Fluoroantimonic Acid: Advancements in Protonation Mechanisms
JUN 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Evolution and Objectives
Fluoroantimonic acid, often hailed as the world's strongest superacid, has a rich history dating back to its discovery in the 1960s. This compound, formed by mixing hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has been a subject of intense scientific interest due to its extraordinary proton-donating capabilities. The evolution of fluoroantimonic acid research has been marked by significant milestones in understanding its structure, properties, and potential applications.
Initially, the focus was on characterizing the acid's extreme acidity, which surpasses that of 100% sulfuric acid by several orders of magnitude. Researchers quickly recognized its potential in various chemical processes, particularly in the petrochemical industry for catalyzing reactions such as isomerization and alkylation of hydrocarbons. As analytical techniques advanced, scientists gained deeper insights into the acid's molecular structure and behavior in solution.
The development of advanced spectroscopic methods, including NMR and Raman spectroscopy, has been crucial in elucidating the protonation mechanisms of fluoroantimonic acid. These techniques have allowed researchers to observe the formation of complex ionic species and understand the acid's interaction with various substrates at a molecular level. This knowledge has been instrumental in optimizing its use in organic synthesis and catalysis.
In recent years, the focus has shifted towards exploring novel applications of fluoroantimonic acid beyond traditional organic chemistry. Researchers are investigating its potential in materials science, particularly in the synthesis of novel fluorinated compounds and in surface modification of materials. Additionally, there is growing interest in harnessing its extreme acidity for energy-related applications, such as in advanced battery technologies and fuel cell catalysts.
The objectives of current and future research on fluoroantimonic acid are multifaceted. One primary goal is to develop safer handling methods and more stable formulations, addressing the challenges posed by its highly corrosive and reactive nature. This includes exploring ionic liquid forms of the acid, which could offer improved stability and ease of use in industrial settings.
Another key objective is to further elucidate the precise mechanisms of protonation, particularly in complex organic systems. This understanding is crucial for designing more efficient and selective catalytic processes. Researchers are also aiming to expand the acid's application scope, investigating its potential in emerging fields such as nanotechnology and advanced materials processing.
Furthermore, there is a growing emphasis on developing more environmentally friendly alternatives that can mimic the extreme acidity of fluoroantimonic acid while reducing its environmental impact. This aligns with the broader trend towards green chemistry and sustainable industrial processes. As research progresses, the goal is to balance the acid's unparalleled reactivity with practical considerations of safety, sustainability, and scalability in industrial applications.
Initially, the focus was on characterizing the acid's extreme acidity, which surpasses that of 100% sulfuric acid by several orders of magnitude. Researchers quickly recognized its potential in various chemical processes, particularly in the petrochemical industry for catalyzing reactions such as isomerization and alkylation of hydrocarbons. As analytical techniques advanced, scientists gained deeper insights into the acid's molecular structure and behavior in solution.
The development of advanced spectroscopic methods, including NMR and Raman spectroscopy, has been crucial in elucidating the protonation mechanisms of fluoroantimonic acid. These techniques have allowed researchers to observe the formation of complex ionic species and understand the acid's interaction with various substrates at a molecular level. This knowledge has been instrumental in optimizing its use in organic synthesis and catalysis.
In recent years, the focus has shifted towards exploring novel applications of fluoroantimonic acid beyond traditional organic chemistry. Researchers are investigating its potential in materials science, particularly in the synthesis of novel fluorinated compounds and in surface modification of materials. Additionally, there is growing interest in harnessing its extreme acidity for energy-related applications, such as in advanced battery technologies and fuel cell catalysts.
The objectives of current and future research on fluoroantimonic acid are multifaceted. One primary goal is to develop safer handling methods and more stable formulations, addressing the challenges posed by its highly corrosive and reactive nature. This includes exploring ionic liquid forms of the acid, which could offer improved stability and ease of use in industrial settings.
Another key objective is to further elucidate the precise mechanisms of protonation, particularly in complex organic systems. This understanding is crucial for designing more efficient and selective catalytic processes. Researchers are also aiming to expand the acid's application scope, investigating its potential in emerging fields such as nanotechnology and advanced materials processing.
Furthermore, there is a growing emphasis on developing more environmentally friendly alternatives that can mimic the extreme acidity of fluoroantimonic acid while reducing its environmental impact. This aligns with the broader trend towards green chemistry and sustainable industrial processes. As research progresses, the goal is to balance the acid's unparalleled reactivity with practical considerations of safety, sustainability, and scalability in industrial applications.
