Fluoroantimonic Acid in Chemical Synthesis: A Guide
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Overview and Objectives
Fluoroantimonic acid, often referred to as the world's strongest superacid, has been a subject of intense research and development in the field of chemical synthesis for several decades. This powerful compound, composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has revolutionized various aspects of organic and inorganic chemistry due to its exceptional proton-donating ability and extreme acidity.
The evolution of fluoroantimonic acid can be traced back to the early 20th century when the concept of superacids was first introduced. However, it wasn't until the 1960s that significant breakthroughs in its synthesis and characterization were achieved. The pioneering work of George A. Olah in the field of superacid chemistry paved the way for the development and application of fluoroantimonic acid in various chemical processes.
Over the years, the understanding of fluoroantimonic acid's unique properties has grown exponentially. Its ability to protonate even extremely weak bases and catalyze reactions that were previously thought impossible has opened up new avenues in synthetic chemistry. The acid's extreme reactivity and corrosiveness have also presented significant challenges in terms of handling and storage, driving innovations in materials science and safety protocols.
The primary objective of research into fluoroantimonic acid in chemical synthesis is to harness its unparalleled acidity for facilitating complex organic transformations. This includes the activation of inert C-H bonds, isomerization of hydrocarbons, and the generation of highly reactive carbocations. Additionally, there is a growing interest in exploring its potential in the synthesis of novel materials, such as superhydrophobic coatings and advanced polymers.
Another key goal is to develop more stable and manageable forms of fluoroantimonic acid, which could expand its applicability in industrial processes. This involves research into supported acid systems, ionic liquids, and other innovative formulations that maintain the acid's potency while mitigating its hazardous nature.
Furthermore, the scientific community aims to deepen the understanding of the fundamental chemistry behind fluoroantimonic acid's extreme acidity. This includes investigating its behavior at the molecular level, its interactions with various substrates, and the mechanisms of reactions it catalyzes. Such knowledge is crucial for optimizing its use in existing applications and discovering new potential uses.
As we look towards the future, the continued exploration of fluoroantimonic acid in chemical synthesis promises to unlock new realms of possibility in the creation of complex molecules, the development of more efficient industrial processes, and the advancement of materials science. The ongoing research in this field is expected to contribute significantly to the broader landscape of chemistry and its applications across various industries.
The evolution of fluoroantimonic acid can be traced back to the early 20th century when the concept of superacids was first introduced. However, it wasn't until the 1960s that significant breakthroughs in its synthesis and characterization were achieved. The pioneering work of George A. Olah in the field of superacid chemistry paved the way for the development and application of fluoroantimonic acid in various chemical processes.
Over the years, the understanding of fluoroantimonic acid's unique properties has grown exponentially. Its ability to protonate even extremely weak bases and catalyze reactions that were previously thought impossible has opened up new avenues in synthetic chemistry. The acid's extreme reactivity and corrosiveness have also presented significant challenges in terms of handling and storage, driving innovations in materials science and safety protocols.
The primary objective of research into fluoroantimonic acid in chemical synthesis is to harness its unparalleled acidity for facilitating complex organic transformations. This includes the activation of inert C-H bonds, isomerization of hydrocarbons, and the generation of highly reactive carbocations. Additionally, there is a growing interest in exploring its potential in the synthesis of novel materials, such as superhydrophobic coatings and advanced polymers.
Another key goal is to develop more stable and manageable forms of fluoroantimonic acid, which could expand its applicability in industrial processes. This involves research into supported acid systems, ionic liquids, and other innovative formulations that maintain the acid's potency while mitigating its hazardous nature.
Furthermore, the scientific community aims to deepen the understanding of the fundamental chemistry behind fluoroantimonic acid's extreme acidity. This includes investigating its behavior at the molecular level, its interactions with various substrates, and the mechanisms of reactions it catalyzes. Such knowledge is crucial for optimizing its use in existing applications and discovering new potential uses.
