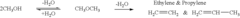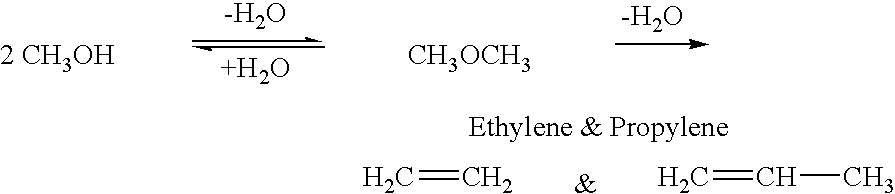How Dimethyl Ether Compares in Combustion Efficiency Metrics?
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DME Combustion Background
Dimethyl ether (DME) has emerged as a promising alternative fuel in recent years, attracting significant attention from researchers and industry professionals alike. The combustion of DME has been a subject of extensive study due to its unique properties and potential advantages over conventional fuels.
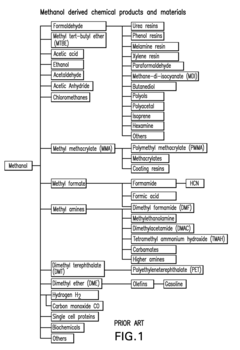
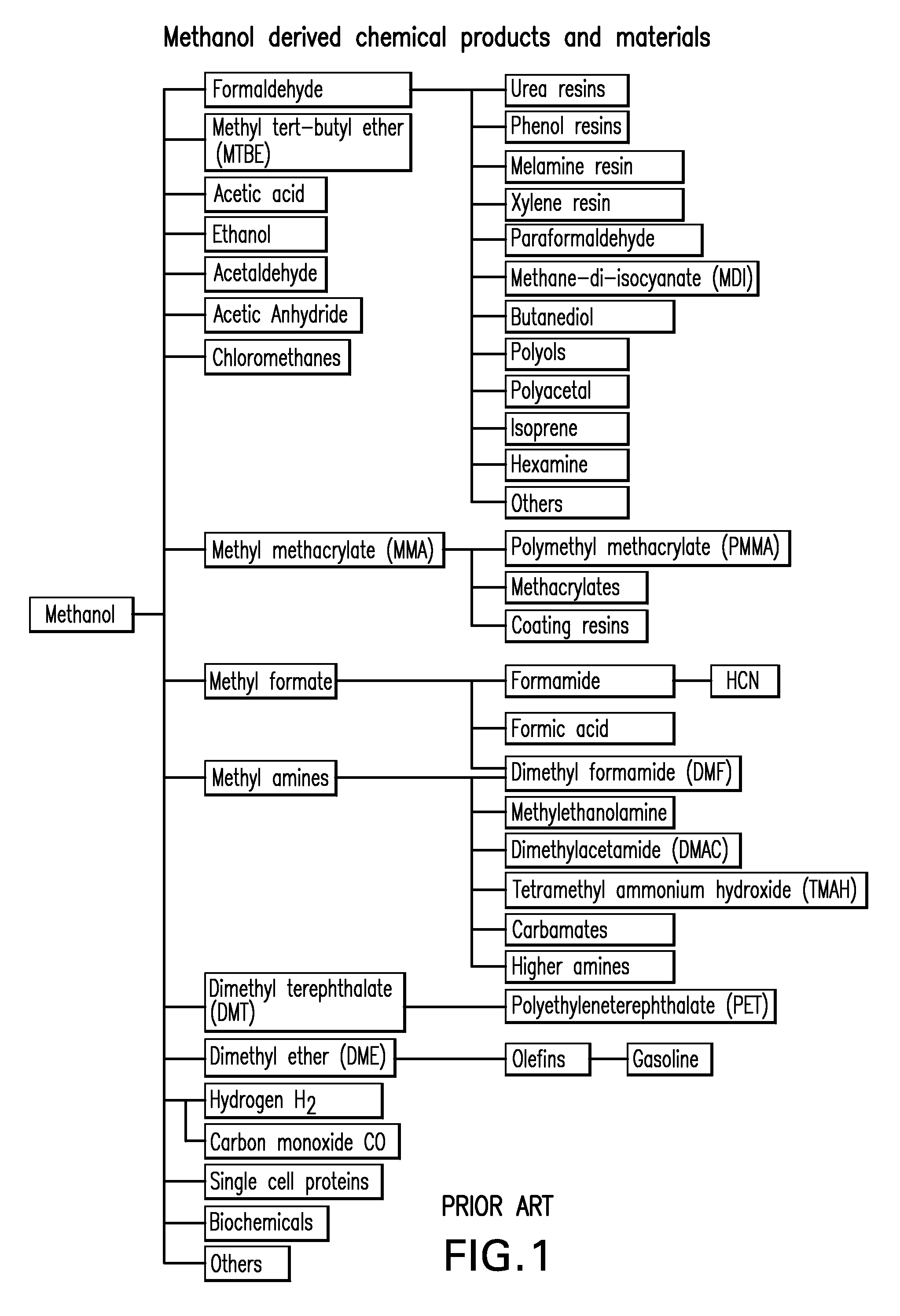
DME is a simple ether compound with the chemical formula CH3OCH3. It is colorless, non-toxic, and easily liquefied under moderate pressure. These characteristics make it an attractive option for various applications, including as a fuel for internal combustion engines and gas turbines.