Industrial Applications and Market Demand
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in various industrial sectors due to its exceptional protonation capabilities. The market demand for this powerful compound has been steadily increasing, driven by its unique properties and diverse applications across multiple industries.
In the petrochemical industry, fluoroantimonic acid has found extensive use in catalytic cracking processes, particularly in the production of high-octane gasoline. Its ability to protonate even weakly basic compounds makes it an invaluable tool for isomerization reactions, enhancing the efficiency of fuel production and quality. This application has led to a growing demand from oil refineries and petrochemical plants seeking to optimize their processes and improve product yields.
The electronics industry has also recognized the potential of fluoroantimonic acid in semiconductor manufacturing. Its strong acidic properties make it useful in etching and cleaning processes for silicon wafers, contributing to the production of more efficient and smaller electronic components. As the demand for advanced electronic devices continues to rise, the market for fluoroantimonic acid in this sector is expected to expand significantly.
In the field of materials science, fluoroantimonic acid has shown promise in the synthesis of novel materials with unique properties. Its extreme acidity enables reactions that were previously challenging or impossible, opening up new avenues for material development. This has sparked interest from research institutions and advanced materials manufacturers, further driving market demand.
The pharmaceutical industry has begun exploring the use of fluoroantimonic acid in drug synthesis, particularly for the production of complex organic compounds. Its ability to facilitate certain reactions under milder conditions than traditional methods has the potential to revolutionize some aspects of pharmaceutical manufacturing, leading to increased efficiency and potentially lower production costs.
Despite its numerous applications, the market for fluoroantimonic acid faces challenges related to its extreme corrosiveness and reactivity. Handling and storage requirements are stringent, necessitating specialized equipment and safety protocols. This has led to a growing demand for safer handling technologies and containment solutions, creating a secondary market for supporting products and services.
As research into protonation mechanisms advances, new applications for fluoroantimonic acid are likely to emerge, potentially expanding its market reach. Industries such as waste treatment, where its strong acidic properties could be harnessed for breaking down resistant compounds, are beginning to show interest. This diversification of applications is expected to contribute to the overall growth of the fluoroantimonic acid market in the coming years.
In the petrochemical industry, fluoroantimonic acid has found extensive use in catalytic cracking processes, particularly in the production of high-octane gasoline. Its ability to protonate even weakly basic compounds makes it an invaluable tool for isomerization reactions, enhancing the efficiency of fuel production and quality. This application has led to a growing demand from oil refineries and petrochemical plants seeking to optimize their processes and improve product yields.
The electronics industry has also recognized the potential of fluoroantimonic acid in semiconductor manufacturing. Its strong acidic properties make it useful in etching and cleaning processes for silicon wafers, contributing to the production of more efficient and smaller electronic components. As the demand for advanced electronic devices continues to rise, the market for fluoroantimonic acid in this sector is expected to expand significantly.
In the field of materials science, fluoroantimonic acid has shown promise in the synthesis of novel materials with unique properties. Its extreme acidity enables reactions that were previously challenging or impossible, opening up new avenues for material development. This has sparked interest from research institutions and advanced materials manufacturers, further driving market demand.
The pharmaceutical industry has begun exploring the use of fluoroantimonic acid in drug synthesis, particularly for the production of complex organic compounds. Its ability to facilitate certain reactions under milder conditions than traditional methods has the potential to revolutionize some aspects of pharmaceutical manufacturing, leading to increased efficiency and potentially lower production costs.
Despite its numerous applications, the market for fluoroantimonic acid faces challenges related to its extreme corrosiveness and reactivity. Handling and storage requirements are stringent, necessitating specialized equipment and safety protocols. This has led to a growing demand for safer handling technologies and containment solutions, creating a secondary market for supporting products and services.
As research into protonation mechanisms advances, new applications for fluoroantimonic acid are likely to emerge, potentially expanding its market reach. Industries such as waste treatment, where its strong acidic properties could be harnessed for breaking down resistant compounds, are beginning to show interest. This diversification of applications is expected to contribute to the overall growth of the fluoroantimonic acid market in the coming years.
Current Challenges in Protonation Mechanisms
Despite significant advancements in protonation mechanisms, researchers in the field of Fluoroantimonic Acid face several persistent challenges. One of the primary obstacles is the extreme reactivity of Fluoroantimonic Acid, which makes it difficult to study and manipulate under standard laboratory conditions. This superacid's corrosive nature limits the types of containment materials and analytical instruments that can be used, hampering detailed mechanistic investigations.
