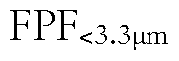How Do Spray Drying Parameters Affect Pharmaceutical Stability?
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Spray Drying Technology Evolution and Objectives
Spray drying technology has evolved significantly since its inception in the late 19th century. Initially developed for the dairy industry to produce milk powder, this technology has undergone substantial transformations to become a cornerstone process in pharmaceutical manufacturing. The 1940s marked the beginning of spray drying applications in pharmaceuticals, primarily for simple formulations. By the 1970s, advancements in equipment design and process control enabled more complex applications, including microencapsulation and controlled release formulations.
The evolution accelerated in the 1990s with the integration of computerized control systems, allowing for precise manipulation of critical process parameters such as inlet temperature, atomization pressure, and feed rate. This period saw the emergence of sophisticated multi-stage drying chambers and improved atomization technologies that significantly enhanced product quality and consistency. The early 2000s brought further refinements with the development of aseptic spray drying capabilities for biopharmaceuticals and the introduction of PAT (Process Analytical Technology) tools for real-time monitoring.
Recent technological advancements have focused on scalability challenges, with the development of modular systems that maintain consistent product attributes across laboratory, pilot, and commercial scales. Computational fluid dynamics modeling has revolutionized equipment design, enabling optimization of air flow patterns and heat transfer efficiency. Additionally, innovations in nozzle technology have expanded the range of achievable particle characteristics, critical for pharmaceutical applications requiring specific dissolution profiles or lung deposition patterns.
The primary objective of modern spray drying technology in pharmaceuticals is to produce stable, high-quality products with predictable performance characteristics. This includes enhancing bioavailability of poorly water-soluble drugs through amorphous solid dispersions, protecting sensitive active ingredients from environmental stressors, and creating engineered particles with tailored properties for specific delivery routes. Process efficiency objectives encompass reducing energy consumption, minimizing product loss, and shortening development timelines through improved predictive capabilities.
Current research aims to establish robust correlations between spray drying parameters and pharmaceutical stability outcomes. This includes understanding how inlet/outlet temperatures influence degradation kinetics, how atomization parameters affect particle morphology and subsequent stability, and how formulation composition interacts with process conditions to determine the physical state of the final product. The ultimate goal is to develop a comprehensive framework that enables rational design of spray drying processes tailored to specific pharmaceutical applications, ensuring optimal stability profiles while maximizing manufacturing efficiency.
The evolution accelerated in the 1990s with the integration of computerized control systems, allowing for precise manipulation of critical process parameters such as inlet temperature, atomization pressure, and feed rate. This period saw the emergence of sophisticated multi-stage drying chambers and improved atomization technologies that significantly enhanced product quality and consistency. The early 2000s brought further refinements with the development of aseptic spray drying capabilities for biopharmaceuticals and the introduction of PAT (Process Analytical Technology) tools for real-time monitoring.
Recent technological advancements have focused on scalability challenges, with the development of modular systems that maintain consistent product attributes across laboratory, pilot, and commercial scales. Computational fluid dynamics modeling has revolutionized equipment design, enabling optimization of air flow patterns and heat transfer efficiency. Additionally, innovations in nozzle technology have expanded the range of achievable particle characteristics, critical for pharmaceutical applications requiring specific dissolution profiles or lung deposition patterns.
The primary objective of modern spray drying technology in pharmaceuticals is to produce stable, high-quality products with predictable performance characteristics. This includes enhancing bioavailability of poorly water-soluble drugs through amorphous solid dispersions, protecting sensitive active ingredients from environmental stressors, and creating engineered particles with tailored properties for specific delivery routes. Process efficiency objectives encompass reducing energy consumption, minimizing product loss, and shortening development timelines through improved predictive capabilities.
Current research aims to establish robust correlations between spray drying parameters and pharmaceutical stability outcomes. This includes understanding how inlet/outlet temperatures influence degradation kinetics, how atomization parameters affect particle morphology and subsequent stability, and how formulation composition interacts with process conditions to determine the physical state of the final product. The ultimate goal is to develop a comprehensive framework that enables rational design of spray drying processes tailored to specific pharmaceutical applications, ensuring optimal stability profiles while maximizing manufacturing efficiency.