As we look towards the future, the continued exploration of fluoroantimonic acid in chemical synthesis promises to unlock new realms of possibility in the creation of complex molecules, the development of more efficient industrial processes, and the advancement of materials science. The ongoing research in this field is expected to contribute significantly to the broader landscape of chemistry and its applications across various industries.
Industrial Applications and Demand Analysis
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in the chemical industry due to its exceptional protonating ability and catalytic properties. The demand for this powerful compound has been steadily increasing across various industrial sectors, driven by its unique capabilities in chemical synthesis and processing.
In the petrochemical industry, fluoroantimonic acid has found extensive applications in hydrocarbon cracking and isomerization processes. Its ability to catalyze reactions at lower temperatures and pressures compared to conventional acids has led to improved efficiency and reduced energy consumption in refineries. This has resulted in a growing demand from oil and gas companies seeking to optimize their production processes and reduce operational costs.
The pharmaceutical sector has also shown increasing interest in fluoroantimonic acid for its potential in synthesizing complex organic compounds. Its strong acidic properties enable the activation of challenging substrates and facilitate novel reaction pathways, opening up possibilities for the development of new drug molecules. As the pharmaceutical industry continues to explore innovative synthesis routes, the demand for fluoroantimonic acid is expected to rise further.
In the field of materials science, fluoroantimonic acid has demonstrated promising applications in the production of advanced polymers and specialty chemicals. Its unique reactivity allows for the modification of polymer structures and the creation of materials with enhanced properties. This has led to a surge in demand from manufacturers of high-performance plastics, coatings, and electronic materials.
The electronics industry has also recognized the potential of fluoroantimonic acid in semiconductor processing and etching applications. Its ability to selectively etch certain materials with high precision has made it valuable in the production of microchips and other electronic components. As the demand for smaller and more powerful electronic devices continues to grow, the use of fluoroantimonic acid in this sector is projected to increase.
Despite its numerous applications, the market for fluoroantimonic acid faces certain challenges. The highly corrosive nature of the compound necessitates specialized handling and storage equipment, which can be costly for some industries. Additionally, environmental and safety concerns associated with its use have led to increased regulatory scrutiny, potentially impacting its adoption in certain regions.
Nevertheless, the overall market trend for fluoroantimonic acid remains positive. The compound's unique properties and the continuous innovation in chemical synthesis techniques are expected to drive sustained demand across multiple industries. As research and development efforts continue to uncover new applications and improve handling methods, the market for fluoroantimonic acid is likely to expand further in the coming years.
In the petrochemical industry, fluoroantimonic acid has found extensive applications in hydrocarbon cracking and isomerization processes. Its ability to catalyze reactions at lower temperatures and pressures compared to conventional acids has led to improved efficiency and reduced energy consumption in refineries. This has resulted in a growing demand from oil and gas companies seeking to optimize their production processes and reduce operational costs.
The pharmaceutical sector has also shown increasing interest in fluoroantimonic acid for its potential in synthesizing complex organic compounds. Its strong acidic properties enable the activation of challenging substrates and facilitate novel reaction pathways, opening up possibilities for the development of new drug molecules. As the pharmaceutical industry continues to explore innovative synthesis routes, the demand for fluoroantimonic acid is expected to rise further.
In the field of materials science, fluoroantimonic acid has demonstrated promising applications in the production of advanced polymers and specialty chemicals. Its unique reactivity allows for the modification of polymer structures and the creation of materials with enhanced properties. This has led to a surge in demand from manufacturers of high-performance plastics, coatings, and electronic materials.
The electronics industry has also recognized the potential of fluoroantimonic acid in semiconductor processing and etching applications. Its ability to selectively etch certain materials with high precision has made it valuable in the production of microchips and other electronic components. As the demand for smaller and more powerful electronic devices continues to grow, the use of fluoroantimonic acid in this sector is projected to increase.
