Fluoroantimonic Acid: A Pillar in Future Chemistry
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid: Evolution and Objectives
Fluoroantimonic acid, often hailed as the world's strongest superacid, has a rich history dating back to its discovery in the early 20th century. This compound, formed by mixing hydrogen fluoride with antimony pentafluoride, has been a subject of fascination for chemists due to its extraordinary acidity and unique properties. The evolution of fluoroantimonic acid research has been marked by significant milestones in understanding its structure, reactivity, and potential applications.
Initially, the focus was on characterizing the acid's extreme acidity, which surpasses that of 100% sulfuric acid by many orders of magnitude. Researchers worked to quantify its acidity on the Hammett acidity function, establishing it as one of the most powerful proton donors known to science. This led to a surge of interest in exploring its chemical behavior and reactivity with various substances.
As analytical techniques advanced, scientists gained deeper insights into the molecular structure of fluoroantimonic acid. The formation of complex fluoroantimonates and the role of hydrogen bonding in stabilizing these structures became key areas of study. This structural understanding paved the way for investigating the acid's potential in catalysis and organic synthesis.
The objectives of fluoroantimonic acid research have evolved significantly over time. Early goals centered on fundamental characterization and safety protocols for handling such a corrosive substance. As its potential became apparent, objectives shifted towards exploring practical applications. These included its use as a superacid catalyst in petrochemical processes, particularly in the isomerization of alkanes and the cracking of hydrocarbons.
More recently, the focus has expanded to include the acid's role in materials science. Researchers are investigating its potential in the synthesis of novel materials, including advanced polymers and nanostructures. The ability of fluoroantimonic acid to protonate even very weak bases has opened up new possibilities in organic synthesis, allowing for reactions that were previously challenging or impossible.
Looking ahead, the objectives for fluoroantimonic acid research are multifaceted. There is a growing interest in developing more stable and manageable forms of the acid for broader industrial application. Environmental considerations are also driving research into recyclable or less hazardous alternatives that maintain similar levels of acidity. Additionally, the potential of fluoroantimonic acid in emerging fields such as energy storage and advanced materials continues to be a key area of exploration.
As we move forward, the evolution of fluoroantimonic acid research aims to balance its extraordinary chemical properties with practical, safe, and sustainable applications. The ongoing objectives include optimizing its use in existing applications while discovering novel uses that could revolutionize various sectors of chemistry and materials science.
Initially, the focus was on characterizing the acid's extreme acidity, which surpasses that of 100% sulfuric acid by many orders of magnitude. Researchers worked to quantify its acidity on the Hammett acidity function, establishing it as one of the most powerful proton donors known to science. This led to a surge of interest in exploring its chemical behavior and reactivity with various substances.
As analytical techniques advanced, scientists gained deeper insights into the molecular structure of fluoroantimonic acid. The formation of complex fluoroantimonates and the role of hydrogen bonding in stabilizing these structures became key areas of study. This structural understanding paved the way for investigating the acid's potential in catalysis and organic synthesis.
The objectives of fluoroantimonic acid research have evolved significantly over time. Early goals centered on fundamental characterization and safety protocols for handling such a corrosive substance. As its potential became apparent, objectives shifted towards exploring practical applications. These included its use as a superacid catalyst in petrochemical processes, particularly in the isomerization of alkanes and the cracking of hydrocarbons.
More recently, the focus has expanded to include the acid's role in materials science. Researchers are investigating its potential in the synthesis of novel materials, including advanced polymers and nanostructures. The ability of fluoroantimonic acid to protonate even very weak bases has opened up new possibilities in organic synthesis, allowing for reactions that were previously challenging or impossible.
Looking ahead, the objectives for fluoroantimonic acid research are multifaceted. There is a growing interest in developing more stable and manageable forms of the acid for broader industrial application. Environmental considerations are also driving research into recyclable or less hazardous alternatives that maintain similar levels of acidity. Additionally, the potential of fluoroantimonic acid in emerging fields such as energy storage and advanced materials continues to be a key area of exploration.
