Fluoroantimonic Acid in New Era Catalysis: What Lies Ahead
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Catalysis Evolution and Objectives
Fluoroantimonic acid, known as the world's strongest superacid, has been a subject of intense research and development in the field of catalysis for several decades. Its exceptional acidity, surpassing even that of 100% sulfuric acid, has positioned it as a powerful tool in various chemical processes. The evolution of fluoroantimonic acid catalysis can be traced back to the mid-20th century when the concept of superacids was first introduced by James Bryant Conant.
The journey of fluoroantimonic acid in catalysis began with its synthesis and characterization, followed by exploratory studies to understand its unique properties. Early research focused on its ability to protonate even very weak bases, which opened up new possibilities in organic synthesis and petrochemical processes. As the understanding of its catalytic potential grew, so did its applications in various industrial sectors.
Throughout the years, the development of fluoroantimonic acid catalysis has been driven by the need for more efficient and selective chemical transformations. Its ability to catalyze reactions under milder conditions and with higher yields compared to traditional acid catalysts has been a key factor in its continued relevance. The evolution of this field has seen significant advancements in reaction mechanisms, catalyst design, and process optimization.
In recent years, the objectives of fluoroantimonic acid catalysis research have shifted towards addressing contemporary challenges in chemistry and materials science. One primary goal is to enhance the sustainability of chemical processes by reducing energy consumption and minimizing waste production. Researchers are exploring ways to utilize fluoroantimonic acid in green chemistry applications, aiming to develop more environmentally friendly catalytic systems.
Another important objective is to expand the scope of reactions that can be catalyzed by fluoroantimonic acid. This includes investigating its potential in asymmetric synthesis, C-H bond activation, and the conversion of biomass into value-added chemicals. The development of novel reaction pathways and the ability to access previously unattainable products are at the forefront of current research efforts.
Furthermore, there is a growing interest in understanding and controlling the molecular-level interactions between fluoroantimonic acid and various substrates. This fundamental knowledge is crucial for designing more efficient and selective catalytic systems. Advanced spectroscopic and computational techniques are being employed to gain deeper insights into reaction mechanisms and transition states.
As we look ahead, the objectives of fluoroantimonic acid catalysis research are likely to focus on overcoming its limitations, such as its extreme corrosiveness and sensitivity to moisture. Developing more stable and easily handled forms of the acid, as well as exploring its synergistic effects with other catalytic systems, are areas of active investigation. The ultimate goal is to harness the full potential of this superacid in catalysis while addressing the practical challenges associated with its use in industrial settings.
The journey of fluoroantimonic acid in catalysis began with its synthesis and characterization, followed by exploratory studies to understand its unique properties. Early research focused on its ability to protonate even very weak bases, which opened up new possibilities in organic synthesis and petrochemical processes. As the understanding of its catalytic potential grew, so did its applications in various industrial sectors.
Throughout the years, the development of fluoroantimonic acid catalysis has been driven by the need for more efficient and selective chemical transformations. Its ability to catalyze reactions under milder conditions and with higher yields compared to traditional acid catalysts has been a key factor in its continued relevance. The evolution of this field has seen significant advancements in reaction mechanisms, catalyst design, and process optimization.
In recent years, the objectives of fluoroantimonic acid catalysis research have shifted towards addressing contemporary challenges in chemistry and materials science. One primary goal is to enhance the sustainability of chemical processes by reducing energy consumption and minimizing waste production. Researchers are exploring ways to utilize fluoroantimonic acid in green chemistry applications, aiming to develop more environmentally friendly catalytic systems.
Another important objective is to expand the scope of reactions that can be catalyzed by fluoroantimonic acid. This includes investigating its potential in asymmetric synthesis, C-H bond activation, and the conversion of biomass into value-added chemicals. The development of novel reaction pathways and the ability to access previously unattainable products are at the forefront of current research efforts.
Furthermore, there is a growing interest in understanding and controlling the molecular-level interactions between fluoroantimonic acid and various substrates. This fundamental knowledge is crucial for designing more efficient and selective catalytic systems. Advanced spectroscopic and computational techniques are being employed to gain deeper insights into reaction mechanisms and transition states.
As we look ahead, the objectives of fluoroantimonic acid catalysis research are likely to focus on overcoming its limitations, such as its extreme corrosiveness and sensitivity to moisture. Developing more stable and easily handled forms of the acid, as well as exploring its synergistic effects with other catalytic systems, are areas of active investigation. The ultimate goal is to harness the full potential of this superacid in catalysis while addressing the practical challenges associated with its use in industrial settings.