The interest in DME as a fuel stems from its favorable combustion properties. It has a high cetane number, typically ranging from 55 to 60, which is significantly higher than that of conventional diesel fuel. This high cetane number results in improved ignition quality and reduced ignition delay, leading to more efficient combustion processes.
One of the key advantages of DME combustion is its clean-burning nature. When compared to traditional fossil fuels, DME produces significantly lower emissions of particulate matter, nitrogen oxides (NOx), and sulfur oxides (SOx). This characteristic makes it an environmentally friendly alternative, particularly in regions with stringent emission regulations.
The molecular structure of DME plays a crucial role in its combustion behavior. The presence of oxygen in the molecule contributes to more complete combustion, reducing the formation of soot and other harmful byproducts. This inherent oxygen content also allows for leaner fuel-air mixtures, potentially improving overall combustion efficiency.
Research into DME combustion has focused on various aspects, including flame propagation, ignition characteristics, and emission profiles. Studies have shown that DME exhibits a wider flammability range compared to conventional fuels, which can lead to more stable combustion under a broader range of operating conditions.
The combustion of DME in compression ignition engines has been of particular interest. Its high volatility and low boiling point facilitate rapid vaporization and mixing with air, leading to more homogeneous fuel-air mixtures. This property can contribute to more uniform combustion and potentially reduce local temperature peaks, which are often associated with NOx formation.
However, the use of DME as a fuel also presents certain challenges. Its lower energy density compared to conventional diesel fuel means that larger fuel tanks or more frequent refueling may be necessary. Additionally, its low viscosity and lubricity properties require modifications to fuel injection systems and the use of appropriate lubricants to ensure long-term reliability.
DME is a simple ether compound with the chemical formula CH3OCH3. It is colorless, non-toxic, and easily liquefied under moderate pressure. These characteristics make it an attractive option for various applications, including as a fuel for internal combustion engines and gas turbines.
The interest in DME as a fuel stems from its favorable combustion properties. It has a high cetane number, typically ranging from 55 to 60, which is significantly higher than that of conventional diesel fuel. This high cetane number results in improved ignition quality and reduced ignition delay, leading to more efficient combustion processes.
One of the key advantages of DME combustion is its clean-burning nature. When compared to traditional fossil fuels, DME produces significantly lower emissions of particulate matter, nitrogen oxides (NOx), and sulfur oxides (SOx). This characteristic makes it an environmentally friendly alternative, particularly in regions with stringent emission regulations.
The molecular structure of DME plays a crucial role in its combustion behavior. The presence of oxygen in the molecule contributes to more complete combustion, reducing the formation of soot and other harmful byproducts. This inherent oxygen content also allows for leaner fuel-air mixtures, potentially improving overall combustion efficiency.
Research into DME combustion has focused on various aspects, including flame propagation, ignition characteristics, and emission profiles. Studies have shown that DME exhibits a wider flammability range compared to conventional fuels, which can lead to more stable combustion under a broader range of operating conditions.
The combustion of DME in compression ignition engines has been of particular interest. Its high volatility and low boiling point facilitate rapid vaporization and mixing with air, leading to more homogeneous fuel-air mixtures. This property can contribute to more uniform combustion and potentially reduce local temperature peaks, which are often associated with NOx formation.
However, the use of DME as a fuel also presents certain challenges. Its lower energy density compared to conventional diesel fuel means that larger fuel tanks or more frequent refueling may be necessary. Additionally, its low viscosity and lubricity properties require modifications to fuel injection systems and the use of appropriate lubricants to ensure long-term reliability.