Pharmaceutical Market Demands for Spray Dried Products
The pharmaceutical industry has witnessed a significant surge in demand for spray dried products over the past decade, driven primarily by the growing need for enhanced drug stability, bioavailability, and targeted delivery systems. Market research indicates that the global pharmaceutical spray drying market is projected to reach $7.3 billion by 2026, growing at a compound annual growth rate of 6.8% from 2021. This growth trajectory underscores the increasing recognition of spray drying as a critical technology in modern pharmaceutical manufacturing.
The demand for spray dried pharmaceutical products is particularly pronounced in the development of solid dispersions for poorly water-soluble drugs, which constitute approximately 40% of approved drugs and nearly 90% of developmental pipeline molecules. These formulations require sophisticated processing techniques to enhance dissolution rates and bioavailability, making spray drying an invaluable technology for addressing this fundamental challenge in drug development.
Inhalable dry powder formulations represent another significant market segment driving demand for spray dried products. The global pulmonary drug delivery market, valued at $36 billion in 2021, is increasingly relying on spray drying technology to produce particles with precise aerodynamic properties necessary for effective lung deposition. This application is particularly crucial for respiratory conditions such as asthma, COPD, and more recently, for potential COVID-19 treatments.
Biopharmaceuticals, including proteins, peptides, and vaccines, constitute a rapidly expanding market segment that benefits substantially from spray drying technology. The ability to preserve biological activity while achieving stable dry formulations has positioned spray drying as a preferred alternative to traditional lyophilization in many applications, offering advantages in processing time, energy efficiency, and scalability.
Market analysis reveals that pharmaceutical companies are increasingly demanding spray dried formulations that offer extended shelf life under ambient conditions, reducing cold chain requirements and associated costs. This trend is particularly evident in emerging markets where cold chain infrastructure may be limited, creating a substantial market opportunity for thermally stable spray dried products.
Contract manufacturing organizations (CMOs) specializing in spray drying services have experienced substantial growth, with the pharmaceutical contract manufacturing market for spray dried products expanding at approximately 8.5% annually. This growth reflects both the technical complexity of spray drying operations and the significant capital investment required for in-house capabilities, driving pharmaceutical companies toward outsourcing these specialized manufacturing processes.
Consumer preferences are also shaping market demands, with increasing interest in patient-friendly dosage forms such as orally disintegrating tablets and taste-masked formulations, both of which frequently utilize spray drying technology in their production. This consumer-driven trend aligns with broader industry movements toward patient-centric drug development and improved medication adherence.
The demand for spray dried pharmaceutical products is particularly pronounced in the development of solid dispersions for poorly water-soluble drugs, which constitute approximately 40% of approved drugs and nearly 90% of developmental pipeline molecules. These formulations require sophisticated processing techniques to enhance dissolution rates and bioavailability, making spray drying an invaluable technology for addressing this fundamental challenge in drug development.
Inhalable dry powder formulations represent another significant market segment driving demand for spray dried products. The global pulmonary drug delivery market, valued at $36 billion in 2021, is increasingly relying on spray drying technology to produce particles with precise aerodynamic properties necessary for effective lung deposition. This application is particularly crucial for respiratory conditions such as asthma, COPD, and more recently, for potential COVID-19 treatments.
Biopharmaceuticals, including proteins, peptides, and vaccines, constitute a rapidly expanding market segment that benefits substantially from spray drying technology. The ability to preserve biological activity while achieving stable dry formulations has positioned spray drying as a preferred alternative to traditional lyophilization in many applications, offering advantages in processing time, energy efficiency, and scalability.
Market analysis reveals that pharmaceutical companies are increasingly demanding spray dried formulations that offer extended shelf life under ambient conditions, reducing cold chain requirements and associated costs. This trend is particularly evident in emerging markets where cold chain infrastructure may be limited, creating a substantial market opportunity for thermally stable spray dried products.
Contract manufacturing organizations (CMOs) specializing in spray drying services have experienced substantial growth, with the pharmaceutical contract manufacturing market for spray dried products expanding at approximately 8.5% annually. This growth reflects both the technical complexity of spray drying operations and the significant capital investment required for in-house capabilities, driving pharmaceutical companies toward outsourcing these specialized manufacturing processes.
