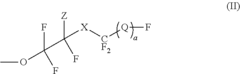
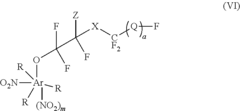
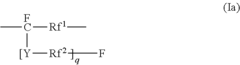Fluoroantimonic Acid and its Role in Emerging Technologies
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid: Background and Objectives
Fluoroantimonic acid, often referred to as the world's strongest superacid, has been a subject of intense scientific interest since its discovery in the 1960s. This compound, with its extraordinary acidity and unique chemical properties, has played a significant role in advancing our understanding of superacidity and its potential applications in various fields of chemistry and technology.
The development of fluoroantimonic acid can be traced back to the pioneering work of George A. Olah, who later won the Nobel Prize in Chemistry for his contributions to carbocation chemistry. Olah's research on superacids laid the foundation for the synthesis and characterization of fluoroantimonic acid, which is formed by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5).
As research progressed, scientists began to uncover the remarkable properties of fluoroantimonic acid. Its ability to protonate even extremely weak bases and its potential to catalyze various chemical reactions quickly garnered attention from both academia and industry. The acid's extreme corrosiveness and reactivity also presented significant challenges in terms of handling and storage, spurring the development of specialized containment materials and safety protocols.
In recent years, the focus on fluoroantimonic acid has shifted towards its potential applications in emerging technologies. Its unique properties make it a promising candidate for use in advanced materials processing, nanotechnology, and energy storage systems. Researchers are exploring its role in the synthesis of novel materials, such as high-performance polymers and advanced ceramics, which could revolutionize industries ranging from aerospace to electronics.
The objectives of current research on fluoroantimonic acid are multifaceted. Scientists aim to further elucidate its fundamental chemical properties and reaction mechanisms, which could lead to new insights in acid-base chemistry and catalysis. There is also a strong emphasis on developing safer and more efficient methods for its production and handling, which is crucial for expanding its practical applications.
Another key goal is to explore the acid's potential in emerging fields such as quantum computing and advanced energy technologies. Its unique electronic properties and ability to generate highly reactive species make it an intriguing candidate for developing new types of quantum materials and high-efficiency catalysts for energy conversion processes.
As we look to the future, the trajectory of fluoroantimonic acid research is likely to be shaped by the growing demand for innovative materials and processes in cutting-edge technologies. The challenge lies in harnessing its extraordinary properties while mitigating its inherent risks, a balance that will require continued collaboration between chemists, materials scientists, and engineers across various disciplines.
The development of fluoroantimonic acid can be traced back to the pioneering work of George A. Olah, who later won the Nobel Prize in Chemistry for his contributions to carbocation chemistry. Olah's research on superacids laid the foundation for the synthesis and characterization of fluoroantimonic acid, which is formed by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5).
As research progressed, scientists began to uncover the remarkable properties of fluoroantimonic acid. Its ability to protonate even extremely weak bases and its potential to catalyze various chemical reactions quickly garnered attention from both academia and industry. The acid's extreme corrosiveness and reactivity also presented significant challenges in terms of handling and storage, spurring the development of specialized containment materials and safety protocols.
In recent years, the focus on fluoroantimonic acid has shifted towards its potential applications in emerging technologies. Its unique properties make it a promising candidate for use in advanced materials processing, nanotechnology, and energy storage systems. Researchers are exploring its role in the synthesis of novel materials, such as high-performance polymers and advanced ceramics, which could revolutionize industries ranging from aerospace to electronics.
The objectives of current research on fluoroantimonic acid are multifaceted. Scientists aim to further elucidate its fundamental chemical properties and reaction mechanisms, which could lead to new insights in acid-base chemistry and catalysis. There is also a strong emphasis on developing safer and more efficient methods for its production and handling, which is crucial for expanding its practical applications.
Another key goal is to explore the acid's potential in emerging fields such as quantum computing and advanced energy technologies. Its unique electronic properties and ability to generate highly reactive species make it an intriguing candidate for developing new types of quantum materials and high-efficiency catalysts for energy conversion processes.