Market Demand for Advanced Catalytic Processes
The market demand for advanced catalytic processes has been steadily growing, driven by the increasing need for more efficient and sustainable chemical production methods. Fluoroantimonic acid, as one of the strongest known superacids, has garnered significant attention in the field of catalysis due to its exceptional proton-donating ability and potential to catalyze reactions that were previously challenging or impossible.
In the petrochemical industry, there is a rising demand for catalysts that can enhance the efficiency of hydrocarbon cracking and isomerization processes. Fluoroantimonic acid-based catalysts have shown promise in these applications, potentially offering higher conversion rates and improved selectivity compared to traditional acid catalysts. This has led to increased interest from major oil and gas companies seeking to optimize their refining processes and reduce energy consumption.
The pharmaceutical sector has also expressed growing interest in advanced catalytic processes involving fluoroantimonic acid. The ability of this superacid to facilitate complex organic transformations could potentially streamline the synthesis of pharmaceutical intermediates and active pharmaceutical ingredients (APIs). This could lead to more cost-effective drug manufacturing processes and potentially enable the development of novel therapeutic compounds.
In the fine chemicals industry, there is a persistent demand for catalysts that can enable highly selective transformations. Fluoroantimonic acid's unique properties make it a candidate for catalyzing challenging reactions, such as the activation of C-H bonds or the formation of carbon-carbon bonds under mild conditions. This could open up new synthetic routes for specialty chemicals and advanced materials.
The growing focus on sustainability and green chemistry has also created a market demand for catalytic processes that can reduce waste generation and improve atom economy. While fluoroantimonic acid itself is highly corrosive and requires careful handling, its potential to enable more efficient reactions could contribute to overall process intensification and waste reduction in certain applications.
However, it is important to note that the adoption of fluoroantimonic acid in industrial catalytic processes faces significant challenges. The extreme corrosiveness of the acid necessitates specialized equipment and safety measures, which can increase implementation costs. Additionally, regulatory concerns regarding the use of such strong acids in industrial settings may limit widespread adoption in certain regions or applications.
Despite these challenges, the potential benefits of fluoroantimonic acid in catalysis continue to drive research and development efforts. As industries seek to push the boundaries of chemical transformations and process efficiency, the demand for advanced catalytic processes, including those involving superacids like fluoroantimonic acid, is expected to grow. This trend is likely to be particularly pronounced in high-value sectors such as specialty chemicals, pharmaceuticals, and advanced materials manufacturing.
In the petrochemical industry, there is a rising demand for catalysts that can enhance the efficiency of hydrocarbon cracking and isomerization processes. Fluoroantimonic acid-based catalysts have shown promise in these applications, potentially offering higher conversion rates and improved selectivity compared to traditional acid catalysts. This has led to increased interest from major oil and gas companies seeking to optimize their refining processes and reduce energy consumption.
The pharmaceutical sector has also expressed growing interest in advanced catalytic processes involving fluoroantimonic acid. The ability of this superacid to facilitate complex organic transformations could potentially streamline the synthesis of pharmaceutical intermediates and active pharmaceutical ingredients (APIs). This could lead to more cost-effective drug manufacturing processes and potentially enable the development of novel therapeutic compounds.
In the fine chemicals industry, there is a persistent demand for catalysts that can enable highly selective transformations. Fluoroantimonic acid's unique properties make it a candidate for catalyzing challenging reactions, such as the activation of C-H bonds or the formation of carbon-carbon bonds under mild conditions. This could open up new synthetic routes for specialty chemicals and advanced materials.
The growing focus on sustainability and green chemistry has also created a market demand for catalytic processes that can reduce waste generation and improve atom economy. While fluoroantimonic acid itself is highly corrosive and requires careful handling, its potential to enable more efficient reactions could contribute to overall process intensification and waste reduction in certain applications.
However, it is important to note that the adoption of fluoroantimonic acid in industrial catalytic processes faces significant challenges. The extreme corrosiveness of the acid necessitates specialized equipment and safety measures, which can increase implementation costs. Additionally, regulatory concerns regarding the use of such strong acids in industrial settings may limit widespread adoption in certain regions or applications.