Consumer preferences are also shaping market demands, with increasing interest in patient-friendly dosage forms such as orally disintegrating tablets and taste-masked formulations, both of which frequently utilize spray drying technology in their production. This consumer-driven trend aligns with broader industry movements toward patient-centric drug development and improved medication adherence.
Current Challenges in Spray Drying Pharmaceutical Stability
Despite significant advancements in spray drying technology for pharmaceutical applications, several critical challenges persist that impact product stability. The primary challenge remains the precise control of process parameters and their complex interrelationships. Temperature management presents a particular difficulty, as many pharmaceuticals contain heat-sensitive compounds that can degrade when exposed to elevated temperatures during the drying process. The delicate balance between inlet temperature, outlet temperature, and residence time creates a narrow processing window that must be carefully navigated.
Moisture content control represents another significant hurdle. Residual moisture in spray-dried pharmaceuticals can accelerate chemical degradation pathways and compromise physical stability. Conversely, excessive drying may lead to structural changes that affect dissolution profiles and bioavailability. This dichotomy creates a challenging optimization problem that varies substantially between different active pharmaceutical ingredients (APIs).
Scale-up issues further complicate pharmaceutical spray drying. Parameters optimized at laboratory scale often perform differently in production environments, leading to inconsistent product characteristics and stability profiles. The fluid dynamics within larger spray dryers differ substantially, affecting particle formation mechanisms and resulting in potential stability variations that are difficult to predict from small-scale studies.
Feed solution characteristics present additional complexity. Viscosity, surface tension, and solubility all influence droplet formation and subsequent drying behavior. Many pharmaceutical formulations contain multiple components with different physicochemical properties, creating heterogeneous drying conditions within individual droplets that can lead to phase separation, crystallization issues, or amorphous state instability.
Equipment limitations also contribute to stability challenges. Current spray dryer designs struggle with uniform heat distribution and consistent atomization across large production volumes. Nozzle clogging remains problematic for certain formulations, leading to process interruptions that can introduce variability in the final product stability profile.
Analytical challenges compound these technical issues. Real-time monitoring of critical quality attributes during spray drying remains limited, making it difficult to implement effective process analytical technology (PAT) approaches. This gap hinders the development of robust control strategies that could mitigate stability risks through adaptive processing.
Regulatory considerations add another layer of complexity. Demonstrating consistent stability profiles across batches requires extensive validation data, particularly when spray drying parameters must be adjusted to accommodate different API characteristics or batch sizes. The pharmaceutical industry faces increasing pressure to establish clear correlations between process parameters and stability outcomes to satisfy quality-by-design regulatory expectations.
Moisture content control represents another significant hurdle. Residual moisture in spray-dried pharmaceuticals can accelerate chemical degradation pathways and compromise physical stability. Conversely, excessive drying may lead to structural changes that affect dissolution profiles and bioavailability. This dichotomy creates a challenging optimization problem that varies substantially between different active pharmaceutical ingredients (APIs).
Scale-up issues further complicate pharmaceutical spray drying. Parameters optimized at laboratory scale often perform differently in production environments, leading to inconsistent product characteristics and stability profiles. The fluid dynamics within larger spray dryers differ substantially, affecting particle formation mechanisms and resulting in potential stability variations that are difficult to predict from small-scale studies.
Feed solution characteristics present additional complexity. Viscosity, surface tension, and solubility all influence droplet formation and subsequent drying behavior. Many pharmaceutical formulations contain multiple components with different physicochemical properties, creating heterogeneous drying conditions within individual droplets that can lead to phase separation, crystallization issues, or amorphous state instability.
Equipment limitations also contribute to stability challenges. Current spray dryer designs struggle with uniform heat distribution and consistent atomization across large production volumes. Nozzle clogging remains problematic for certain formulations, leading to process interruptions that can introduce variability in the final product stability profile.
Analytical challenges compound these technical issues. Real-time monitoring of critical quality attributes during spray drying remains limited, making it difficult to implement effective process analytical technology (PAT) approaches. This gap hinders the development of robust control strategies that could mitigate stability risks through adaptive processing.
Regulatory considerations add another layer of complexity. Demonstrating consistent stability profiles across batches requires extensive validation data, particularly when spray drying parameters must be adjusted to accommodate different API characteristics or batch sizes. The pharmaceutical industry faces increasing pressure to establish clear correlations between process parameters and stability outcomes to satisfy quality-by-design regulatory expectations.