Another challenge lies in the complexity of the protonation process itself. The exact mechanism by which Fluoroantimonic Acid transfers protons to substrates is not fully understood, particularly in cases involving complex organic molecules or in non-aqueous environments. This lack of comprehensive understanding impedes the development of more efficient and selective protonation methodologies.
The formation and stability of reaction intermediates present additional hurdles. Due to the highly acidic environment created by Fluoroantimonic Acid, many intermediate species are extremely short-lived, making their detection and characterization exceptionally challenging. This limitation hinders the elucidation of complete reaction pathways and the optimization of protonation processes.
Furthermore, controlling the selectivity of protonation reactions remains a significant challenge. In systems with multiple potential protonation sites, achieving site-specific protonation with Fluoroantimonic Acid is often difficult. This lack of selectivity can lead to unwanted side reactions and reduced yields in synthetic applications.
The environmental and safety concerns associated with Fluoroantimonic Acid also pose substantial challenges to its widespread use in research and industry. The acid's extreme corrosiveness and potential for generating toxic fumes necessitate stringent safety protocols, limiting its applicability in large-scale processes or in less controlled environments.
Lastly, the development of computational models that accurately predict protonation behavior in Fluoroantimonic Acid systems remains an ongoing challenge. The extreme acidity and unique solvation properties of this superacid make it difficult to apply standard computational chemistry methods, necessitating the development of specialized modeling approaches.
Another challenge lies in the complexity of the protonation process itself. The exact mechanism by which Fluoroantimonic Acid transfers protons to substrates is not fully understood, particularly in cases involving complex organic molecules or in non-aqueous environments. This lack of comprehensive understanding impedes the development of more efficient and selective protonation methodologies.
The formation and stability of reaction intermediates present additional hurdles. Due to the highly acidic environment created by Fluoroantimonic Acid, many intermediate species are extremely short-lived, making their detection and characterization exceptionally challenging. This limitation hinders the elucidation of complete reaction pathways and the optimization of protonation processes.
Furthermore, controlling the selectivity of protonation reactions remains a significant challenge. In systems with multiple potential protonation sites, achieving site-specific protonation with Fluoroantimonic Acid is often difficult. This lack of selectivity can lead to unwanted side reactions and reduced yields in synthetic applications.
The environmental and safety concerns associated with Fluoroantimonic Acid also pose substantial challenges to its widespread use in research and industry. The acid's extreme corrosiveness and potential for generating toxic fumes necessitate stringent safety protocols, limiting its applicability in large-scale processes or in less controlled environments.
Lastly, the development of computational models that accurately predict protonation behavior in Fluoroantimonic Acid systems remains an ongoing challenge. The extreme acidity and unique solvation properties of this superacid make it difficult to apply standard computational chemistry methods, necessitating the development of specialized modeling approaches.
State-of-the-Art Protonation Techniques
01 Superacid properties of fluoroantimonic acid
Fluoroantimonic acid is known as one of the strongest superacids, capable of protonating even very weak bases. Its extreme acidity is due to the formation of complex anions between fluoride and antimony pentafluoride. This property allows it to protonate a wide range of compounds, making it useful in various chemical reactions and industrial processes.- Superacid properties of fluoroantimonic acid: Fluoroantimonic acid is known as one of the strongest superacids, capable of protonating even very weak bases. Its extreme acidity is due to the formation of complex anions between fluorine and antimony, which greatly stabilize the conjugate base. This property allows it to protonate a wide range of compounds that are typically considered non-basic.
- Protonation of hydrocarbons and other organic compounds: Fluoroantimonic acid can protonate various hydrocarbons and organic compounds that are not typically considered bases. This includes alkanes, aromatics, and even some organometallic compounds. The protonation often leads to carbocations, which can initiate various chemical reactions, including isomerization and polymerization.
- Application in chemical synthesis and catalysis: The strong protonating ability of fluoroantimonic acid makes it useful in various chemical synthesis processes and as a catalyst. It can initiate reactions that are difficult or impossible with weaker acids, such as the cracking of hydrocarbons or the synthesis of certain organofluorine compounds. Its use in catalysis often involves the generation of highly reactive intermediates through protonation.
- Protonation mechanism in non-aqueous media: The protonation mechanism of fluoroantimonic acid in non-aqueous media is of particular interest. In these conditions, the acid can exhibit even stronger acidity due to the lack of solvent leveling effects. The protonation often involves the formation of ion pairs or larger aggregates, which can influence the reactivity and selectivity of the protonation process.