Despite these challenges, the potential benefits of fluoroantimonic acid in catalysis continue to drive research and development efforts. As industries seek to push the boundaries of chemical transformations and process efficiency, the demand for advanced catalytic processes, including those involving superacids like fluoroantimonic acid, is expected to grow. This trend is likely to be particularly pronounced in high-value sectors such as specialty chemicals, pharmaceuticals, and advanced materials manufacturing.
Current State and Challenges in Superacid Catalysis
Superacid catalysis, particularly involving fluoroantimonic acid, has reached a critical juncture in its development. The current state of this field is characterized by significant advancements in understanding the fundamental mechanisms of superacid-catalyzed reactions, yet it faces substantial challenges in practical applications and scalability.
Recent research has demonstrated the exceptional catalytic activity of fluoroantimonic acid in various organic transformations, including alkylations, isomerizations, and cracking reactions. Its ability to protonate even weak bases and generate highly reactive carbocations has opened new avenues in synthetic chemistry. However, the extreme corrosiveness and moisture sensitivity of fluoroantimonic acid pose significant handling and safety concerns, limiting its widespread industrial adoption.
One of the primary challenges in superacid catalysis is the development of more stable and easily manageable superacid systems. Efforts are underway to create solid superacids or supported liquid superacids that maintain the catalytic activity of fluoroantimonic acid while mitigating its hazardous properties. These approaches aim to combine the high acidity of fluoroantimonic acid with the practical advantages of heterogeneous catalysts.
Another significant challenge lies in the selectivity of superacid-catalyzed reactions. While fluoroantimonic acid excels in activating unreactive substrates, controlling the reaction pathways to achieve desired products remains difficult. Researchers are exploring the use of designer solvents, co-catalysts, and tailored reaction conditions to enhance selectivity without compromising catalytic activity.
The environmental impact of superacid catalysis presents a further challenge. The production and disposal of fluoroantimonic acid and related superacids raise ecological concerns. Developing greener alternatives or closed-loop systems for superacid recycling is crucial for the sustainable future of this technology.
In the realm of materials science, the potential of superacids in the synthesis of novel materials, such as advanced polymers and nanostructures, is being actively explored. However, integrating superacid catalysis into existing industrial processes without major infrastructure overhauls remains a significant hurdle.
The computational modeling of superacid systems has made substantial progress, aiding in the understanding of reaction mechanisms and the design of new catalytic systems. Nevertheless, the extreme acidity of these systems pushes the limits of current computational methods, necessitating the development of more sophisticated modeling techniques.
As research in superacid catalysis progresses, bridging the gap between laboratory discoveries and industrial applications remains a central challenge. Overcoming these obstacles will be crucial in realizing the full potential of fluoroantimonic acid and related superacids in next-generation catalytic processes.
Recent research has demonstrated the exceptional catalytic activity of fluoroantimonic acid in various organic transformations, including alkylations, isomerizations, and cracking reactions. Its ability to protonate even weak bases and generate highly reactive carbocations has opened new avenues in synthetic chemistry. However, the extreme corrosiveness and moisture sensitivity of fluoroantimonic acid pose significant handling and safety concerns, limiting its widespread industrial adoption.
One of the primary challenges in superacid catalysis is the development of more stable and easily manageable superacid systems. Efforts are underway to create solid superacids or supported liquid superacids that maintain the catalytic activity of fluoroantimonic acid while mitigating its hazardous properties. These approaches aim to combine the high acidity of fluoroantimonic acid with the practical advantages of heterogeneous catalysts.
Another significant challenge lies in the selectivity of superacid-catalyzed reactions. While fluoroantimonic acid excels in activating unreactive substrates, controlling the reaction pathways to achieve desired products remains difficult. Researchers are exploring the use of designer solvents, co-catalysts, and tailored reaction conditions to enhance selectivity without compromising catalytic activity.
The environmental impact of superacid catalysis presents a further challenge. The production and disposal of fluoroantimonic acid and related superacids raise ecological concerns. Developing greener alternatives or closed-loop systems for superacid recycling is crucial for the sustainable future of this technology.
In the realm of materials science, the potential of superacids in the synthesis of novel materials, such as advanced polymers and nanostructures, is being actively explored. However, integrating superacid catalysis into existing industrial processes without major infrastructure overhauls remains a significant hurdle.
The computational modeling of superacid systems has made substantial progress, aiding in the understanding of reaction mechanisms and the design of new catalytic systems. Nevertheless, the extreme acidity of these systems pushes the limits of current computational methods, necessitating the development of more sophisticated modeling techniques.