Current Parameter Optimization Approaches
01 Temperature and humidity control in spray drying
Controlling temperature and humidity parameters during spray drying is critical for pharmaceutical stability. Optimal inlet and outlet temperatures must be maintained to prevent degradation of heat-sensitive compounds while ensuring proper drying. Relative humidity levels affect moisture content in the final product, which directly impacts stability. Advanced monitoring systems can help maintain these parameters within specified ranges to enhance product shelf life and maintain therapeutic efficacy.- Temperature and humidity control in spray drying: Temperature and humidity parameters are critical in spray drying pharmaceuticals to ensure stability. Controlling inlet and outlet temperatures affects moisture content and prevents degradation of heat-sensitive compounds. Maintaining optimal relative humidity during the process helps achieve consistent particle size distribution and prevents moisture-induced instability. These parameters must be carefully calibrated based on the specific pharmaceutical formulation to maximize stability and shelf life.
- Excipient selection for enhanced stability: The choice of excipients significantly impacts the stability of spray-dried pharmaceuticals. Stabilizing agents such as sugars (trehalose, mannitol) and polymers can protect active ingredients during the drying process and subsequent storage. These excipients form protective matrices around the active pharmaceutical ingredients, preventing degradation from oxidation, hydrolysis, and other destabilizing mechanisms. Proper excipient selection and ratio optimization are essential for maintaining pharmaceutical integrity throughout the product lifecycle.
- Atomization techniques and nozzle design: Atomization parameters and nozzle design significantly influence the stability of spray-dried pharmaceuticals. The atomization method (rotary, pressure, or ultrasonic) affects droplet size, which in turn determines drying efficiency and particle characteristics. Nozzle design features such as orifice diameter, pressure, and spray angle impact particle morphology and size distribution. Optimizing these parameters helps achieve consistent particle properties, uniform drying, and ultimately enhanced pharmaceutical stability.
- Drying chamber configuration and airflow patterns: The design of the drying chamber and airflow patterns significantly impact pharmaceutical stability during spray drying. Chamber geometry, residence time, and airflow direction affect heat and mass transfer efficiency. Co-current, counter-current, or mixed flow configurations provide different drying environments that must be matched to product sensitivity. Optimizing these parameters helps prevent thermal degradation while ensuring complete drying, resulting in stable pharmaceutical products with consistent quality attributes.
- Post-drying handling and storage conditions: Post-drying handling and storage conditions are crucial for maintaining the stability of spray-dried pharmaceuticals. Parameters such as cooling rate, collection method, and packaging environment significantly impact product quality. Controlling residual moisture through appropriate secondary drying steps and implementing protective packaging prevents degradation mechanisms. Storage temperature, humidity control, and protection from light and oxygen exposure are essential considerations for ensuring long-term pharmaceutical stability after the spray drying process.
02 Excipient selection for enhanced stability
The choice of excipients significantly influences the stability of spray-dried pharmaceuticals. Stabilizing agents such as sugars (trehalose, mannitol), polymers (HPMC, PVP), and surfactants can protect active ingredients during the drying process and subsequent storage. These excipients form protective matrices around the active pharmaceutical ingredients, preventing degradation from oxidation, hydrolysis, and other destabilizing mechanisms. Proper excipient selection can extend product shelf life and maintain therapeutic efficacy.Expand Specific Solutions03 Atomization techniques and nozzle design
Atomization parameters and nozzle design significantly impact pharmaceutical stability in spray-dried products. The droplet size distribution, determined by nozzle type (rotary, pressure, ultrasonic) and operating conditions, affects drying kinetics and particle morphology. Optimized atomization prevents thermal degradation by creating uniform droplets that dry consistently. Advanced nozzle designs can produce particles with specific characteristics that enhance stability through controlled surface area, porosity, and density.Expand Specific Solutions04 Process optimization for amorphous stability
Spray drying parameters can be optimized to produce stable amorphous forms of pharmaceuticals. Rapid drying kinetics prevent crystallization, resulting in amorphous solid dispersions with enhanced solubility and bioavailability. Critical parameters include solution concentration, feed rate, and drying gas flow rate. These factors must be carefully balanced to prevent phase separation and maintain the amorphous state during storage. Stabilization techniques may include co-processing with polymers that increase the glass transition temperature of the final product.Expand Specific Solutions05 Post-drying handling and packaging considerations