As we move forward, the evolution of fluoroantimonic acid research aims to balance its extraordinary chemical properties with practical, safe, and sustainable applications. The ongoing objectives include optimizing its use in existing applications while discovering novel uses that could revolutionize various sectors of chemistry and materials science.
Market Demand Analysis
Fluoroantimonic acid, the strongest known superacid, is gaining significant attention in the chemical industry due to its unique properties and potential applications. The market demand for this powerful compound is driven by several factors, including its use in advanced materials synthesis, catalysis, and energy storage technologies.
In the petrochemical sector, fluoroantimonic acid shows promise as a catalyst for hydrocarbon cracking and isomerization processes. Its ability to protonate even weak bases makes it an attractive option for improving the efficiency of fuel production and refining. This application alone represents a substantial market opportunity, as the global petrochemical industry continues to grow and seek more efficient production methods.
The electronics industry is another key driver of demand for fluoroantimonic acid. Its use in the etching of silicon wafers for semiconductor production is becoming increasingly important as manufacturers strive to create smaller and more powerful electronic components. The ongoing trend towards miniaturization in electronics suggests a sustained demand for this superacid in the coming years.
In materials science, fluoroantimonic acid is finding applications in the synthesis of novel polymers and advanced composites. Its extreme acidity allows for unique chemical reactions that can produce materials with enhanced properties, such as improved strength, thermal stability, or chemical resistance. As industries like aerospace, automotive, and construction seek innovative materials to meet evolving performance requirements, the demand for fluoroantimonic acid in this sector is expected to grow.
The energy storage market presents another significant opportunity for fluoroantimonic acid. Research into its potential use in next-generation batteries, particularly for electric vehicles and grid-scale energy storage, is ongoing. If successful, this could lead to a substantial increase in demand, driven by the global push towards renewable energy and electrification.
However, it's important to note that the market for fluoroantimonic acid faces certain challenges. Its extreme corrosiveness and reactivity require specialized handling and storage, which can increase costs and limit its widespread adoption. Additionally, environmental and safety concerns may lead to regulatory restrictions in some regions, potentially impacting market growth.
Despite these challenges, the overall market trajectory for fluoroantimonic acid appears positive. As research continues to uncover new applications and industries recognize its potential benefits, demand is expected to rise. The development of safer handling methods and more environmentally friendly production processes could further expand its market potential, solidifying its position as a key component in future chemical technologies.
In the petrochemical sector, fluoroantimonic acid shows promise as a catalyst for hydrocarbon cracking and isomerization processes. Its ability to protonate even weak bases makes it an attractive option for improving the efficiency of fuel production and refining. This application alone represents a substantial market opportunity, as the global petrochemical industry continues to grow and seek more efficient production methods.
The electronics industry is another key driver of demand for fluoroantimonic acid. Its use in the etching of silicon wafers for semiconductor production is becoming increasingly important as manufacturers strive to create smaller and more powerful electronic components. The ongoing trend towards miniaturization in electronics suggests a sustained demand for this superacid in the coming years.
In materials science, fluoroantimonic acid is finding applications in the synthesis of novel polymers and advanced composites. Its extreme acidity allows for unique chemical reactions that can produce materials with enhanced properties, such as improved strength, thermal stability, or chemical resistance. As industries like aerospace, automotive, and construction seek innovative materials to meet evolving performance requirements, the demand for fluoroantimonic acid in this sector is expected to grow.
The energy storage market presents another significant opportunity for fluoroantimonic acid. Research into its potential use in next-generation batteries, particularly for electric vehicles and grid-scale energy storage, is ongoing. If successful, this could lead to a substantial increase in demand, driven by the global push towards renewable energy and electrification.
However, it's important to note that the market for fluoroantimonic acid faces certain challenges. Its extreme corrosiveness and reactivity require specialized handling and storage, which can increase costs and limit its widespread adoption. Additionally, environmental and safety concerns may lead to regulatory restrictions in some regions, potentially impacting market growth.
Despite these challenges, the overall market trajectory for fluoroantimonic acid appears positive. As research continues to uncover new applications and industries recognize its potential benefits, demand is expected to rise. The development of safer handling methods and more environmentally friendly production processes could further expand its market potential, solidifying its position as a key component in future chemical technologies.