Market Demand Analysis
The market demand for dimethyl ether (DME) as an alternative fuel has been steadily growing, driven by increasing environmental concerns and the need for cleaner energy sources. DME's potential as a substitute for diesel fuel in compression ignition engines has garnered significant attention from both the automotive and energy sectors. The global DME market is expected to expand at a compound annual growth rate of 9.6% from 2021 to 2028, reflecting the rising interest in this fuel.
In terms of combustion efficiency, DME offers several advantages that contribute to its market appeal. Its high cetane number, typically ranging from 55 to 60, surpasses that of conventional diesel fuel, resulting in improved ignition quality and potentially higher engine efficiency. This characteristic makes DME particularly attractive for heavy-duty vehicle applications, where fuel efficiency is a critical factor.
The transportation sector, especially in regions with stringent emission regulations, represents a significant market for DME. Countries like China, Japan, and Sweden have been at the forefront of DME adoption, with pilot projects and commercial applications demonstrating its viability as a cleaner alternative to diesel. The potential for reduced particulate matter and NOx emissions compared to conventional diesel has positioned DME as a promising solution for meeting increasingly strict environmental standards.
Industrial applications also contribute to the growing demand for DME. Its use as a propellant in aerosol products and as a refrigerant in cooling systems has expanded the market beyond the transportation sector. The versatility of DME as a chemical feedstock further enhances its market potential, with applications in the production of olefins and other valuable chemicals.
The power generation sector presents another avenue for DME market growth. As countries seek to diversify their energy mix and reduce reliance on coal and oil, DME's potential as a cleaner-burning fuel for gas turbines and combined cycle power plants is being explored. This application could significantly impact the market demand, especially in regions with abundant natural gas resources that can be converted to DME.
Despite the promising outlook, challenges remain in the widespread adoption of DME. The lack of established infrastructure for production, distribution, and storage poses a significant barrier to market expansion. Additionally, the current cost of DME production compared to conventional fuels impacts its economic competitiveness, although this gap is expected to narrow as production scales up and technologies improve.
In conclusion, the market demand for DME is driven by its favorable combustion efficiency metrics and environmental benefits. As technology advances and regulatory pressures for cleaner fuels intensify, the DME market is poised for substantial growth across various sectors, particularly in transportation, industrial applications, and power generation.
In terms of combustion efficiency, DME offers several advantages that contribute to its market appeal. Its high cetane number, typically ranging from 55 to 60, surpasses that of conventional diesel fuel, resulting in improved ignition quality and potentially higher engine efficiency. This characteristic makes DME particularly attractive for heavy-duty vehicle applications, where fuel efficiency is a critical factor.
The transportation sector, especially in regions with stringent emission regulations, represents a significant market for DME. Countries like China, Japan, and Sweden have been at the forefront of DME adoption, with pilot projects and commercial applications demonstrating its viability as a cleaner alternative to diesel. The potential for reduced particulate matter and NOx emissions compared to conventional diesel has positioned DME as a promising solution for meeting increasingly strict environmental standards.
Industrial applications also contribute to the growing demand for DME. Its use as a propellant in aerosol products and as a refrigerant in cooling systems has expanded the market beyond the transportation sector. The versatility of DME as a chemical feedstock further enhances its market potential, with applications in the production of olefins and other valuable chemicals.
The power generation sector presents another avenue for DME market growth. As countries seek to diversify their energy mix and reduce reliance on coal and oil, DME's potential as a cleaner-burning fuel for gas turbines and combined cycle power plants is being explored. This application could significantly impact the market demand, especially in regions with abundant natural gas resources that can be converted to DME.
Despite the promising outlook, challenges remain in the widespread adoption of DME. The lack of established infrastructure for production, distribution, and storage poses a significant barrier to market expansion. Additionally, the current cost of DME production compared to conventional fuels impacts its economic competitiveness, although this gap is expected to narrow as production scales up and technologies improve.
In conclusion, the market demand for DME is driven by its favorable combustion efficiency metrics and environmental benefits. As technology advances and regulatory pressures for cleaner fuels intensify, the DME market is poised for substantial growth across various sectors, particularly in transportation, industrial applications, and power generation.
DME Combustion Challenges
Dimethyl ether (DME) combustion presents several unique challenges that impact its efficiency metrics when compared to conventional fuels. One of the primary issues is DME's low energy density, which necessitates larger fuel storage and injection systems to achieve equivalent power output. This characteristic can lead to increased vehicle weight and reduced overall efficiency in transportation applications.