Post-drying handling and packaging significantly impact the long-term stability of spray-dried pharmaceuticals. Controlled cooling rates prevent moisture absorption and crystallization. Immediate packaging under inert gas atmospheres can protect hygroscopic or oxidation-sensitive products. Specialized packaging materials with appropriate moisture and oxygen barriers extend shelf life. Storage conditions, including temperature and humidity control, must be specified based on stability studies to maintain product integrity throughout its intended shelf life.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The pharmaceutical spray drying market is currently in a growth phase, with increasing adoption across the industry due to its advantages in enhancing drug stability and bioavailability. The global market size is expanding rapidly, driven by the rising demand for advanced drug delivery systems and biopharmaceuticals. Technologically, spray drying has reached moderate maturity, with leading companies like Novartis, AbbVie, and Boehringer Ingelheim investing significantly in optimizing parameters for pharmaceutical stability. Vertex Pharmaceuticals and Hovione Scientia have developed proprietary technologies to address stability challenges, while academic institutions like Ghent University collaborate with industry partners to advance fundamental understanding. Companies such as MedImmune and Novo Nordisk are focusing on protein stability during spray drying, representing the frontier of technological innovation in this field.
Boehringer Ingelheim Pharma GmbH & Co., KG
Technical Solution: Boehringer Ingelheim has developed a comprehensive Quality by Design (QbD) approach to spray drying that focuses on understanding critical process parameters (CPPs) affecting pharmaceutical stability. Their technology utilizes a multi-factorial design of experiments (DoE) methodology to systematically evaluate how inlet/outlet temperatures, feed concentration, atomization pressure, and drying gas flow rate interact to affect product stability[1]. Their proprietary process analytical technology (PAT) includes in-line near-infrared spectroscopy and particle size analysis to monitor moisture content and particle characteristics in real-time during spray drying operations[3]. This allows for adaptive process control that maintains optimal conditions for thermolabile compounds. Boehringer's approach particularly excels in stabilizing amorphous solid dispersions of poorly water-soluble drugs by carefully controlling the glass transition temperature (Tg) through precise manipulation of drying kinetics and excipient selection[5].
Strengths: Superior process control through advanced PAT implementation enables consistent product quality even with sensitive compounds. Their systematic DoE approach provides robust understanding of multi-parameter interactions affecting stability. Weaknesses: Their highly specialized equipment configurations may limit technology transfer to contract manufacturing organizations, and the complex process monitoring systems require significant technical expertise to operate effectively.
Hovione Scientia Ltd.
Technical Solution: Hovione has pioneered an advanced spray drying platform specifically designed to enhance pharmaceutical stability through precise control of particle engineering. Their technology employs a proprietary atomization system that creates uniform droplets with controlled size distribution, significantly reducing thermal degradation risks for heat-sensitive compounds[2]. Hovione's approach incorporates computational fluid dynamics (CFD) modeling to predict temperature and humidity profiles throughout the drying chamber, enabling optimization of process parameters before physical trials begin[4]. Their spray drying systems feature adaptive feed rate control that responds to real-time measurements of chamber conditions, maintaining the optimal evaporation rate to prevent thermal stress while ensuring complete solvent removal. For protein and peptide formulations, Hovione has developed specialized low-temperature spray drying protocols that operate at reduced pressures, allowing efficient drying at temperatures 15-20°C lower than conventional processes[6]. This has demonstrated preservation of up to 95% of biological activity in sensitive biomolecules compared to standard spray drying approaches.
Strengths: Exceptional particle engineering capabilities allow precise control over physical properties critical to stability. Their CFD modeling significantly reduces development time and material consumption during optimization. Weaknesses: The specialized equipment and modeling expertise required represents a significant capital investment. Their low-temperature approaches, while effective for stability, typically operate at lower throughput rates than conventional spray drying.
