in chemical resilience continue to evolve, researchers are exploring ways to enhance the environmental profile of sodium acetate. This includes developing more efficient production methods that reduce energy consumption and emissions, as well as investigating sustainable sourcing options for raw materials. Additionally, efforts are being made to optimize the use of sodium acetate in various applications to maximize its benefits while minimizing potential negative impacts on the environment.
In conclusion, while sodium acetate offers several environmental advantages in terms of its applications and biodegradability, its overall environmental impact is complex and multifaceted. As the compound plays an increasingly important role in driving innovations for chemical resilience, ongoing research and careful management practices will be essential to ensure its sustainable use and minimize any potential adverse effects on the environment.
Safety Regulations
The safety regulations surrounding sodium acetate and its applications in chemical resilience have evolved significantly in recent years, reflecting the growing importance of this compound in various industries. Regulatory bodies worldwide have recognized the need for comprehensive guidelines to ensure the safe handling, storage, and use of sodium acetate, particularly in light of its innovative applications in enhancing chemical resilience.
One of the key areas of focus in safety regulations is the proper storage and handling of sodium acetate. Given its hygroscopic nature, regulations typically mandate that sodium acetate be stored in tightly sealed containers in cool, dry environments. This is crucial to prevent moisture absorption, which can lead to caking and potential chemical changes. Many jurisdictions now require specific labeling and packaging standards to ensure clear identification and proper handling instructions.
Transportation regulations for sodium acetate have also been strengthened, particularly for bulk shipments. While sodium acetate is generally considered non-hazardous, its potential to form flammable vapors when heated has led to specific guidelines for its transport. These often include requirements for proper ventilation, temperature control, and segregation from incompatible materials during shipping.
In industrial settings, occupational safety regulations have been updated to address the unique properties of sodium acetate. This includes mandates for personal protective equipment (PPE) when handling large quantities, as well as guidelines for emergency response procedures in case of spills or accidental releases. Many regulatory frameworks now require regular training for personnel working with sodium acetate, focusing on its chemical properties and potential hazards.
Environmental regulations have also evolved to address the potential impacts of sodium acetate. While it is generally considered environmentally benign, regulations often focus on preventing large-scale releases into aquatic environments due to its potential to alter pH levels. Waste disposal guidelines typically classify sodium acetate waste based on concentration and form, with specific protocols for its treatment and disposal.
As sodium acetate finds new applications in chemical resilience, such as in phase change materials for thermal energy storage, regulatory bodies are adapting their frameworks to address these novel uses. This includes developing new testing protocols to assess the long-term stability and safety of sodium acetate-based materials in various environmental conditions.
The regulatory landscape also reflects an increased focus on the lifecycle management of sodium acetate products. This encompasses regulations governing the sourcing of raw materials, manufacturing processes, and end-of-life considerations for products incorporating sodium acetate. Such comprehensive approaches aim to ensure the sustainable and safe use of this versatile compound throughout its entire lifecycle.
One of the key areas of focus in safety regulations is the proper storage and handling of sodium acetate. Given its hygroscopic nature, regulations typically mandate that sodium acetate be stored in tightly sealed containers in cool, dry environments. This is crucial to prevent moisture absorption, which can lead to caking and potential chemical changes. Many jurisdictions now require specific labeling and packaging standards to ensure clear identification and proper handling instructions.
Transportation regulations for sodium acetate have also been strengthened, particularly for bulk shipments. While sodium acetate is generally considered non-hazardous, its potential to form flammable vapors when heated has led to specific guidelines for its transport. These often include requirements for proper ventilation, temperature control, and segregation from incompatible materials during shipping.
In industrial settings, occupational safety regulations have been updated to address the unique properties of sodium acetate. This includes mandates for personal protective equipment (PPE) when handling large quantities, as well as guidelines for emergency response procedures in case of spills or accidental releases. Many regulatory frameworks now require regular training for personnel working with sodium acetate, focusing on its chemical properties and potential hazards.
Environmental regulations have also evolved to address the potential impacts of sodium acetate. While it is generally considered environmentally benign, regulations often focus on preventing large-scale releases into aquatic environments due to its potential to alter pH levels. Waste disposal guidelines typically classify sodium acetate waste based on concentration and form, with specific protocols for its treatment and disposal.
As sodium acetate finds new applications in chemical resilience, such as in phase change materials for thermal energy storage, regulatory bodies are adapting their frameworks to address these novel uses. This includes developing new testing protocols to assess the long-term stability and safety of sodium acetate-based materials in various environmental conditions.
The regulatory landscape also reflects an increased focus on the lifecycle management of sodium acetate products. This encompasses regulations governing the sourcing of raw materials, manufacturing processes, and end-of-life considerations for products incorporating sodium acetate. Such comprehensive approaches aim to ensure the sustainable and safe use of this versatile compound throughout its entire lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!