As research in superacid catalysis progresses, bridging the gap between laboratory discoveries and industrial applications remains a central challenge. Overcoming these obstacles will be crucial in realizing the full potential of fluoroantimonic acid and related superacids in next-generation catalytic processes.
Existing Applications of Fluoroantimonic Acid
01 Synthesis and production methods
Various methods for synthesizing and producing fluoroantimonic acid are described. These include techniques for generating the superacid through the reaction of hydrogen fluoride with antimony pentafluoride, as well as processes for purification and stabilization of the resulting compound.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Specialized equipment and safety measures are required due to the extreme reactivity of the acid.
- Applications in organic synthesis and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It can facilitate alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids.
- Use in materials science and surface treatment: Fluoroantimonic acid finds applications in materials science for surface etching and modification of various substrates. It can be used to create specialized coatings or to modify the properties of materials such as polymers or metals. The acid's strong etching ability makes it suitable for certain semiconductor processing steps.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and storage procedures. Safety measures include the use of specialized containment materials, personal protective equipment, and strict protocols for handling and disposal. Proper training and safety systems are essential for working with this superacid.
- Analytical and research applications: Fluoroantimonic acid is used in certain analytical and research applications where its superacidic properties are beneficial. It can be employed in spectroscopic studies, as a reagent in specialized chemical analyses, or as a tool for investigating superacid chemistry and related phenomena in academic and industrial research settings.
02 Applications in catalysis
Fluoroantimonic acid is utilized as a powerful catalyst in various chemical reactions. Its superacidic properties make it effective for promoting reactions such as isomerization, alkylation, and polymerization of hydrocarbons and other organic compounds.Expand Specific Solutions03 Use in materials science
The superacid finds applications in materials science, particularly in the development of advanced materials. It is used in the preparation of specialized polymers, surface treatments, and the modification of various substrates to enhance their properties.Expand Specific Solutions04 Safety and handling considerations
Due to its extremely corrosive and reactive nature, special safety measures and handling procedures are required for fluoroantimonic acid. This includes the use of specialized containment materials, protective equipment, and protocols for storage and transportation.Expand Specific Solutions05 Analytical and characterization techniques
Various analytical and characterization techniques are employed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analyses, and specialized equipment designed to handle and measure properties of superacids.Expand Specific Solutions
Key Players in Superacid Catalysis Industry
The field of Fluoroantimonic Acid in New Era Catalysis is in a nascent stage of development, with significant potential for growth. The market size is currently limited but expected to expand as research progresses. Technological maturity varies among key players, with academic institutions like California Institute of Technology, Yale University, and Massachusetts Institute of Technology leading in fundamental research. Industry giants such as BASF SE, DuPont de Nemours, Inc., and ExxonMobil Chemical Patents, Inc. are investing in applied research and development. Research organizations like Centre National de la Recherche Scientifique and Chinese Academy of Sciences are bridging the gap between academia and industry, fostering innovation in this emerging field.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has made significant strides in applying fluoroantimonic acid to enhance petroleum refining processes. Their research team has developed a novel catalytic cracking method that utilizes a stabilized form of fluoroantimonic acid to achieve higher conversion rates and improved selectivity in the production of high-value petrochemicals. This innovative approach allows for the efficient processing of heavy crude oil fractions, resulting in increased yields of desirable products such as gasoline and diesel fuel. Sinopec's technology also incorporates advanced containment and neutralization systems to address the corrosive nature of the superacid, ensuring safe and environmentally responsible operations.
Strengths: Improved refining efficiency, ability to process lower-quality feedstocks, and potential for increased profitability. Weaknesses: High initial investment for retrofitting existing refineries, potential environmental risks, and the need for specialized handling procedures.
Novartis AG
Technical Solution: Novartis AG has made significant advancements in utilizing fluoroantimonic acid for pharmaceutical applications. Their research focuses on employing the superacid as a powerful activating agent in the synthesis of complex drug molecules, particularly those containing fluorine. By carefully controlling the reaction conditions and using specially designed protective groups, Novartis has developed a method to selectively introduce fluorine atoms into challenging positions of pharmaceutical compounds. This approach has enabled the creation of novel fluorinated drug candidates with enhanced metabolic stability and improved pharmacokinetic properties. Additionally, Novartis is exploring the potential of fluoroantimonic acid-catalyzed reactions in the production of PET imaging agents for advanced diagnostic applications.