Critical Patents in Spray Drying Stability Enhancement
Deamorphization of spray-dried formulations via spray-blending
PatentWO2014141069A1
Innovation
- A method involving spray-drying a feedstock with a high drug content followed by blending with vehicle particles to minimize API dissolution and maintain crystallinity, using a process called spray-blending, which simultaneously prepares and blends particles to achieve uniform, crystalline API formulations with reduced amorphous content.
Combination pharmaceutical compositions and methods thereof
PatentPendingUS20220096503A1
Innovation
- A combination pharmaceutical composition is developed by dissolving hydrophobic and hydrophilic therapeutic agents with lipid excipients in an alcoholic solvent, followed by controlled solvent removal through spray drying, creating a stable powder with a unified multi-drug motif structure that maintains long-range order and extended plasma drug concentrations.
Regulatory Compliance for Spray Dried Pharmaceuticals
Regulatory compliance represents a critical aspect of spray dried pharmaceutical development and manufacturing. The FDA, EMA, and other global regulatory bodies have established stringent guidelines that manufacturers must adhere to when utilizing spray drying technology for pharmaceutical products. These regulations primarily focus on ensuring product quality, safety, and consistency throughout the manufacturing process.
Quality by Design (QbD) principles have become increasingly important in regulatory frameworks, requiring manufacturers to demonstrate thorough understanding of how spray drying parameters affect critical quality attributes. This includes establishing design spaces where parameters can be adjusted while maintaining product stability and efficacy. Regulatory agencies expect comprehensive documentation of parameter-stability relationships and their impact on final product characteristics.
Process validation requirements specifically address spray drying operations, mandating that manufacturers validate that their spray drying processes consistently produce pharmaceuticals meeting predetermined quality criteria. This typically involves three stages: process design, process qualification, and continued process verification, with stability data playing a crucial role in each stage.
Good Manufacturing Practice (GMP) guidelines contain specific provisions for spray drying operations, including requirements for equipment qualification, cleaning validation, and environmental monitoring. Manufacturers must demonstrate that their spray drying facilities and processes comply with these GMP requirements, particularly regarding contamination control and process reproducibility.
Stability testing protocols for spray dried pharmaceuticals must follow ICH guidelines (particularly ICH Q1A-Q1E), which outline conditions for accelerated and long-term stability studies. Regulatory submissions require comprehensive stability data demonstrating how the selected spray drying parameters contribute to product shelf-life and stability profile under various storage conditions.
Change management procedures are especially relevant for spray dried pharmaceuticals, as modifications to spray drying parameters may be classified as minor, moderate, or major changes depending on their potential impact on product stability. Each classification triggers different regulatory notification and approval requirements, with stability data often needed to support proposed changes.
Emerging regulatory trends indicate increasing scrutiny of continuous manufacturing processes, including continuous spray drying operations. Regulatory agencies are developing new frameworks for real-time release testing and process analytical technology (PAT) implementation in spray drying, with emphasis on how these approaches can enhance stability monitoring and quality assurance.
International harmonization efforts are underway to standardize regulatory requirements for spray dried pharmaceuticals across different markets, though significant regional variations persist in stability testing requirements, validation expectations, and post-approval change management procedures.
Quality by Design (QbD) principles have become increasingly important in regulatory frameworks, requiring manufacturers to demonstrate thorough understanding of how spray drying parameters affect critical quality attributes. This includes establishing design spaces where parameters can be adjusted while maintaining product stability and efficacy. Regulatory agencies expect comprehensive documentation of parameter-stability relationships and their impact on final product characteristics.
Process validation requirements specifically address spray drying operations, mandating that manufacturers validate that their spray drying processes consistently produce pharmaceuticals meeting predetermined quality criteria. This typically involves three stages: process design, process qualification, and continued process verification, with stability data playing a crucial role in each stage.
Good Manufacturing Practice (GMP) guidelines contain specific provisions for spray drying operations, including requirements for equipment qualification, cleaning validation, and environmental monitoring. Manufacturers must demonstrate that their spray drying facilities and processes comply with these GMP requirements, particularly regarding contamination control and process reproducibility.
Stability testing protocols for spray dried pharmaceuticals must follow ICH guidelines (particularly ICH Q1A-Q1E), which outline conditions for accelerated and long-term stability studies. Regulatory submissions require comprehensive stability data demonstrating how the selected spray drying parameters contribute to product shelf-life and stability profile under various storage conditions.