Current State and Challenges
Fluoroantimonic acid, often hailed as the world's strongest superacid, currently stands at the forefront of chemical research and industrial applications. Its unparalleled acidity, with a Hammett acidity function estimated at -21.6, far surpasses that of pure sulfuric acid. This extraordinary property has positioned fluoroantimonic acid as a potential game-changer in various fields of chemistry and materials science.
The current state of fluoroantimonic acid research is characterized by intense exploration of its catalytic properties. Scientists worldwide are investigating its potential to facilitate challenging chemical transformations, particularly in the petrochemical industry. Its ability to protonate even weak bases has opened new avenues for hydrocarbon cracking and isomerization processes, potentially revolutionizing fuel production and refining techniques.
However, the widespread adoption and application of fluoroantimonic acid face significant challenges. Foremost among these is its extreme reactivity, which necessitates specialized handling and storage conditions. The acid's corrosive nature limits the materials that can be used in its containment and application, posing substantial engineering hurdles for large-scale industrial use.
Safety concerns present another major obstacle. The acid's ability to react violently with water and many organic compounds requires stringent safety protocols and specialized training for personnel. This not only increases operational costs but also raises regulatory hurdles in many jurisdictions, potentially limiting its commercial viability.
Environmental considerations also pose challenges to the expanded use of fluoroantimonic acid. Its production and use involve highly toxic and environmentally harmful compounds, including hydrogen fluoride. Developing sustainable production methods and ensuring safe disposal or recycling of waste products remain critical challenges for researchers and industry professionals.
From a geographical perspective, research and development in fluoroantimonic acid technology are primarily concentrated in advanced chemical research facilities in North America, Europe, and East Asia. This concentration reflects the high level of expertise and sophisticated infrastructure required to work with such a potent substance.
Looking ahead, the key technical challenges revolve around developing more stable formulations of the acid, creating more resistant containment materials, and designing safer handling systems. Researchers are also exploring ways to harness the acid's extreme properties in more controlled and targeted applications, potentially opening up new fields of chemistry previously thought impossible.
In conclusion, while fluoroantimonic acid holds immense promise as a pillar of future chemistry, its current state is marked by a delicate balance between groundbreaking potential and formidable challenges. Overcoming these hurdles will require concerted efforts from chemists, engineers, and materials scientists, potentially ushering in a new era of superacid-enabled technologies and discoveries.
The current state of fluoroantimonic acid research is characterized by intense exploration of its catalytic properties. Scientists worldwide are investigating its potential to facilitate challenging chemical transformations, particularly in the petrochemical industry. Its ability to protonate even weak bases has opened new avenues for hydrocarbon cracking and isomerization processes, potentially revolutionizing fuel production and refining techniques.
However, the widespread adoption and application of fluoroantimonic acid face significant challenges. Foremost among these is its extreme reactivity, which necessitates specialized handling and storage conditions. The acid's corrosive nature limits the materials that can be used in its containment and application, posing substantial engineering hurdles for large-scale industrial use.
Safety concerns present another major obstacle. The acid's ability to react violently with water and many organic compounds requires stringent safety protocols and specialized training for personnel. This not only increases operational costs but also raises regulatory hurdles in many jurisdictions, potentially limiting its commercial viability.
Environmental considerations also pose challenges to the expanded use of fluoroantimonic acid. Its production and use involve highly toxic and environmentally harmful compounds, including hydrogen fluoride. Developing sustainable production methods and ensuring safe disposal or recycling of waste products remain critical challenges for researchers and industry professionals.
From a geographical perspective, research and development in fluoroantimonic acid technology are primarily concentrated in advanced chemical research facilities in North America, Europe, and East Asia. This concentration reflects the high level of expertise and sophisticated infrastructure required to work with such a potent substance.
Looking ahead, the key technical challenges revolve around developing more stable formulations of the acid, creating more resistant containment materials, and designing safer handling systems. Researchers are also exploring ways to harness the acid's extreme properties in more controlled and targeted applications, potentially opening up new fields of chemistry previously thought impossible.