Strengths: Access to new chemical space for drug discovery, potential for improved drug efficacy and safety profiles. Weaknesses: Challenges in scaling up production, stringent safety and containment requirements, and potential regulatory hurdles.
Core Innovations in Fluoroantimonic Acid Research
Mixtures of ionic liquids with lewis acids
PatentInactiveEP1658268A1
Innovation
- Development of new ionic liquid materials formed by mixing triflate or bis(trifluoromethylsulfonyl)imide salts of imidazolium, pyridinium, ammonium, or phosphonium ions with Lewis acids like AICI3, AIBr3, SnCl2, FeCl3, and ZnCl2 at molar ratios greater than 1:1, resulting in higher acidity and stability, allowing for increased Lewis acid content without exceeding 100°C melting points.
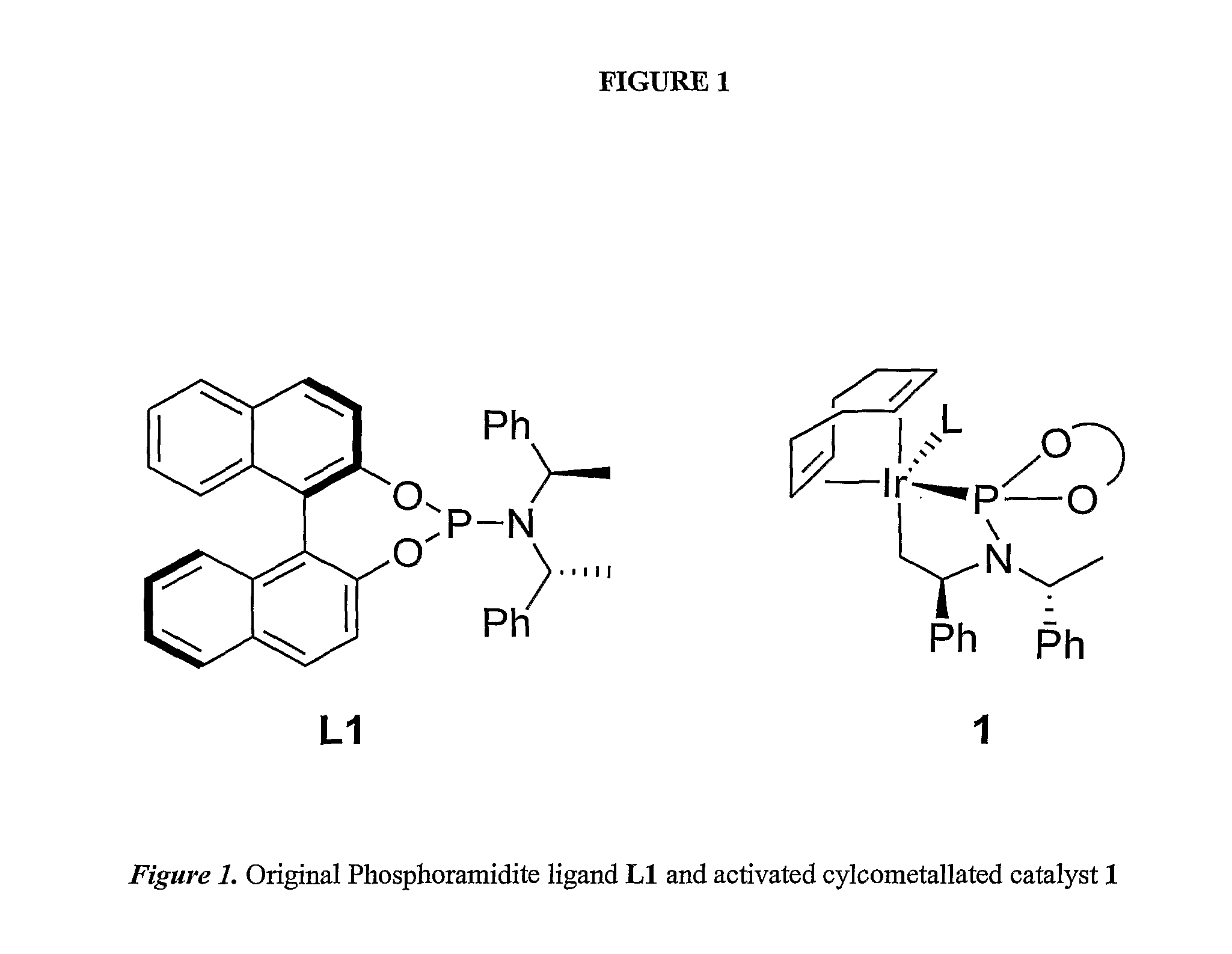
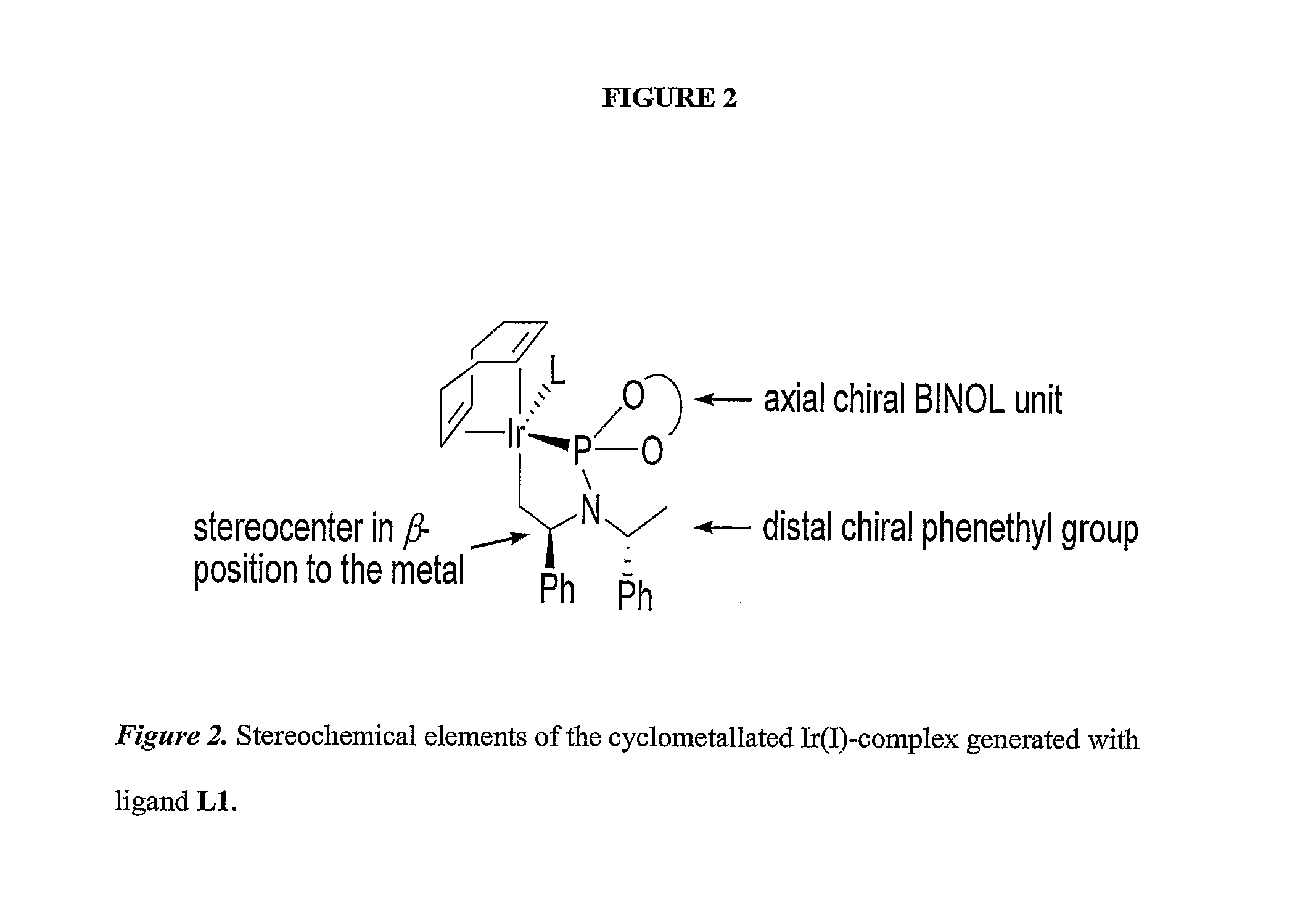
Enantioselective Phosphoramidite Compounds and Catalysts
PatentInactiveUS20070259774A1
Innovation
- Phosphoramidite compounds are used to form catalyst complexes with transition metals like iridium, rhodium, ruthenium, nickel, palladium, platinum, copper, or silver for enantioselective and regioselective hydrogenation, transfer hydrogenation, and allylic substitution reactions, including the formation of allylic amines and ethers.