As we look to the future, the trajectory of fluoroantimonic acid research is likely to be shaped by the growing demand for innovative materials and processes in cutting-edge technologies. The challenge lies in harnessing its extraordinary properties while mitigating its inherent risks, a balance that will require continued collaboration between chemists, materials scientists, and engineers across various disciplines.
Market Analysis for Superacid Applications
The market for superacid applications, particularly those involving fluoroantimonic acid, is experiencing significant growth driven by emerging technologies and industrial processes. Fluoroantimonic acid, known as the strongest superacid, finds applications across various sectors due to its unique properties and extreme acidity.
In the petrochemical industry, fluoroantimonic acid plays a crucial role in catalytic cracking and isomerization processes. The demand for high-octane fuels and specialized petrochemical products has led to increased adoption of superacid-based catalysts. This trend is expected to continue as refineries seek to optimize their processes and improve product quality.
The electronics sector represents another key market for fluoroantimonic acid applications. As the demand for smaller, more powerful electronic devices grows, manufacturers are exploring advanced etching techniques for semiconductor production. Fluoroantimonic acid's ability to etch silicon and other materials with high precision makes it valuable in the fabrication of microchips and integrated circuits.
In materials science, fluoroantimonic acid is utilized in the synthesis of novel compounds and materials. Research institutions and specialty chemical companies are investigating its potential in creating new polymers, advanced ceramics, and other high-performance materials. This niche market segment shows promise for future growth as new applications are discovered.
The pharmaceutical industry is also exploring the use of fluoroantimonic acid in drug synthesis and the production of complex organic compounds. While currently limited due to safety concerns, ongoing research may lead to breakthroughs in pharmaceutical manufacturing processes, potentially opening up new market opportunities.
Despite its potential, the market for fluoroantimonic acid faces challenges related to safety, handling, and environmental concerns. Stringent regulations and the need for specialized equipment and facilities limit widespread adoption. However, advancements in containment technologies and safer handling procedures are gradually addressing these issues, potentially expanding the market.
Geographically, North America and Europe lead in fluoroantimonic acid applications, primarily due to their established petrochemical and electronics industries. Asia-Pacific, particularly China and South Korea, is emerging as a significant market driven by rapid industrialization and technological advancements in semiconductor manufacturing.
The global market for superacids, including fluoroantimonic acid, is projected to grow steadily over the next decade. While exact figures are difficult to ascertain due to the specialized nature of the market, industry analysts estimate a compound annual growth rate in the mid-single digits. This growth is expected to be fueled by ongoing research and development efforts, as well as the expansion of high-tech manufacturing sectors in emerging economies.
In the petrochemical industry, fluoroantimonic acid plays a crucial role in catalytic cracking and isomerization processes. The demand for high-octane fuels and specialized petrochemical products has led to increased adoption of superacid-based catalysts. This trend is expected to continue as refineries seek to optimize their processes and improve product quality.
The electronics sector represents another key market for fluoroantimonic acid applications. As the demand for smaller, more powerful electronic devices grows, manufacturers are exploring advanced etching techniques for semiconductor production. Fluoroantimonic acid's ability to etch silicon and other materials with high precision makes it valuable in the fabrication of microchips and integrated circuits.
In materials science, fluoroantimonic acid is utilized in the synthesis of novel compounds and materials. Research institutions and specialty chemical companies are investigating its potential in creating new polymers, advanced ceramics, and other high-performance materials. This niche market segment shows promise for future growth as new applications are discovered.
The pharmaceutical industry is also exploring the use of fluoroantimonic acid in drug synthesis and the production of complex organic compounds. While currently limited due to safety concerns, ongoing research may lead to breakthroughs in pharmaceutical manufacturing processes, potentially opening up new market opportunities.
Despite its potential, the market for fluoroantimonic acid faces challenges related to safety, handling, and environmental concerns. Stringent regulations and the need for specialized equipment and facilities limit widespread adoption. However, advancements in containment technologies and safer handling procedures are gradually addressing these issues, potentially expanding the market.