In conclusion, while fluoroantimonic acid holds immense promise as a pillar of future chemistry, its current state is marked by a delicate balance between groundbreaking potential and formidable challenges. Overcoming these hurdles will require concerted efforts from chemists, engineers, and materials scientists, potentially ushering in a new era of superacid-enabled technologies and discoveries.
Existing Applications
01 Synthesis and preparation of fluoroantimonic acid
Fluoroantimonic acid is synthesized by combining hydrogen fluoride and antimony pentafluoride. The process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the resulting superacid.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in organic synthesis and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids, making it valuable in the production of certain chemicals and materials.
- Use in materials science and surface treatment: Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, improve adhesion properties, and create specialized coatings. The acid's unique properties make it suitable for treating metals, semiconductors, and other materials in specific industrial processes.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and storage procedures. Safety measures include the use of appropriate containment materials, personal protective equipment, and controlled environments. Proper disposal methods and emergency response protocols are essential when working with this highly hazardous substance.
- Analytical and characterization techniques: Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These methods include spectroscopic analysis, electrochemical measurements, and specialized apparatus for handling and analyzing superacids. Such techniques are crucial for understanding the acid's properties, reaction mechanisms, and potential applications in research and industry.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid is utilized as a powerful catalyst in various organic synthesis reactions. Its extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids. It has found applications in alkylation, isomerization, and polymerization processes in the chemical and petrochemical industries.Expand Specific Solutions03 Use in materials science and surface treatment
The superacidic properties of fluoroantimonic acid make it useful in materials science applications. It can be used for surface treatment of metals and other materials, enhancing their properties or creating unique surface characteristics. This has implications in fields such as corrosion resistance, adhesion improvement, and advanced materials development.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and storage procedures. Research has been conducted on developing safer methods for its use, including containment systems, protective equipment, and neutralization techniques. Safety protocols and risk assessments are crucial when working with this superacid.Expand Specific Solutions05 Analytical and characterization techniques
Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analyses, and computational modeling. Such techniques are essential for understanding the behavior of this superacid and optimizing its use in different applications.Expand Specific Solutions
Key Industry Players
The field of Fluoroantimonic Acid is in its early development stage, with significant potential for growth in various chemical applications. The market size is relatively small but expanding, driven by increasing demand in specialized industries. Technologically, it's still evolving, with companies like DuPont de Nemours, Inc., 3M Innovative Properties Co., and Pfizer Inc. leading research efforts. Academic institutions such as Hunan University and Beijing Normal University are also contributing to advancements. The competitive landscape is characterized by a mix of established chemical companies and research-oriented organizations, each striving to unlock the full potential of this powerful superacid in future chemistry applications.
3M Innovative Properties Co.
Technical Solution: 3M has developed a novel approach to harnessing the power of fluoroantimonic acid in materials science. Their technology focuses on using the acid's extreme acidity to modify surface properties of various materials, particularly in the field of advanced coatings. 3M's process involves carefully controlled exposure of substrate materials to dilute fluoroantimonic acid solutions, resulting in unique surface characteristics such as enhanced chemical resistance, improved adhesion properties, and novel optical effects. The company has also explored the use of fluoroantimonic acid in the development of super-acid catalysts for specific industrial processes, aiming to increase efficiency and reduce waste in chemical manufacturing.
Strengths: Diverse application potential across multiple industries, strong focus on practical industrial applications. Weaknesses: Challenges in scaling up production and ensuring consistent results across different material substrates.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for the synthesis and handling of fluoroantimonic acid, utilizing advanced containment systems and specialized materials resistant to extreme acidity. Their method involves the controlled reaction of hydrogen fluoride with antimony pentafluoride under precisely regulated conditions. DuPont's approach incorporates innovative safety measures, including remote handling techniques and advanced monitoring systems, to manage the highly corrosive nature of the acid. The company has also invested in developing applications for fluoroantimonic acid in organic synthesis, particularly in the creation of novel fluorinated compounds for various industries.