- Safety and handling considerations: Due to its extreme reactivity, the use of fluoroantimonic acid requires special safety and handling procedures. It reacts violently with water and many organic compounds, producing toxic and corrosive fumes. Specialized equipment and techniques are necessary for its preparation, storage, and use in chemical processes. Understanding its protonation mechanisms is crucial for safe handling and effective application.
02 Protonation mechanism in organic synthesis
The protonation mechanism of fluoroantimonic acid plays a crucial role in organic synthesis. It can initiate carbocation formation in alkanes and aromatics, leading to various transformations such as isomerization, alkylation, and polymerization. The strong proton-donating ability of fluoroantimonic acid enables reactions that are difficult or impossible with conventional acids.Expand Specific Solutions03 Applications in materials science
Fluoroantimonic acid's protonation mechanisms are utilized in materials science, particularly in the modification of surfaces and the synthesis of novel materials. Its ability to protonate and activate various substrates allows for the creation of functionalized surfaces, nanostructures, and advanced polymers with unique properties.Expand Specific Solutions04 Catalytic applications
The protonation mechanisms of fluoroantimonic acid are exploited in catalytic processes. Its strong acidic nature and ability to generate highly reactive intermediates make it an effective catalyst for various reactions, including isomerization of hydrocarbons, polymerization of olefins, and certain types of cracking reactions in petrochemical industries.Expand Specific Solutions05 Safety and handling considerations
Due to its extreme reactivity and corrosive nature, the use of fluoroantimonic acid requires special safety and handling procedures. Its protonation mechanisms can lead to violent reactions with water and many organic compounds. Specialized equipment and strict protocols are necessary for its safe use in research and industrial settings.Expand Specific Solutions
Key Players in Superacid Research and Production
The field of Fluoroantimonic Acid and its protonation mechanisms is in a mature stage of development, with ongoing research focused on refining and expanding applications. The market size for this superacid is relatively niche but growing, driven by its use in specialized chemical processes and research. Technologically, the field is well-established, with institutions like Hunan University, The Scripps Research Institute, and Fuzhou University leading academic research. Companies such as 3M Innovative Properties Co. and MedImmune LLC are actively involved in industrial applications, while organizations like the Council of Scientific & Industrial Research contribute to broader scientific understanding. The competitive landscape is characterized by a mix of academic institutions and industrial players, each contributing to advancements in different aspects of fluoroantimonic acid technology.
The Scripps Research Institute
Technical Solution: The Scripps Research Institute has developed a novel approach to studying Fluoroantimonic Acid's protonation mechanisms. Their research focuses on using advanced spectroscopic techniques, including ultrafast infrared spectroscopy and nuclear magnetic resonance (NMR) spectroscopy, to observe the real-time dynamics of proton transfer in Fluoroantimonic Acid systems. They have also implemented computational modeling to complement their experimental findings, providing a comprehensive understanding of the acid's behavior at the molecular level.
Strengths: Cutting-edge spectroscopic techniques, combined experimental and computational approach. Weaknesses: High cost of specialized equipment, complexity in data interpretation.
3M Innovative Properties Co.
Technical Solution: 3M Innovative Properties Co. has developed a proprietary method for synthesizing and stabilizing Fluoroantimonic Acid for industrial applications. Their approach involves a controlled reaction environment that allows for the precise manipulation of the acid's protonation mechanisms. They have also engineered specialized containment materials that can withstand the extreme corrosiveness of Fluoroantimonic Acid, enabling safer handling and storage. Additionally, 3M has developed novel catalytic systems that leverage the acid's superacidity for enhancing chemical reactions in various industrial processes.
Strengths: Industrial-scale production capabilities, innovative containment solutions. Weaknesses: Limited to applied research, potential environmental concerns.
Breakthrough Innovations in Fluoroantimonic Acid
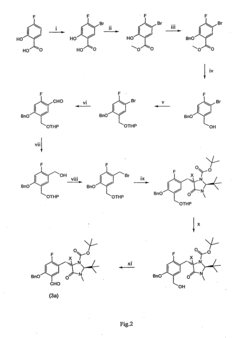
Method for producing precursors for l-3,4-dihydroxy-6- [18f] fluorophenyl alaine and 2- [18f] fluoro-l-tyrosine and the alpha-methylated derivatives thereof, precursor, and method for producing l-3, 4dihydroxy-6- [18f] fluorophenylalanine and 2- [18f] fluoro-l-tyrosine and the alpha-methylated derivatives from the precursor
PatentInactiveUS20100261913A1
Innovation
- A method that produces these compounds in an enantiopure manner through three radioactive steps, achieving enantiomeric purities of ≥98%, suitable for automated synthesis using a novel precursor and specific chemical reactions, including halogenation, protection, reduction, and fluorination steps.