Despite its numerous applications, the market for fluoroantimonic acid faces certain challenges. The highly corrosive nature of the compound necessitates specialized handling and storage equipment, which can be costly for some industries. Additionally, environmental and safety concerns associated with its use have led to increased regulatory scrutiny, potentially impacting its adoption in certain regions.
Nevertheless, the overall market trend for fluoroantimonic acid remains positive. The compound's unique properties and the continuous innovation in chemical synthesis techniques are expected to drive sustained demand across multiple industries. As research and development efforts continue to uncover new applications and improve handling methods, the market for fluoroantimonic acid is likely to expand further in the coming years.
Current Challenges in Fluoroantimonic Acid Synthesis
Fluoroantimonic acid synthesis faces several significant challenges that hinder its widespread application in chemical processes. One of the primary obstacles is the extreme reactivity and corrosiveness of the acid, which necessitates specialized handling and storage equipment. This requirement substantially increases production costs and limits the scale at which the acid can be synthesized safely.
The synthesis process itself presents another major challenge. Fluoroantimonic acid is typically produced by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5). However, controlling the reaction between these highly reactive components is exceptionally difficult. The process requires precise temperature and pressure control, as well as stringent safety measures to prevent accidental releases or explosions.
Furthermore, the purity of the final product is a critical concern. Trace impurities can significantly affect the acid's performance in chemical synthesis applications. Achieving and maintaining high purity levels during large-scale production remains a technical hurdle, often requiring multiple purification steps that further complicate the manufacturing process.
Environmental and safety concerns also pose substantial challenges to fluoroantimonic acid synthesis. The acid's extreme corrosiveness and toxicity make it a significant environmental hazard. Developing containment systems and waste treatment protocols that can effectively manage these risks without compromising production efficiency is an ongoing challenge for manufacturers.
The sourcing of raw materials, particularly high-purity antimony pentafluoride, presents another obstacle. The global supply chain for this precursor is limited, and its production involves its own set of technical and environmental challenges. This dependency on scarce raw materials introduces vulnerabilities in the production process and can lead to supply chain disruptions.
Scaling up production to meet industrial demands while maintaining safety and quality standards is perhaps the most pressing challenge. The highly specialized nature of the synthesis process makes it difficult to adapt conventional chemical engineering principles to large-scale production. Innovative reactor designs and process control systems are needed to overcome these scaling limitations.
Lastly, the development of more efficient catalytic systems for fluoroantimonic acid synthesis remains an active area of research. Current methods often result in low yields or require extreme conditions, making the process economically unfavorable for many potential applications. Improving catalytic efficiency could significantly reduce production costs and expand the acid's use in chemical synthesis.
The synthesis process itself presents another major challenge. Fluoroantimonic acid is typically produced by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5). However, controlling the reaction between these highly reactive components is exceptionally difficult. The process requires precise temperature and pressure control, as well as stringent safety measures to prevent accidental releases or explosions.
Furthermore, the purity of the final product is a critical concern. Trace impurities can significantly affect the acid's performance in chemical synthesis applications. Achieving and maintaining high purity levels during large-scale production remains a technical hurdle, often requiring multiple purification steps that further complicate the manufacturing process.
Environmental and safety concerns also pose substantial challenges to fluoroantimonic acid synthesis. The acid's extreme corrosiveness and toxicity make it a significant environmental hazard. Developing containment systems and waste treatment protocols that can effectively manage these risks without compromising production efficiency is an ongoing challenge for manufacturers.
The sourcing of raw materials, particularly high-purity antimony pentafluoride, presents another obstacle. The global supply chain for this precursor is limited, and its production involves its own set of technical and environmental challenges. This dependency on scarce raw materials introduces vulnerabilities in the production process and can lead to supply chain disruptions.
Scaling up production to meet industrial demands while maintaining safety and quality standards is perhaps the most pressing challenge. The highly specialized nature of the synthesis process makes it difficult to adapt conventional chemical engineering principles to large-scale production. Innovative reactor designs and process control systems are needed to overcome these scaling limitations.