Strengths: Extensive experience in handling hazardous materials, strong R&D capabilities, and established industrial-scale production facilities. Weaknesses: High production costs and limited commercial applications due to the acid's extreme reactivity.
Core Innovations
Ionic liquids
PatentActiveEP1954680A1
Innovation
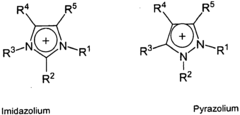
- Development of novel ionic liquid compositions comprising fluorinated anions, specifically cations such as pyridinium, imidazolium, and phosphonium with anions like tetrafluoroethanesulfonate, which are designed to function as solvents for various chemical reactions and polymerization processes, offering improved solvation properties and low volatility.
Ionic liquids
PatentActiveEP1954680B1
Innovation
- The development of novel compositions of fluorinated ionic liquids, specifically cation-anion pairs where the cation is selected from various imidazolium derivatives and the anion is a fluorinated sulfonate, offering improved properties as solvents in chemical reactions and polymerization processes.
Safety and Handling Protocols
Fluoroantimonic acid, being one of the strongest known superacids, requires exceptionally stringent safety and handling protocols. The extreme corrosiveness and reactivity of this compound necessitate specialized equipment and highly trained personnel for its manipulation. All handling must be conducted in a controlled environment, typically within a dry box or glove box filled with an inert atmosphere such as argon or nitrogen, to prevent any contact with moisture or air.
Personal protective equipment (PPE) is crucial when working with fluoroantimonic acid. This includes chemical-resistant suits made from materials like Teflon or Viton, which can withstand the acid's corrosive nature. Full-face respirators with appropriate acid gas cartridges are mandatory, as are multiple layers of chemical-resistant gloves. Eye protection in the form of sealed goggles or a face shield is essential, even when working within a glove box.
Storage of fluoroantimonic acid presents unique challenges due to its extreme reactivity. It must be kept in containers made from materials that can resist its corrosive effects, such as polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA). These containers should be sealed tightly and stored in a cool, dry area away from any potential reactants or incompatible materials. Regular inspections of storage containers are necessary to ensure their integrity and prevent any leaks or degradation.
Disposal of fluoroantimonic acid and any materials contaminated by it requires specialized procedures. Neutralization is typically performed using a carefully controlled addition of a base, such as sodium hydroxide or calcium hydroxide, in a well-ventilated area or fume hood. The resulting neutralized solution must then be treated as hazardous waste and disposed of according to local regulations and guidelines.
Emergency response protocols for fluoroantimonic acid incidents must be well-established and regularly practiced. This includes having appropriate spill kits readily available, containing materials capable of absorbing and neutralizing the acid. Decontamination showers and eyewash stations should be easily accessible in areas where the acid is handled or stored. All personnel working with or near fluoroantimonic acid must be thoroughly trained in emergency procedures and the use of safety equipment.
Given the extreme hazards associated with fluoroantimonic acid, its use should be limited to essential applications where no safer alternatives exist. Ongoing research into safer superacid alternatives and improved handling techniques is crucial for minimizing risks while harnessing the unique properties of this powerful compound in future chemical applications.
Personal protective equipment (PPE) is crucial when working with fluoroantimonic acid. This includes chemical-resistant suits made from materials like Teflon or Viton, which can withstand the acid's corrosive nature. Full-face respirators with appropriate acid gas cartridges are mandatory, as are multiple layers of chemical-resistant gloves. Eye protection in the form of sealed goggles or a face shield is essential, even when working within a glove box.
Storage of fluoroantimonic acid presents unique challenges due to its extreme reactivity. It must be kept in containers made from materials that can resist its corrosive effects, such as polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA). These containers should be sealed tightly and stored in a cool, dry area away from any potential reactants or incompatible materials. Regular inspections of storage containers are necessary to ensure their integrity and prevent any leaks or degradation.
Disposal of fluoroantimonic acid and any materials contaminated by it requires specialized procedures. Neutralization is typically performed using a carefully controlled addition of a base, such as sodium hydroxide or calcium hydroxide, in a well-ventilated area or fume hood. The resulting neutralized solution must then be treated as hazardous waste and disposed of according to local regulations and guidelines.