The low lubricity of DME poses another significant challenge, particularly for fuel injection systems. Traditional fuel injection components designed for diesel or gasoline may experience accelerated wear when used with DME, potentially leading to reduced combustion efficiency over time. This issue necessitates the development of specialized materials and coatings for fuel system components to ensure long-term reliability and consistent performance.
DME's high cetane number, while generally advantageous for compression ignition engines, can sometimes lead to premature ignition in certain engine designs. This phenomenon, known as knock, can reduce combustion efficiency and potentially cause engine damage if not properly managed. Careful optimization of engine timing and combustion chamber design is required to fully leverage DME's rapid ignition characteristics while avoiding negative consequences.
The relatively low flame temperature of DME combustion, compared to conventional fuels, presents both advantages and challenges. While it contributes to lower NOx emissions, it can also result in reduced thermal efficiency in some engine configurations. Engineers must carefully balance these factors to achieve optimal combustion efficiency while meeting increasingly stringent emissions regulations.
DME's propensity to form peroxides during storage and handling introduces additional complexities in fuel system design and maintenance. These peroxides can lead to increased corrosion and degradation of fuel system components, potentially impacting long-term combustion efficiency if not properly addressed. Specialized storage and handling protocols, as well as the use of antioxidant additives, may be necessary to mitigate these effects.
The need for pressurization to maintain DME in a liquid state at ambient temperatures adds complexity to fuel storage and distribution systems. This requirement can impact overall system efficiency, particularly in mobile applications where weight and space are critical factors. Innovative storage solutions and optimized pressure management systems are essential to maximize the practical efficiency of DME as a fuel.
The low lubricity of DME poses another significant challenge, particularly for fuel injection systems. Traditional fuel injection components designed for diesel or gasoline may experience accelerated wear when used with DME, potentially leading to reduced combustion efficiency over time. This issue necessitates the development of specialized materials and coatings for fuel system components to ensure long-term reliability and consistent performance.
DME's high cetane number, while generally advantageous for compression ignition engines, can sometimes lead to premature ignition in certain engine designs. This phenomenon, known as knock, can reduce combustion efficiency and potentially cause engine damage if not properly managed. Careful optimization of engine timing and combustion chamber design is required to fully leverage DME's rapid ignition characteristics while avoiding negative consequences.
The relatively low flame temperature of DME combustion, compared to conventional fuels, presents both advantages and challenges. While it contributes to lower NOx emissions, it can also result in reduced thermal efficiency in some engine configurations. Engineers must carefully balance these factors to achieve optimal combustion efficiency while meeting increasingly stringent emissions regulations.
DME's propensity to form peroxides during storage and handling introduces additional complexities in fuel system design and maintenance. These peroxides can lead to increased corrosion and degradation of fuel system components, potentially impacting long-term combustion efficiency if not properly addressed. Specialized storage and handling protocols, as well as the use of antioxidant additives, may be necessary to mitigate these effects.
The need for pressurization to maintain DME in a liquid state at ambient temperatures adds complexity to fuel storage and distribution systems. This requirement can impact overall system efficiency, particularly in mobile applications where weight and space are critical factors. Innovative storage solutions and optimized pressure management systems are essential to maximize the practical efficiency of DME as a fuel.
Current DME Solutions
01 Combustion efficiency improvement methods
Various methods are employed to enhance the combustion efficiency of dimethyl ether (DME). These include optimizing the air-fuel ratio, improving fuel injection systems, and utilizing advanced combustion chamber designs. Such techniques aim to achieve more complete combustion, reduce emissions, and increase overall engine performance when using DME as a fuel.- Combustion efficiency improvement methods: Various methods are employed to enhance the combustion efficiency of dimethyl ether (DME). These include optimizing the air-fuel ratio, improving fuel injection systems, and utilizing advanced combustion chamber designs. Such techniques aim to achieve more complete combustion, reduce emissions, and increase overall engine performance when using DME as a fuel.
- DME production and purification: Efficient production and purification of dimethyl ether are crucial for its use as a clean-burning fuel. Advanced catalytic processes and separation techniques are developed to produce high-purity DME from various feedstocks, including natural gas, coal, and biomass. These methods aim to increase yield and reduce impurities, thereby improving the overall combustion efficiency of the final product.
- DME blending with other fuels: Blending dimethyl ether with other fuels is explored to optimize combustion characteristics. This approach can involve mixing DME with conventional diesel fuel, biodiesel, or other alternative fuels. The goal is to leverage the beneficial properties of DME, such as its high cetane number, while addressing challenges related to its low viscosity and energy density.