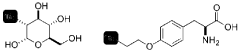
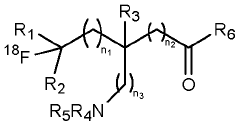
18f labeled amino acids, derivatives thereof and method of making same
PatentWO2018035610A1
Innovation
- Development of novel 18F-labeled amino acids and derivatives that can be synthesized through direct fluorination of unactivated tertiary or secondary C-H bonds in unprotected amino acids, using [18F]NFSI with a decatungstate catalyst and light source in an aqueous solvent, eliminating the need for precursor synthesis and protective groups.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, poses significant safety and environmental challenges that require careful consideration and management. The extreme corrosiveness and reactivity of this compound necessitate stringent safety protocols in laboratory and industrial settings. Personal protective equipment (PPE) is crucial, including chemical-resistant suits, gloves, and respiratory protection. Specialized containment systems and handling procedures are essential to prevent accidental releases or exposure.
The environmental impact of fluoroantimonic acid is a major concern due to its potential to cause severe ecological damage. Its highly acidic nature can lead to soil and water contamination, resulting in long-lasting environmental effects. Proper disposal methods are critical to mitigate these risks. Neutralization techniques using appropriate bases must be employed before disposal, and specialized waste management facilities are required to handle the resulting byproducts.
Occupational health and safety regulations surrounding the use of fluoroantimonic acid are particularly stringent. Regular safety training, risk assessments, and emergency response planning are mandatory for facilities working with this superacid. Monitoring systems for detecting potential leaks or emissions are essential to ensure worker safety and environmental protection.
The transportation of fluoroantimonic acid presents unique challenges due to its extreme reactivity. Specialized containers with multiple layers of protection are required to prevent any potential breaches during transit. Strict regulations govern the movement of this substance, including route planning to minimize risks to populated areas and environmentally sensitive regions.
Research into safer alternatives and improved handling techniques is ongoing. This includes the development of less hazardous catalysts that can potentially replace fluoroantimonic acid in certain applications, as well as advanced containment technologies to enhance safety during use and storage. Additionally, efforts are being made to improve emergency response capabilities specific to superacid incidents.
Public awareness and education about the risks associated with fluoroantimonic acid are crucial, particularly in communities near facilities that use or produce this compound. Transparent communication and community engagement strategies are essential to address concerns and ensure preparedness in case of accidents.
As advancements in protonation mechanisms continue, it is imperative that safety and environmental considerations evolve in parallel. This includes ongoing assessment of long-term environmental impacts, refinement of safety protocols, and investment in research to minimize risks associated with the production and use of fluoroantimonic acid.
The environmental impact of fluoroantimonic acid is a major concern due to its potential to cause severe ecological damage. Its highly acidic nature can lead to soil and water contamination, resulting in long-lasting environmental effects. Proper disposal methods are critical to mitigate these risks. Neutralization techniques using appropriate bases must be employed before disposal, and specialized waste management facilities are required to handle the resulting byproducts.
Occupational health and safety regulations surrounding the use of fluoroantimonic acid are particularly stringent. Regular safety training, risk assessments, and emergency response planning are mandatory for facilities working with this superacid. Monitoring systems for detecting potential leaks or emissions are essential to ensure worker safety and environmental protection.
The transportation of fluoroantimonic acid presents unique challenges due to its extreme reactivity. Specialized containers with multiple layers of protection are required to prevent any potential breaches during transit. Strict regulations govern the movement of this substance, including route planning to minimize risks to populated areas and environmentally sensitive regions.
Research into safer alternatives and improved handling techniques is ongoing. This includes the development of less hazardous catalysts that can potentially replace fluoroantimonic acid in certain applications, as well as advanced containment technologies to enhance safety during use and storage. Additionally, efforts are being made to improve emergency response capabilities specific to superacid incidents.
Public awareness and education about the risks associated with fluoroantimonic acid are crucial, particularly in communities near facilities that use or produce this compound. Transparent communication and community engagement strategies are essential to address concerns and ensure preparedness in case of accidents.