Emergency response protocols for fluoroantimonic acid incidents must be well-established and regularly practiced. This includes having appropriate spill kits readily available, containing materials capable of absorbing and neutralizing the acid. Decontamination showers and eyewash stations should be easily accessible in areas where the acid is handled or stored. All personnel working with or near fluoroantimonic acid must be thoroughly trained in emergency procedures and the use of safety equipment.
Given the extreme hazards associated with fluoroantimonic acid, its use should be limited to essential applications where no safer alternatives exist. Ongoing research into safer superacid alternatives and improved handling techniques is crucial for minimizing risks while harnessing the unique properties of this powerful compound in future chemical applications.
Environmental Impact Assessment
Fluoroantimonic acid, known as the world's strongest superacid, poses significant environmental concerns due to its extreme reactivity and corrosive nature. The production, handling, and disposal of this compound require stringent safety measures to prevent potential ecological damage.
The primary environmental risk associated with fluoroantimonic acid is its potential for water and soil contamination. Even minute quantities of this superacid can drastically alter the pH of water bodies, leading to severe impacts on aquatic ecosystems. The acid's ability to react violently with water makes it particularly dangerous if accidentally released into the environment.
Air pollution is another critical concern. The volatile nature of fluoroantimonic acid can result in the release of toxic fumes, including hydrogen fluoride and antimony compounds. These emissions can contribute to air quality degradation and pose health risks to both humans and wildlife in the surrounding areas.
The long-term effects of fluoroantimonic acid on soil chemistry and microbial communities are not fully understood. However, it is likely that soil exposed to this superacid would experience significant changes in pH, nutrient availability, and microbial diversity, potentially leading to long-lasting ecological imbalances.
Waste management and disposal of materials contaminated with fluoroantimonic acid present additional environmental challenges. Specialized treatment facilities are required to neutralize and safely dispose of this highly reactive substance, as conventional waste treatment methods are inadequate.
The production process of fluoroantimonic acid also raises environmental concerns. The synthesis involves highly toxic and reactive precursors, such as hydrogen fluoride and antimony pentafluoride. Ensuring the containment of these materials throughout the production cycle is crucial to prevent environmental contamination.
Given these environmental risks, the future use of fluoroantimonic acid in chemistry applications must be balanced against its potential ecological impact. Research into safer alternatives or improved containment and handling technologies will be essential to mitigate environmental risks while harnessing the unique properties of this superacid for scientific and industrial advancements.
The primary environmental risk associated with fluoroantimonic acid is its potential for water and soil contamination. Even minute quantities of this superacid can drastically alter the pH of water bodies, leading to severe impacts on aquatic ecosystems. The acid's ability to react violently with water makes it particularly dangerous if accidentally released into the environment.
Air pollution is another critical concern. The volatile nature of fluoroantimonic acid can result in the release of toxic fumes, including hydrogen fluoride and antimony compounds. These emissions can contribute to air quality degradation and pose health risks to both humans and wildlife in the surrounding areas.
The long-term effects of fluoroantimonic acid on soil chemistry and microbial communities are not fully understood. However, it is likely that soil exposed to this superacid would experience significant changes in pH, nutrient availability, and microbial diversity, potentially leading to long-lasting ecological imbalances.
Waste management and disposal of materials contaminated with fluoroantimonic acid present additional environmental challenges. Specialized treatment facilities are required to neutralize and safely dispose of this highly reactive substance, as conventional waste treatment methods are inadequate.
The production process of fluoroantimonic acid also raises environmental concerns. The synthesis involves highly toxic and reactive precursors, such as hydrogen fluoride and antimony pentafluoride. Ensuring the containment of these materials throughout the production cycle is crucial to prevent environmental contamination.
Given these environmental risks, the future use of fluoroantimonic acid in chemistry applications must be balanced against its potential ecological impact. Research into safer alternatives or improved containment and handling technologies will be essential to mitigate environmental risks while harnessing the unique properties of this superacid for scientific and industrial advancements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!