- Engine modifications for DME use: Adapting engines for efficient DME combustion involves various modifications. These can include changes to fuel injection systems, adjustments to compression ratios, and alterations to engine control units. Such modifications are designed to accommodate the unique properties of DME and maximize its combustion efficiency in internal combustion engines.
- Emissions reduction in DME combustion: Efforts to reduce emissions from DME combustion focus on optimizing the combustion process and implementing aftertreatment technologies. This includes developing low-temperature combustion strategies, utilizing exhaust gas recirculation, and employing catalytic converters specifically designed for DME exhaust. These approaches aim to minimize pollutants while maintaining high combustion efficiency.
02 DME production and purification
Efficient production and purification of dimethyl ether play a crucial role in its combustion efficiency. Advanced synthesis methods, catalytic processes, and purification techniques are developed to produce high-quality DME with minimal impurities. These improvements in DME quality contribute to better combustion characteristics and increased efficiency in various applications.Expand Specific Solutions03 DME as an alternative fuel
Dimethyl ether is explored as an alternative fuel for various applications, including internal combustion engines and power generation. Its properties, such as high cetane number and low emissions, make it an attractive option. Research focuses on adapting existing engines and developing new technologies to maximize DME's combustion efficiency and overall performance as a clean-burning fuel.Expand Specific Solutions04 Catalytic combustion of DME
Catalytic combustion techniques are investigated to enhance the efficiency of dimethyl ether combustion. Various catalysts and catalyst support materials are studied to promote complete oxidation, reduce ignition temperature, and improve overall combustion characteristics. This approach aims to achieve higher energy conversion efficiency and lower emissions in DME-based systems.Expand Specific Solutions05 DME blending and additives
The combustion efficiency of dimethyl ether can be improved through blending with other fuels or the use of additives. Research explores optimal blend ratios with conventional fuels and the development of specific additives to enhance DME's combustion properties. These strategies aim to overcome some of the challenges associated with pure DME combustion and expand its applicability in various energy systems.Expand Specific Solutions
Key Industry Players
The combustion efficiency of dimethyl ether (DME) is gaining attention in a competitive landscape characterized by evolving energy technologies. The market is in a growth phase, with increasing interest from both academic institutions and industry players. Companies like Weichai Power, SK Energy, and Ford Motor Co. are exploring DME's potential as an alternative fuel. Research institutions such as the University of Southern California and Guangdong University of Technology are contributing to technological advancements. The market size is expanding, driven by the need for cleaner fuel options. While DME technology is maturing, it is not yet fully commercialized, with ongoing research and development efforts focused on improving efficiency and reducing emissions.
Weichai Power
Technical Solution: Weichai Power has made significant strides in DME combustion technology, particularly for heavy-duty engines. They have developed a high-pressure common rail fuel injection system specifically optimized for DME, capable of operating at pressures up to 2000 bar[4]. This system allows for precise control of fuel injection timing and quantity, crucial for maximizing DME's combustion efficiency. Weichai's DME engines have demonstrated thermal efficiencies of up to 45%, comparable to advanced diesel engines[5]. They have also implemented a unique exhaust gas recirculation (EGR) strategy tailored for DME combustion, which further reduces NOx emissions while maintaining high efficiency. In collaboration with academic partners, Weichai has conducted extensive computational fluid dynamics (CFD) simulations to optimize combustion chamber geometry for DME, resulting in improved mixture formation and combustion stability[6].
Strengths: Specialized DME engine technology, high thermal efficiency, advanced fuel injection system. Weaknesses: Limited to heavy-duty applications, potential challenges in widespread infrastructure adoption.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has been at the forefront of dimethyl ether (DME) research and application. They have developed a proprietary process for large-scale DME production from coal and natural gas. Their technology involves a single-step synthesis process that directly converts syngas to DME, improving efficiency by up to 10% compared to traditional two-step processes[1]. Sinopec has also conducted extensive research on DME as a clean alternative fuel for diesel engines, demonstrating a reduction in particulate matter emissions by up to 90% and NOx emissions by 90% in heavy-duty vehicles[2]. Their DME fuel formulation includes additives that enhance lubricity and reduce wear on engine components, addressing one of the key challenges in DME adoption[3].
Strengths: Integrated production and application research, large-scale production capability, improved emission profile. Weaknesses: Dependency on coal/natural gas feedstock, potential competition from other alternative fuels.