As advancements in protonation mechanisms continue, it is imperative that safety and environmental considerations evolve in parallel. This includes ongoing assessment of long-term environmental impacts, refinement of safety protocols, and investment in research to minimize risks associated with the production and use of fluoroantimonic acid.
Regulatory Framework for Superacid Usage
The regulatory framework for superacid usage, particularly concerning fluoroantimonic acid, is a critical aspect of its application in various industries. Given the extreme corrosiveness and reactivity of fluoroantimonic acid, stringent regulations have been established to ensure safe handling, storage, and disposal.
In the United States, the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) play pivotal roles in regulating superacids. The EPA, under the Toxic Substances Control Act (TSCA), requires manufacturers and importers to report any new chemical substances, including superacids, before they are introduced into commerce. This process involves a thorough evaluation of the substance's potential risks to human health and the environment.
OSHA, on the other hand, sets standards for workplace safety when handling hazardous materials like fluoroantimonic acid. These standards include requirements for personal protective equipment (PPE), proper ventilation systems, and emergency response procedures. The Hazard Communication Standard (HCS) mandates that employers provide information about the hazards of chemicals through safety data sheets (SDS) and appropriate labeling.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to chemical hazard classification and communication. Under this system, fluoroantimonic acid is classified as a highly corrosive substance, requiring specific hazard pictograms, signal words, and precautionary statements on its packaging and documentation.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation imposes additional requirements on the manufacture, import, and use of superacids within EU member states. This includes mandatory registration of substances, evaluation of their properties and potential risks, and in some cases, authorization for specific uses.
Transportation of fluoroantimonic acid is subject to strict regulations under various international agreements, such as the International Maritime Dangerous Goods (IMDG) Code for sea transport and the International Air Transport Association (IATA) Dangerous Goods Regulations for air transport. These regulations specify packaging requirements, labeling standards, and documentation procedures to ensure safe transportation of such highly hazardous materials.
Research institutions and industrial facilities working with fluoroantimonic acid must adhere to these regulatory frameworks and often implement additional safety protocols. This includes specialized training for personnel, regular safety audits, and the development of detailed standard operating procedures (SOPs) for handling and disposal of the superacid.
As advancements in protonation mechanisms continue to expand the potential applications of fluoroantimonic acid, regulatory bodies are likely to adapt their frameworks to address emerging safety concerns and environmental impacts. This ongoing evolution of regulations underscores the importance of staying informed about the latest regulatory updates and best practices in superacid management.
In the United States, the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) play pivotal roles in regulating superacids. The EPA, under the Toxic Substances Control Act (TSCA), requires manufacturers and importers to report any new chemical substances, including superacids, before they are introduced into commerce. This process involves a thorough evaluation of the substance's potential risks to human health and the environment.
OSHA, on the other hand, sets standards for workplace safety when handling hazardous materials like fluoroantimonic acid. These standards include requirements for personal protective equipment (PPE), proper ventilation systems, and emergency response procedures. The Hazard Communication Standard (HCS) mandates that employers provide information about the hazards of chemicals through safety data sheets (SDS) and appropriate labeling.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to chemical hazard classification and communication. Under this system, fluoroantimonic acid is classified as a highly corrosive substance, requiring specific hazard pictograms, signal words, and precautionary statements on its packaging and documentation.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation imposes additional requirements on the manufacture, import, and use of superacids within EU member states. This includes mandatory registration of substances, evaluation of their properties and potential risks, and in some cases, authorization for specific uses.
Transportation of fluoroantimonic acid is subject to strict regulations under various international agreements, such as the International Maritime Dangerous Goods (IMDG) Code for sea transport and the International Air Transport Association (IATA) Dangerous Goods Regulations for air transport. These regulations specify packaging requirements, labeling standards, and documentation procedures to ensure safe transportation of such highly hazardous materials.
Research institutions and industrial facilities working with fluoroantimonic acid must adhere to these regulatory frameworks and often implement additional safety protocols. This includes specialized training for personnel, regular safety audits, and the development of detailed standard operating procedures (SOPs) for handling and disposal of the superacid.
As advancements in protonation mechanisms continue to expand the potential applications of fluoroantimonic acid, regulatory bodies are likely to adapt their frameworks to address emerging safety concerns and environmental impacts. This ongoing evolution of regulations underscores the importance of staying informed about the latest regulatory updates and best practices in superacid management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!