Geographically, North America and Europe lead in fluoroantimonic acid applications, primarily due to their established petrochemical and electronics industries. Asia-Pacific, particularly China and South Korea, is emerging as a significant market driven by rapid industrialization and technological advancements in semiconductor manufacturing.
The global market for superacids, including fluoroantimonic acid, is projected to grow steadily over the next decade. While exact figures are difficult to ascertain due to the specialized nature of the market, industry analysts estimate a compound annual growth rate in the mid-single digits. This growth is expected to be fueled by ongoing research and development efforts, as well as the expansion of high-tech manufacturing sectors in emerging economies.
Current Challenges in Fluoroantimonic Acid Production
The production of fluoroantimonic acid faces several significant challenges that hinder its widespread application in emerging technologies. One of the primary obstacles is the extreme reactivity of this superacid, which makes handling and storage exceptionally difficult. Fluoroantimonic acid reacts violently with water and most organic compounds, requiring specialized containment materials and strict safety protocols.
The synthesis process itself presents considerable challenges. The reaction between hydrogen fluoride and antimony pentafluoride must be carried out under carefully controlled conditions to prevent side reactions and ensure product purity. The highly corrosive nature of the reactants and the product necessitates the use of specialized equipment, such as fluoropolymer-lined reactors, which significantly increases production costs.
Another major challenge is the limited availability of high-purity antimony pentafluoride, a key precursor in fluoroantimonic acid production. The global supply of antimony is relatively scarce, and the production of antimony pentafluoride requires additional processing steps, further complicating the supply chain and increasing costs.
Scale-up of production processes poses significant engineering challenges. The heat generated during the synthesis reaction must be carefully managed to prevent runaway reactions and maintain product quality. Additionally, the transfer and storage of large quantities of fluoroantimonic acid require advanced containment systems and rigorous safety measures, which can be prohibitively expensive for large-scale operations.
Environmental and safety concerns also present substantial hurdles. The production and use of fluoroantimonic acid generate hazardous waste that requires specialized disposal methods. Stringent regulations surrounding the handling and transport of such highly corrosive materials add complexity and cost to the production process.
The extreme acidity of fluoroantimonic acid also limits its compatibility with many standard analytical techniques, making quality control and characterization challenging. Developing reliable methods for assessing purity and composition without compromising sample integrity or equipment is an ongoing area of research.
Lastly, the limited commercial demand for fluoroantimonic acid in its pure form has resulted in a lack of incentive for large-scale production optimization. This creates a cyclical problem where the high costs and technical challenges associated with production limit potential applications, which in turn restricts investment in improving production methods.
The synthesis process itself presents considerable challenges. The reaction between hydrogen fluoride and antimony pentafluoride must be carried out under carefully controlled conditions to prevent side reactions and ensure product purity. The highly corrosive nature of the reactants and the product necessitates the use of specialized equipment, such as fluoropolymer-lined reactors, which significantly increases production costs.
Another major challenge is the limited availability of high-purity antimony pentafluoride, a key precursor in fluoroantimonic acid production. The global supply of antimony is relatively scarce, and the production of antimony pentafluoride requires additional processing steps, further complicating the supply chain and increasing costs.
Scale-up of production processes poses significant engineering challenges. The heat generated during the synthesis reaction must be carefully managed to prevent runaway reactions and maintain product quality. Additionally, the transfer and storage of large quantities of fluoroantimonic acid require advanced containment systems and rigorous safety measures, which can be prohibitively expensive for large-scale operations.
Environmental and safety concerns also present substantial hurdles. The production and use of fluoroantimonic acid generate hazardous waste that requires specialized disposal methods. Stringent regulations surrounding the handling and transport of such highly corrosive materials add complexity and cost to the production process.
The extreme acidity of fluoroantimonic acid also limits its compatibility with many standard analytical techniques, making quality control and characterization challenging. Developing reliable methods for assessing purity and composition without compromising sample integrity or equipment is an ongoing area of research.