DME Combustion Innovations
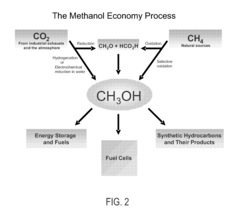
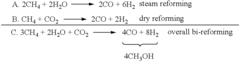
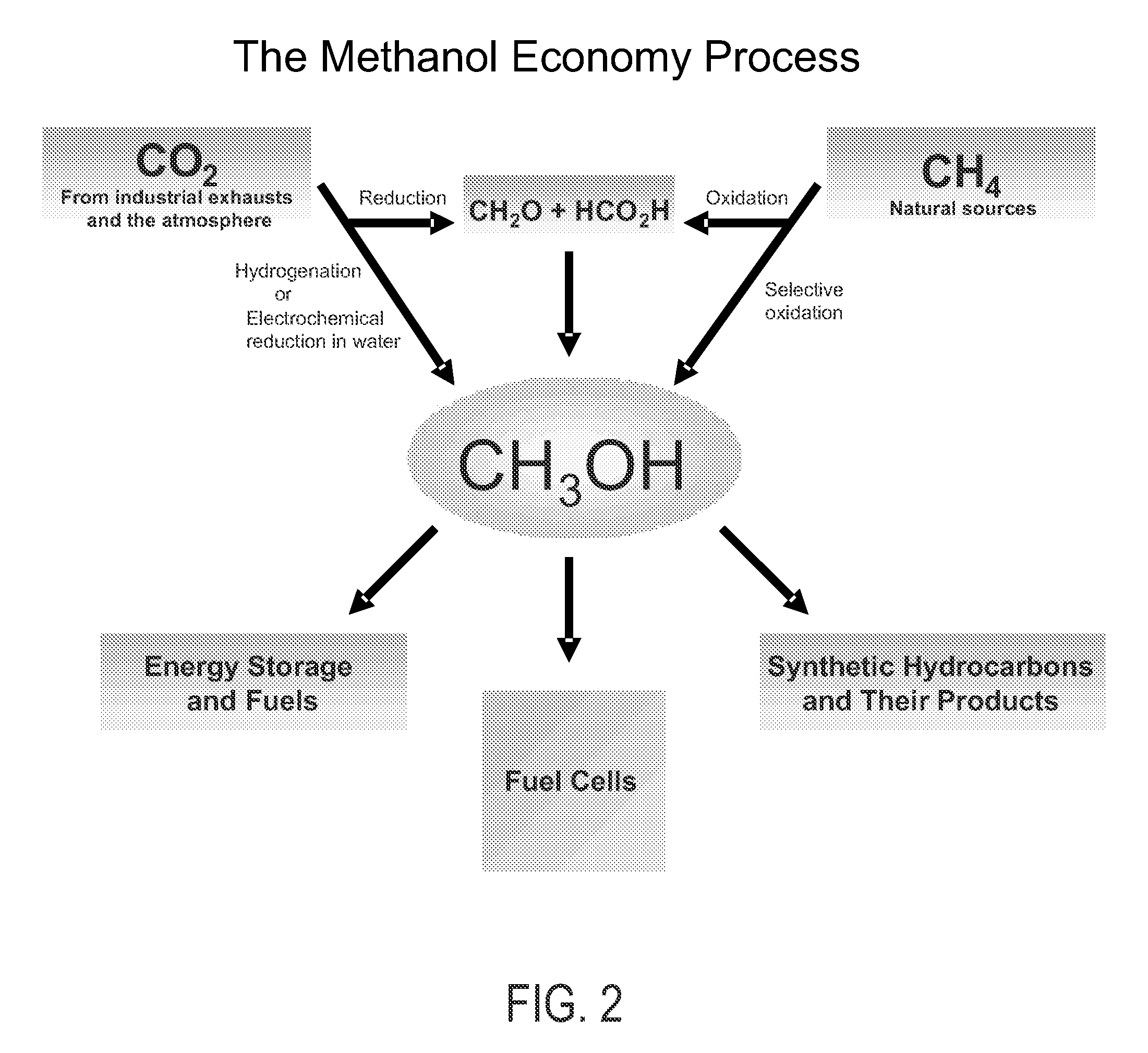
Conversion of carbon dioxide to methanol and/or dimethyl ether using bi-reforming of methane or natural gas
PatentActiveUS7906559B2
Innovation
- A bi-reforming process combining steam and dry reforming of methane to achieve a specific CO/H2 molar ratio of 1:2, allowing for the efficient conversion of carbon dioxide and methane to methanol and dimethyl ether without producing CO2 or other by-products, using a catalyst such as V2O5 and NiO on a silica carrier.