Lastly, the development of more efficient catalytic systems for fluoroantimonic acid synthesis remains an active area of research. Current methods often result in low yields or require extreme conditions, making the process economically unfavorable for many potential applications. Improving catalytic efficiency could significantly reduce production costs and expand the acid's use in chemical synthesis.
Existing Synthesis Methods and Protocols
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in chemical reactions and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acid catalysts.
- Use in materials science and surface treatment: Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials such as polymers and ceramics.
- Safety considerations and handling procedures: Due to its extreme corrosiveness and reactivity, handling fluoroantimonic acid requires stringent safety measures. Specialized equipment, containment systems, and personal protective gear are necessary when working with this superacid. Proper disposal and neutralization procedures must be followed to prevent environmental contamination.
- Analytical and characterization techniques: Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analysis, and specialized apparatus for measuring superacidity. Such techniques are crucial for understanding the properties and behavior of this powerful superacid in different applications.
02 Applications in chemical reactions and catalysis
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids, making it valuable in organic synthesis and industrial chemical production.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials. The acid's unique properties make it suitable for developing advanced materials with specific characteristics.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized safety measures and handling procedures. This includes the use of appropriate containment materials, personal protective equipment, and controlled environments. Proper storage, transportation, and disposal methods are crucial to prevent accidents and environmental contamination.Expand Specific Solutions05 Analytical and characterization techniques
Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These methods include spectroscopic analysis, electrochemical measurements, and computational modeling. Such techniques are essential for understanding the acid's properties, reaction mechanisms, and potential applications in different fields.Expand Specific Solutions
Key Manufacturers and Research Institutions
The competitive landscape for Fluoroantimonic Acid in Chemical Synthesis is characterized by a mature market with established players and ongoing research. The industry is in a growth phase, driven by increasing demand in pharmaceutical and materials science sectors. Key players like Merck Sharp & Dohme Corp., F. Hoffmann-La Roche Ltd., and BASF Corp. are leading commercial applications, while academic institutions such as The University of Chicago and Zhejiang University are advancing fundamental research. The technology's maturity varies across applications, with some areas well-established and others still emerging. Companies like AGC, Inc. and Solvay China Co. Ltd. are focusing on industrial-scale production, while smaller firms like MedPacto, Inc. and Hinova Pharmaceuticals, Inc. are exploring niche applications in drug discovery.
Merck Sharp & Dohme Corp.
Technical Solution: Merck has developed an innovative application of fluoroantimonic acid in pharmaceutical synthesis. Their approach utilizes the superacid as a catalyst in the production of complex drug intermediates and active pharmaceutical ingredients. Merck's process incorporates a series of carefully controlled reaction steps that take advantage of the unique properties of fluoroantimonic acid to achieve specific molecular transformations. They have also implemented rigorous safety protocols and containment systems to ensure the safe handling of this highly reactive substance in a pharmaceutical manufacturing environment.
Strengths: Application in high-value pharmaceutical synthesis, rigorous safety protocols, potential for novel drug development. Weaknesses: Regulatory challenges, high costs associated with pharmaceutical-grade processes.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN has pioneered the use of fluoroantimonic acid in the synthesis of fluoropolymers and other fluorine-containing compounds. Their proprietary process involves a carefully controlled reaction environment that allows for the efficient production of these specialized materials. DAIKIN's approach includes the use of custom-designed reaction vessels and handling equipment to manage the extreme reactivity of fluoroantimonic acid. They have also developed a unique purification process to ensure the highest quality of their final products.
Strengths: Specialized in fluorine chemistry, custom-designed equipment, high-quality products. Weaknesses: Narrow focus on fluorine compounds, high equipment maintenance costs.