Environmental Impact and Safety Considerations
The use of fluoroantimonic acid in catalysis raises significant environmental and safety concerns due to its extreme corrosiveness and reactivity. As the strongest known superacid, it poses severe risks to human health and the environment if not handled properly. Exposure can cause severe burns, tissue damage, and potentially fatal respiratory issues. The acid's highly reactive nature also presents explosion hazards when in contact with water or organic compounds.
Environmental impacts of fluoroantimonic acid are particularly worrisome. Its components, hydrogen fluoride and antimony pentafluoride, can persist in the environment and bioaccumulate in living organisms. Accidental releases could lead to soil and water contamination, affecting ecosystems and potentially entering the food chain. The acid's ability to generate toxic fumes further exacerbates its environmental footprint, potentially contributing to air pollution and acid rain formation.
Safety considerations for handling fluoroantimonic acid are paramount in industrial settings. Specialized containment systems, typically made of fluoropolymers like PTFE, are essential to prevent corrosion and leaks. Personal protective equipment, including fully encapsulating chemical suits and self-contained breathing apparatus, is mandatory for workers. Stringent protocols for storage, transport, and disposal must be implemented to minimize risks of accidents or environmental contamination.
The future of fluoroantimonic acid in catalysis hinges on developing safer handling methods and mitigating its environmental impact. Research into alternative superacids or modified versions with reduced toxicity is ongoing. Innovations in reactor design and process engineering aim to minimize exposure risks and improve containment. Additionally, the development of more efficient recovery and recycling techniques could reduce the overall environmental footprint of processes utilizing this superacid.
Regulatory frameworks governing the use of fluoroantimonic acid are likely to become more stringent as awareness of its potential hazards grows. Industries employing this superacid may face increased scrutiny and compliance requirements. This could drive further innovation in safety technologies and push for the development of greener alternatives in catalysis applications.
Balancing the catalytic potential of fluoroantimonic acid with its environmental and safety challenges will be crucial for its future in industrial processes. As sustainability becomes increasingly important in chemical manufacturing, the continued use of this superacid will depend on significant advancements in risk mitigation strategies and the development of more environmentally friendly alternatives.
Environmental impacts of fluoroantimonic acid are particularly worrisome. Its components, hydrogen fluoride and antimony pentafluoride, can persist in the environment and bioaccumulate in living organisms. Accidental releases could lead to soil and water contamination, affecting ecosystems and potentially entering the food chain. The acid's ability to generate toxic fumes further exacerbates its environmental footprint, potentially contributing to air pollution and acid rain formation.
Safety considerations for handling fluoroantimonic acid are paramount in industrial settings. Specialized containment systems, typically made of fluoropolymers like PTFE, are essential to prevent corrosion and leaks. Personal protective equipment, including fully encapsulating chemical suits and self-contained breathing apparatus, is mandatory for workers. Stringent protocols for storage, transport, and disposal must be implemented to minimize risks of accidents or environmental contamination.
The future of fluoroantimonic acid in catalysis hinges on developing safer handling methods and mitigating its environmental impact. Research into alternative superacids or modified versions with reduced toxicity is ongoing. Innovations in reactor design and process engineering aim to minimize exposure risks and improve containment. Additionally, the development of more efficient recovery and recycling techniques could reduce the overall environmental footprint of processes utilizing this superacid.
Regulatory frameworks governing the use of fluoroantimonic acid are likely to become more stringent as awareness of its potential hazards grows. Industries employing this superacid may face increased scrutiny and compliance requirements. This could drive further innovation in safety technologies and push for the development of greener alternatives in catalysis applications.
Balancing the catalytic potential of fluoroantimonic acid with its environmental and safety challenges will be crucial for its future in industrial processes. As sustainability becomes increasingly important in chemical manufacturing, the continued use of this superacid will depend on significant advancements in risk mitigation strategies and the development of more environmentally friendly alternatives.