Lastly, the limited commercial demand for fluoroantimonic acid in its pure form has resulted in a lack of incentive for large-scale production optimization. This creates a cyclical problem where the high costs and technical challenges associated with production limit potential applications, which in turn restricts investment in improving production methods.
Existing Applications of Fluoroantimonic Acid
01 Synthesis and production of fluoroantimonic acid
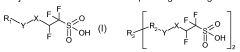
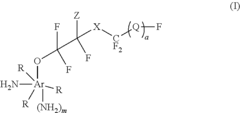
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized by combining hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of these highly reactive compounds under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the resulting superacid.
- Applications in organic synthesis and catalysis: Fluoroantimonic acid is utilized as a powerful catalyst in various organic synthesis reactions. Its extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids. It has found applications in alkylation, isomerization, and polymerization processes in the chemical and petrochemical industries.
- Use in materials science and surface treatment: The superacidic properties of fluoroantimonic acid make it useful in materials science applications. It can be used for surface etching, modification of materials, and in the preparation of certain advanced materials. Its ability to protonate even weak bases has led to its use in specialized surface treatments and material processing techniques.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and storage procedures. Safety measures include the use of specialized containment materials, personal protective equipment, and strict protocols for handling and disposal. Research has been conducted to develop safer handling methods and to mitigate the risks associated with its use.
- Analytical and characterization methods: Various analytical techniques have been developed to characterize fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analyses, and specialized titration techniques. Research has focused on understanding its behavior in different solvents and its interactions with various substrates, contributing to the development of new applications and improved handling methods.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates processes such as alkylation, isomerization, and polymerization of hydrocarbons. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials such as polymers and metals.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, special safety measures and handling procedures are required when working with fluoroantimonic acid. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage, transport, and disposal.Expand Specific Solutions05 Analytical and characterization techniques
Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These methods include spectroscopic techniques, electrochemical analysis, and specialized apparatus designed to handle and analyze this highly reactive superacid under controlled conditions.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Research
The competitive landscape for Fluoroantimonic Acid in emerging technologies is characterized by a nascent industry in its early development stage. The market size remains relatively small, primarily driven by research and specialized applications. Technologically, it's still in the experimental phase, with varying levels of maturity across different sectors. Companies like DuPont de Nemours, BASF Corp., and Merck & Co. are leading in industrial applications, while academic institutions such as Central South University and Yale University are pushing boundaries in research. The involvement of diverse players, from pharmaceutical giants like Pfizer to chemical specialists like DAIKIN INDUSTRIES, indicates a broad potential for future applications, though significant challenges in handling and commercialization remain.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for synthesizing and handling fluoroantimonic acid, utilizing specialized corrosion-resistant materials and containment systems. Their approach involves a controlled reaction between hydrogen fluoride and antimony pentafluoride under precise temperature and pressure conditions. DuPont has also engineered safety protocols and neutralization techniques for managing this superacid in industrial applications, particularly for catalyzing certain petrochemical reactions and in the production of specialized polymers.
Strengths: Extensive experience in handling hazardous chemicals, strong R&D capabilities, established safety protocols. Weaknesses: High production costs, limited commercial applications due to the acid's extreme reactivity.
BASF Corp.
Technical Solution: BASF has developed a novel application of fluoroantimonic acid in the field of advanced materials synthesis. Their approach utilizes the superacid's extreme protonating ability to create unique nanostructured materials with enhanced properties. By carefully controlling the acid's interaction with precursor compounds, BASF has been able to produce materials with tailored porosity, surface area, and chemical reactivity. This technology has potential applications in catalysis, energy storage, and advanced filtration systems.
Strengths: Strong expertise in materials science, diverse product portfolio for potential applications. Weaknesses: Challenges in scaling up production, potential environmental concerns.