Breakthrough Technologies in Superacid Chemistry
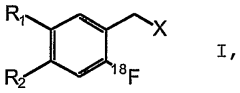
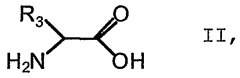
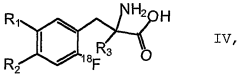
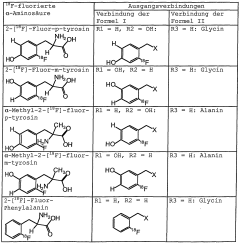
Method for the production of <18>f-fluorinated alpha acids
PatentWO2003099746A1
Innovation
- A stereoselective nucleophilic asymmetric synthesis process using an achiral or chiral Ni(II) complex of a Schiff base with a chiral catalyst and auxiliary base, employing phase transfer catalysis at room temperature to facilitate high-yield production of 18F-fluorinated α-amino acids and derivatives, such as 2-[18F]-Fluoro-L-tyrosine, in fully automated modules.
Use of fluorinated derivatives of 4-aminopyridine in therapeutics and medical imaging
PatentWO2013173746A2
Innovation
- Development of novel compounds that bind to potassium channels, including fluorinated derivatives of 4-aminopyridine, which can be used for both therapeutic treatment and in vivo imaging to diagnose and assess demyelination in the central nervous system.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant safety and environmental challenges in chemical synthesis applications. Its extreme corrosiveness and reactivity necessitate stringent safety protocols and specialized handling equipment. Personal protective equipment (PPE) is crucial, including chemical-resistant suits, gloves, and full-face respirators with appropriate filters. All personnel working with this acid must undergo comprehensive safety training and adhere to strict operational procedures.
Containment is a primary concern due to the acid's ability to react violently with water and many common materials. Specialized storage and reaction vessels, typically made of fluoropolymers like PTFE, are essential to prevent leaks and contamination. Proper ventilation systems with acid-resistant ductwork and scrubbers are necessary to capture and neutralize any vapors or fumes generated during use.
Environmental considerations are equally critical. The potential for soil and water contamination is severe, as even small releases can cause significant ecological damage. Waste management protocols must be rigorously enforced, with all acid-containing materials treated as hazardous waste. Neutralization procedures using appropriate bases should be conducted in controlled environments before disposal.
Emergency response planning is vital when working with fluoroantimonic acid. Facilities must have well-defined spill containment and cleanup procedures, including specialized absorbents and neutralizing agents. Regular drills and training sessions should be conducted to ensure all personnel are prepared for potential incidents.
Long-term exposure risks to human health and the environment are not fully understood, necessitating ongoing research and monitoring. Chronic low-level exposure may pose risks to respiratory and dermal health, while potential bioaccumulation in ecosystems remains a concern. As such, the use of fluoroantimonic acid should be limited to essential applications where no safer alternatives exist.
Regulatory compliance is a complex aspect of working with this superacid. Different countries and regions may have varying requirements for its use, storage, and transport. Organizations must stay informed about relevant regulations and ensure full compliance, including proper documentation, reporting, and obtaining necessary permits.
Containment is a primary concern due to the acid's ability to react violently with water and many common materials. Specialized storage and reaction vessels, typically made of fluoropolymers like PTFE, are essential to prevent leaks and contamination. Proper ventilation systems with acid-resistant ductwork and scrubbers are necessary to capture and neutralize any vapors or fumes generated during use.
Environmental considerations are equally critical. The potential for soil and water contamination is severe, as even small releases can cause significant ecological damage. Waste management protocols must be rigorously enforced, with all acid-containing materials treated as hazardous waste. Neutralization procedures using appropriate bases should be conducted in controlled environments before disposal.
Emergency response planning is vital when working with fluoroantimonic acid. Facilities must have well-defined spill containment and cleanup procedures, including specialized absorbents and neutralizing agents. Regular drills and training sessions should be conducted to ensure all personnel are prepared for potential incidents.
Long-term exposure risks to human health and the environment are not fully understood, necessitating ongoing research and monitoring. Chronic low-level exposure may pose risks to respiratory and dermal health, while potential bioaccumulation in ecosystems remains a concern. As such, the use of fluoroantimonic acid should be limited to essential applications where no safer alternatives exist.