Efficient and selective chemical recycling of carbon dioxide to methanol, dimethyl ether and derived products
PatentActiveUS7608743B2
Innovation
- A process that converts carbon dioxide from industrial or natural sources, including the atmosphere, into methanol and dimethyl ether through catalytic, photochemical, or electrochemical hydrogenation, followed by recycling the carbon dioxide in a carbon-neutral cycle, allowing for the production of methanol without the need for synthesis gas and reducing greenhouse gas emissions.
Environmental Impact
The environmental impact of dimethyl ether (DME) as a combustion fuel is a critical consideration in assessing its viability as an alternative to conventional fossil fuels. DME demonstrates several advantages in terms of reduced emissions and overall environmental footprint compared to traditional diesel and gasoline.
One of the most significant environmental benefits of DME is its lower carbon dioxide (CO2) emissions during combustion. When burned, DME produces approximately 10-15% less CO2 compared to diesel fuel, contributing to a reduction in greenhouse gas emissions. This characteristic makes DME an attractive option for mitigating climate change impacts associated with transportation and industrial processes.
Furthermore, DME combustion results in negligible particulate matter (PM) emissions. The absence of carbon-carbon bonds in DME's molecular structure leads to cleaner burning, significantly reducing the formation of soot and other harmful particulates. This property is particularly beneficial in urban environments, where air quality is a major concern and particulate pollution from diesel engines poses serious health risks.
DME also exhibits lower nitrogen oxide (NOx) emissions compared to conventional diesel fuel. The reduction in NOx emissions can be attributed to DME's higher cetane number and oxygen content, which promote more complete combustion and lower peak combustion temperatures. This characteristic is crucial for addressing air quality issues and reducing the formation of smog in urban areas.
In terms of sulfur emissions, DME offers a substantial advantage. It contains virtually no sulfur, eliminating sulfur dioxide (SO2) emissions that contribute to acid rain and respiratory problems. This property makes DME particularly attractive for use in regions with stringent sulfur emission regulations.
The production of DME can also have positive environmental implications, especially when derived from renewable sources. Bio-based DME, produced from biomass or waste materials, has the potential to be carbon-neutral or even carbon-negative, further enhancing its environmental credentials. Additionally, the production process for DME is relatively simple and can be integrated with existing infrastructure, minimizing the environmental impact associated with new production facilities.
However, it is important to consider the full lifecycle environmental impact of DME, including its production and distribution. While DME offers clear advantages in terms of combustion emissions, the environmental footprint of its production process, especially when derived from fossil fuels, must be carefully evaluated to ensure a net positive environmental impact.
In conclusion, DME demonstrates significant potential for reducing the environmental impact of combustion processes, particularly in terms of greenhouse gas emissions, particulate matter, and air pollutants. Its clean-burning properties and potential for renewable production make it a promising alternative fuel from an environmental perspective, though comprehensive lifecycle assessments are necessary to fully understand its overall environmental impact.
One of the most significant environmental benefits of DME is its lower carbon dioxide (CO2) emissions during combustion. When burned, DME produces approximately 10-15% less CO2 compared to diesel fuel, contributing to a reduction in greenhouse gas emissions. This characteristic makes DME an attractive option for mitigating climate change impacts associated with transportation and industrial processes.
Furthermore, DME combustion results in negligible particulate matter (PM) emissions. The absence of carbon-carbon bonds in DME's molecular structure leads to cleaner burning, significantly reducing the formation of soot and other harmful particulates. This property is particularly beneficial in urban environments, where air quality is a major concern and particulate pollution from diesel engines poses serious health risks.
DME also exhibits lower nitrogen oxide (NOx) emissions compared to conventional diesel fuel. The reduction in NOx emissions can be attributed to DME's higher cetane number and oxygen content, which promote more complete combustion and lower peak combustion temperatures. This characteristic is crucial for addressing air quality issues and reducing the formation of smog in urban areas.
In terms of sulfur emissions, DME offers a substantial advantage. It contains virtually no sulfur, eliminating sulfur dioxide (SO2) emissions that contribute to acid rain and respiratory problems. This property makes DME particularly attractive for use in regions with stringent sulfur emission regulations.