Innovations in Fluoroantimonic Acid Synthesis
Latent acids and their use
PatentWO2011104127A1
Innovation
- The use of substituted 1,1,2-trifluoroalkylsulfonic acids, specifically 1,1,2-trifluoromethylsulfonic acids, which are synthesized from 2,2,3,3-tetrafluoropropanol, a readily available industrial-scale compound, to create latent acids that are highly active and soluble in solvents like propylene glycol monomethyl ether acetate, enabling high-resolution photoresist imaging with low mask edge errors and wide exposure latitude.
Fluoroether-functionalized aminoaromatic compounds and derivatives thereof
PatentInactiveUS20120296122A1
Innovation
- The development of fluoroether-functionalized aminoaromatic compounds, represented by specific chemical structures, which can be synthesized through a process involving fluoroether-functionalized nitroaromatic compounds under hydrogen pressure with solvents and catalysts, forming compounds suitable for use as monomers and co-monomers in polyamides and polyoxadiazoles.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant safety and environmental challenges that must be carefully addressed in its handling and application within emerging technologies. The extreme corrosiveness and reactivity of this compound necessitate stringent safety protocols and specialized containment systems to prevent accidents and exposure.
Workers dealing with fluoroantimonic acid must undergo extensive training and utilize advanced personal protective equipment, including chemical-resistant suits, gloves, and respiratory protection. Facilities handling this superacid require robust ventilation systems, spill containment measures, and emergency response plans tailored to its unique properties.
Environmental considerations are paramount when working with fluoroantimonic acid. Its potential for severe ecological damage if released into the environment cannot be overstated. Proper disposal methods must be meticulously followed, often involving neutralization processes and specialized waste management facilities equipped to handle such hazardous materials.
The transportation of fluoroantimonic acid poses additional risks, requiring specialized containers and strict adherence to hazardous materials transportation regulations. Emergency response teams along transportation routes must be prepared to handle potential incidents involving this superacid.
Research into safer alternatives and improved handling techniques is ongoing, with a focus on developing less hazardous compounds that can achieve similar results in industrial and technological applications. This includes exploring ionic liquids and other novel chemical systems that may offer comparable performance with reduced environmental and safety risks.
The use of fluoroantimonic acid in emerging technologies must be carefully weighed against its potential hazards. Industries must conduct thorough risk assessments and implement comprehensive safety management systems to mitigate the dangers associated with its use. This includes regular audits, continuous monitoring, and the development of safer, more sustainable practices.
Regulatory bodies play a crucial role in overseeing the use of fluoroantimonic acid, establishing guidelines for its handling, storage, and disposal. As emerging technologies continue to explore applications for this powerful superacid, collaboration between industry, researchers, and regulatory agencies is essential to ensure that safety and environmental protection remain at the forefront of its development and utilization.
Workers dealing with fluoroantimonic acid must undergo extensive training and utilize advanced personal protective equipment, including chemical-resistant suits, gloves, and respiratory protection. Facilities handling this superacid require robust ventilation systems, spill containment measures, and emergency response plans tailored to its unique properties.
Environmental considerations are paramount when working with fluoroantimonic acid. Its potential for severe ecological damage if released into the environment cannot be overstated. Proper disposal methods must be meticulously followed, often involving neutralization processes and specialized waste management facilities equipped to handle such hazardous materials.
The transportation of fluoroantimonic acid poses additional risks, requiring specialized containers and strict adherence to hazardous materials transportation regulations. Emergency response teams along transportation routes must be prepared to handle potential incidents involving this superacid.
Research into safer alternatives and improved handling techniques is ongoing, with a focus on developing less hazardous compounds that can achieve similar results in industrial and technological applications. This includes exploring ionic liquids and other novel chemical systems that may offer comparable performance with reduced environmental and safety risks.
The use of fluoroantimonic acid in emerging technologies must be carefully weighed against its potential hazards. Industries must conduct thorough risk assessments and implement comprehensive safety management systems to mitigate the dangers associated with its use. This includes regular audits, continuous monitoring, and the development of safer, more sustainable practices.