Economic Viability of Fluoroantimonic Acid Catalysis
The economic viability of fluoroantimonic acid catalysis is a critical factor in determining its potential for widespread industrial adoption. As one of the strongest known superacids, fluoroantimonic acid has shown remarkable catalytic properties in various chemical reactions. However, its implementation on a commercial scale faces several economic challenges that must be carefully evaluated.
The production costs associated with fluoroantimonic acid are significantly higher than those of conventional catalysts. This is primarily due to the expensive raw materials required, such as antimony pentafluoride and hydrogen fluoride, as well as the specialized equipment needed to handle these highly corrosive substances. The synthesis process itself is also energy-intensive, further contributing to the overall production expenses.
Despite these high initial costs, the exceptional catalytic activity of fluoroantimonic acid may offer substantial benefits in terms of reaction efficiency and product yield. In certain applications, the use of this superacid catalyst could potentially reduce reaction times, lower energy requirements, and improve selectivity, leading to increased productivity and reduced operational costs.
The longevity and recyclability of fluoroantimonic acid catalysts are crucial factors in assessing their economic viability. While the acid's extreme reactivity contributes to its catalytic prowess, it also poses challenges in terms of catalyst stability and reusability. Developing effective methods for catalyst recovery and regeneration could significantly enhance the economic attractiveness of fluoroantimonic acid catalysis.
Safety considerations and environmental regulations play a substantial role in the economic equation. The highly corrosive and toxic nature of fluoroantimonic acid necessitates extensive safety measures and specialized containment systems, which can add considerable costs to industrial operations. Compliance with stringent environmental regulations may require additional investments in waste treatment and disposal facilities.
Market demand for products that can be uniquely or more efficiently produced using fluoroantimonic acid catalysis will be a key driver of its economic viability. Industries such as petrochemicals, pharmaceuticals, and fine chemicals may find particular value in the acid's catalytic properties, potentially justifying the higher costs associated with its use.
The scalability of fluoroantimonic acid catalysis processes is another critical factor. While laboratory-scale experiments have demonstrated impressive results, translating these successes to industrial-scale production presents both technical and economic challenges. Overcoming these scaling issues will be essential for realizing the full economic potential of this catalytic system.
In conclusion, the economic viability of fluoroantimonic acid catalysis hinges on a delicate balance between its high costs and potential benefits. As research progresses and new applications emerge, the economic landscape for this powerful catalyst may shift, potentially opening up new opportunities for industrial implementation.
The production costs associated with fluoroantimonic acid are significantly higher than those of conventional catalysts. This is primarily due to the expensive raw materials required, such as antimony pentafluoride and hydrogen fluoride, as well as the specialized equipment needed to handle these highly corrosive substances. The synthesis process itself is also energy-intensive, further contributing to the overall production expenses.
Despite these high initial costs, the exceptional catalytic activity of fluoroantimonic acid may offer substantial benefits in terms of reaction efficiency and product yield. In certain applications, the use of this superacid catalyst could potentially reduce reaction times, lower energy requirements, and improve selectivity, leading to increased productivity and reduced operational costs.
The longevity and recyclability of fluoroantimonic acid catalysts are crucial factors in assessing their economic viability. While the acid's extreme reactivity contributes to its catalytic prowess, it also poses challenges in terms of catalyst stability and reusability. Developing effective methods for catalyst recovery and regeneration could significantly enhance the economic attractiveness of fluoroantimonic acid catalysis.
Safety considerations and environmental regulations play a substantial role in the economic equation. The highly corrosive and toxic nature of fluoroantimonic acid necessitates extensive safety measures and specialized containment systems, which can add considerable costs to industrial operations. Compliance with stringent environmental regulations may require additional investments in waste treatment and disposal facilities.
Market demand for products that can be uniquely or more efficiently produced using fluoroantimonic acid catalysis will be a key driver of its economic viability. Industries such as petrochemicals, pharmaceuticals, and fine chemicals may find particular value in the acid's catalytic properties, potentially justifying the higher costs associated with its use.
The scalability of fluoroantimonic acid catalysis processes is another critical factor. While laboratory-scale experiments have demonstrated impressive results, translating these successes to industrial-scale production presents both technical and economic challenges. Overcoming these scaling issues will be essential for realizing the full economic potential of this catalytic system.
In conclusion, the economic viability of fluoroantimonic acid catalysis hinges on a delicate balance between its high costs and potential benefits. As research progresses and new applications emerge, the economic landscape for this powerful catalyst may shift, potentially opening up new opportunities for industrial implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!