The production of DME can also have positive environmental implications, especially when derived from renewable sources. Bio-based DME, produced from biomass or waste materials, has the potential to be carbon-neutral or even carbon-negative, further enhancing its environmental credentials. Additionally, the production process for DME is relatively simple and can be integrated with existing infrastructure, minimizing the environmental impact associated with new production facilities.
However, it is important to consider the full lifecycle environmental impact of DME, including its production and distribution. While DME offers clear advantages in terms of combustion emissions, the environmental footprint of its production process, especially when derived from fossil fuels, must be carefully evaluated to ensure a net positive environmental impact.
In conclusion, DME demonstrates significant potential for reducing the environmental impact of combustion processes, particularly in terms of greenhouse gas emissions, particulate matter, and air pollutants. Its clean-burning properties and potential for renewable production make it a promising alternative fuel from an environmental perspective, though comprehensive lifecycle assessments are necessary to fully understand its overall environmental impact.
Economic Feasibility
The economic feasibility of dimethyl ether (DME) as a combustion fuel is a critical factor in its potential adoption and widespread use. When comparing DME to conventional fuels in terms of combustion efficiency metrics, several economic aspects come into play. Firstly, the production costs of DME are relatively competitive, especially when derived from natural gas or biomass. The synthesis process is well-established and can be integrated into existing petrochemical infrastructure, reducing initial capital investments.
However, the economic viability of DME is heavily influenced by the price of feedstock materials. Fluctuations in natural gas or biomass prices can significantly impact the overall production costs, affecting its competitiveness against traditional fuels. Additionally, the current lack of widespread DME distribution infrastructure presents a substantial economic challenge. Significant investments would be required to establish a robust supply chain, including storage facilities, transportation networks, and refueling stations.
From an end-user perspective, the economic feasibility of DME depends on the cost of engine modifications or new engine designs optimized for DME combustion. While DME can be used in modified diesel engines, the expenses associated with these adaptations must be considered in the overall economic assessment. The potential for improved fuel efficiency and reduced emissions could offset these costs over time, but the initial investment may be a barrier to adoption.
The economic feasibility is also tied to regulatory frameworks and government incentives. Policies promoting cleaner fuels and stricter emission standards can create a more favorable economic environment for DME adoption. Tax incentives, subsidies, or carbon pricing mechanisms could significantly enhance the economic attractiveness of DME as an alternative fuel.
Market demand and scale of production play crucial roles in determining economic feasibility. As production volumes increase, economies of scale can lead to reduced costs per unit, making DME more competitive. However, this requires substantial market penetration and consistent demand growth, which are yet to be fully realized for DME as a fuel.
Lastly, the long-term economic feasibility of DME must consider the evolving energy landscape. As renewable energy sources become more prevalent and electric vehicle technology advances, the economic competitiveness of DME as a combustion fuel may face challenges. Its viability will depend on its ability to carve out niche applications where its combustion efficiency metrics offer distinct advantages over alternative energy solutions.
However, the economic viability of DME is heavily influenced by the price of feedstock materials. Fluctuations in natural gas or biomass prices can significantly impact the overall production costs, affecting its competitiveness against traditional fuels. Additionally, the current lack of widespread DME distribution infrastructure presents a substantial economic challenge. Significant investments would be required to establish a robust supply chain, including storage facilities, transportation networks, and refueling stations.
From an end-user perspective, the economic feasibility of DME depends on the cost of engine modifications or new engine designs optimized for DME combustion. While DME can be used in modified diesel engines, the expenses associated with these adaptations must be considered in the overall economic assessment. The potential for improved fuel efficiency and reduced emissions could offset these costs over time, but the initial investment may be a barrier to adoption.
The economic feasibility is also tied to regulatory frameworks and government incentives. Policies promoting cleaner fuels and stricter emission standards can create a more favorable economic environment for DME adoption. Tax incentives, subsidies, or carbon pricing mechanisms could significantly enhance the economic attractiveness of DME as an alternative fuel.
Market demand and scale of production play crucial roles in determining economic feasibility. As production volumes increase, economies of scale can lead to reduced costs per unit, making DME more competitive. However, this requires substantial market penetration and consistent demand growth, which are yet to be fully realized for DME as a fuel.
Lastly, the long-term economic feasibility of DME must consider the evolving energy landscape. As renewable energy sources become more prevalent and electric vehicle technology advances, the economic competitiveness of DME as a combustion fuel may face challenges. Its viability will depend on its ability to carve out niche applications where its combustion efficiency metrics offer distinct advantages over alternative energy solutions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!