Regulatory compliance is a complex aspect of working with this superacid. Different countries and regions may have varying requirements for its use, storage, and transport. Organizations must stay informed about relevant regulations and ensure full compliance, including proper documentation, reporting, and obtaining necessary permits.
Regulatory Framework for Superacid Handling
The regulatory framework for handling superacids, particularly fluoroantimonic acid, is complex and stringent due to the extreme corrosiveness and reactivity of these substances. Governments and international organizations have established comprehensive guidelines to ensure safe handling, storage, and disposal of superacids in chemical synthesis processes. These regulations typically encompass several key areas, including workplace safety, environmental protection, and transportation.
In the United States, the Occupational Safety and Health Administration (OSHA) has set forth specific standards for handling highly corrosive substances. These include mandatory use of personal protective equipment (PPE), implementation of engineering controls such as fume hoods and ventilation systems, and regular safety training for personnel. The Environmental Protection Agency (EPA) regulates the storage and disposal of superacids under the Resource Conservation and Recovery Act (RCRA), classifying them as hazardous waste and mandating strict protocols for their management.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. For superacids like fluoroantimonic acid, this system requires clear labeling with appropriate hazard pictograms, signal words, and safety data sheets. The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes additional requirements on manufacturers and importers to assess and manage the risks associated with these substances.
Transportation of superacids is governed by various national and international regulations. In the United States, the Department of Transportation (DOT) classifies fluoroantimonic acid as a Class 8 corrosive material, subject to specific packaging, labeling, and documentation requirements. Similarly, the International Air Transport Association (IATA) and the International Maritime Dangerous Goods (IMDG) Code provide detailed guidelines for the air and sea transport of such hazardous materials.
Research institutions and industrial facilities working with superacids must adhere to strict protocols for emergency response and spill management. This includes the development of comprehensive safety plans, installation of specialized containment and neutralization systems, and regular drills to ensure preparedness for potential incidents. Many jurisdictions also require facilities to obtain specific permits and undergo regular inspections to maintain compliance with safety regulations.
As the field of chemical synthesis evolves, regulatory frameworks continue to adapt to address emerging challenges and incorporate new safety technologies. Ongoing research into safer alternatives and improved handling techniques may lead to future modifications in the regulatory landscape, potentially easing some restrictions while maintaining rigorous safety standards.
In the United States, the Occupational Safety and Health Administration (OSHA) has set forth specific standards for handling highly corrosive substances. These include mandatory use of personal protective equipment (PPE), implementation of engineering controls such as fume hoods and ventilation systems, and regular safety training for personnel. The Environmental Protection Agency (EPA) regulates the storage and disposal of superacids under the Resource Conservation and Recovery Act (RCRA), classifying them as hazardous waste and mandating strict protocols for their management.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. For superacids like fluoroantimonic acid, this system requires clear labeling with appropriate hazard pictograms, signal words, and safety data sheets. The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes additional requirements on manufacturers and importers to assess and manage the risks associated with these substances.
Transportation of superacids is governed by various national and international regulations. In the United States, the Department of Transportation (DOT) classifies fluoroantimonic acid as a Class 8 corrosive material, subject to specific packaging, labeling, and documentation requirements. Similarly, the International Air Transport Association (IATA) and the International Maritime Dangerous Goods (IMDG) Code provide detailed guidelines for the air and sea transport of such hazardous materials.
Research institutions and industrial facilities working with superacids must adhere to strict protocols for emergency response and spill management. This includes the development of comprehensive safety plans, installation of specialized containment and neutralization systems, and regular drills to ensure preparedness for potential incidents. Many jurisdictions also require facilities to obtain specific permits and undergo regular inspections to maintain compliance with safety regulations.
As the field of chemical synthesis evolves, regulatory frameworks continue to adapt to address emerging challenges and incorporate new safety technologies. Ongoing research into safer alternatives and improved handling techniques may lead to future modifications in the regulatory landscape, potentially easing some restrictions while maintaining rigorous safety standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!