Regulatory bodies play a crucial role in overseeing the use of fluoroantimonic acid, establishing guidelines for its handling, storage, and disposal. As emerging technologies continue to explore applications for this powerful superacid, collaboration between industry, researchers, and regulatory agencies is essential to ensure that safety and environmental protection remain at the forefront of its development and utilization.
Potential Impact on Catalysis Industry
Fluoroantimonic acid, known as the world's strongest superacid, is poised to revolutionize the catalysis industry. Its exceptional proton-donating ability and extreme acidity make it a powerful catalyst for various chemical reactions, particularly in hydrocarbon processing and petrochemical industries.
In the field of hydrocarbon isomerization, fluoroantimonic acid catalysts demonstrate superior performance compared to traditional solid acid catalysts. They enable lower reaction temperatures and pressures, resulting in significant energy savings and improved process efficiency. This could lead to more economical production of high-octane gasoline components and specialty chemicals.
The petrochemical industry stands to benefit greatly from fluoroantimonic acid catalysts in alkylation processes. These catalysts show promise in producing high-quality alkylates with improved selectivity and yield. This could potentially transform the production of aviation fuels and high-performance lubricants, offering both economic and environmental advantages.
In the realm of fine chemical synthesis, fluoroantimonic acid catalysts open up new possibilities for complex organic transformations. Their ability to activate inert C-H bonds and facilitate challenging carbon-carbon bond formations could lead to more efficient and selective synthetic routes for pharmaceuticals and agrochemicals.
The potential impact extends to the polymer industry as well. Fluoroantimonic acid catalysts show promise in catalyzing polymerization reactions, potentially enabling the synthesis of novel polymers with unique properties. This could drive innovation in materials science, leading to advanced materials for various applications.
However, the widespread adoption of fluoroantimonic acid in industrial catalysis faces challenges. Its extreme corrosiveness necessitates specialized handling and equipment, which may require significant investment in infrastructure. Additionally, safety concerns and environmental considerations need to be carefully addressed before large-scale implementation.
Despite these challenges, the potential benefits of fluoroantimonic acid catalysts are driving research and development efforts. Innovations in containment technologies and catalyst immobilization techniques are being pursued to overcome the practical limitations. As these challenges are addressed, the catalysis industry may see a paradigm shift, with fluoroantimonic acid catalysts enabling more efficient, selective, and sustainable chemical processes across various sectors.
In the field of hydrocarbon isomerization, fluoroantimonic acid catalysts demonstrate superior performance compared to traditional solid acid catalysts. They enable lower reaction temperatures and pressures, resulting in significant energy savings and improved process efficiency. This could lead to more economical production of high-octane gasoline components and specialty chemicals.
The petrochemical industry stands to benefit greatly from fluoroantimonic acid catalysts in alkylation processes. These catalysts show promise in producing high-quality alkylates with improved selectivity and yield. This could potentially transform the production of aviation fuels and high-performance lubricants, offering both economic and environmental advantages.
In the realm of fine chemical synthesis, fluoroantimonic acid catalysts open up new possibilities for complex organic transformations. Their ability to activate inert C-H bonds and facilitate challenging carbon-carbon bond formations could lead to more efficient and selective synthetic routes for pharmaceuticals and agrochemicals.
The potential impact extends to the polymer industry as well. Fluoroantimonic acid catalysts show promise in catalyzing polymerization reactions, potentially enabling the synthesis of novel polymers with unique properties. This could drive innovation in materials science, leading to advanced materials for various applications.
However, the widespread adoption of fluoroantimonic acid in industrial catalysis faces challenges. Its extreme corrosiveness necessitates specialized handling and equipment, which may require significant investment in infrastructure. Additionally, safety concerns and environmental considerations need to be carefully addressed before large-scale implementation.
Despite these challenges, the potential benefits of fluoroantimonic acid catalysts are driving research and development efforts. Innovations in containment technologies and catalyst immobilization techniques are being pursued to overcome the practical limitations. As these challenges are addressed, the catalysis industry may see a paradigm shift, with fluoroantimonic acid catalysts enabling more efficient, selective, and sustainable chemical processes across various sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!