Change management procedures are especially relevant for spray dried pharmaceuticals, as modifications to spray drying parameters may be classified as minor, moderate, or major changes depending on their potential impact on product stability. Each classification triggers different regulatory notification and approval requirements, with stability data often needed to support proposed changes.
Emerging regulatory trends indicate increasing scrutiny of continuous manufacturing processes, including continuous spray drying operations. Regulatory agencies are developing new frameworks for real-time release testing and process analytical technology (PAT) implementation in spray drying, with emphasis on how these approaches can enhance stability monitoring and quality assurance.
International harmonization efforts are underway to standardize regulatory requirements for spray dried pharmaceuticals across different markets, though significant regional variations persist in stability testing requirements, validation expectations, and post-approval change management procedures.
Scale-up Considerations and Process Validation
Scaling up spray drying processes from laboratory to commercial production presents significant challenges that can impact pharmaceutical stability. The transition requires meticulous attention to maintaining critical process parameters across different equipment scales. When scaling up, the ratio between the atomization surface area and drying chamber volume changes substantially, potentially altering particle formation dynamics and moisture content distribution. These variations can directly influence the physical stability of amorphous solid dispersions and crystalline materials, necessitating comprehensive process validation protocols.
Process validation for spray drying operations typically follows a three-stage approach: process design, process qualification, and continued process verification. During process design, Quality by Design (QbD) principles are employed to establish a design space where critical quality attributes remain consistent despite scale variations. This involves multivariate analysis of how atomization pressure, feed concentration, and thermal parameters interact differently at larger scales. Risk assessment tools such as Failure Mode and Effects Analysis (FMEA) help identify potential failure points during scale-up that could compromise pharmaceutical stability.
Equipment-specific considerations are paramount during scale-up. Commercial spray dryers feature different atomization mechanisms, more complex air flow patterns, and varied residence time distributions compared to laboratory models. These differences can significantly impact solvent evaporation rates and thermal exposure of active pharmaceutical ingredients (APIs), potentially triggering degradation pathways not observed at smaller scales. Process analytical technology (PAT) implementation becomes essential for real-time monitoring of critical parameters such as particle size distribution, moisture content, and temperature profiles.
Regulatory expectations for spray drying scale-up have evolved to emphasize process understanding rather than fixed parameters. Manufacturers must demonstrate how the relationship between critical process parameters and critical quality attributes is maintained across scales. This requires establishing acceptable ranges for parameters like atomization gas flow rate, liquid feed rate, and inlet/outlet temperatures that may need adjustment between scales while still ensuring equivalent product stability profiles. Statistical approaches such as Design of Experiments (DoE) are increasingly utilized to develop robust scale-up models that predict stability outcomes.
Material handling considerations also change dramatically with scale. Larger batches involve extended processing times, potentially exposing thermolabile compounds to degradative conditions for longer periods. Additionally, the increased mechanical stress in industrial spray dryers can induce structural changes in sensitive molecules. Validation protocols must therefore include accelerated and long-term stability studies specifically designed to detect scale-dependent degradation pathways, with particular attention to oxidative, thermal, and moisture-induced instability mechanisms that may be amplified during commercial production.
Process validation for spray drying operations typically follows a three-stage approach: process design, process qualification, and continued process verification. During process design, Quality by Design (QbD) principles are employed to establish a design space where critical quality attributes remain consistent despite scale variations. This involves multivariate analysis of how atomization pressure, feed concentration, and thermal parameters interact differently at larger scales. Risk assessment tools such as Failure Mode and Effects Analysis (FMEA) help identify potential failure points during scale-up that could compromise pharmaceutical stability.
Equipment-specific considerations are paramount during scale-up. Commercial spray dryers feature different atomization mechanisms, more complex air flow patterns, and varied residence time distributions compared to laboratory models. These differences can significantly impact solvent evaporation rates and thermal exposure of active pharmaceutical ingredients (APIs), potentially triggering degradation pathways not observed at smaller scales. Process analytical technology (PAT) implementation becomes essential for real-time monitoring of critical parameters such as particle size distribution, moisture content, and temperature profiles.
Regulatory expectations for spray drying scale-up have evolved to emphasize process understanding rather than fixed parameters. Manufacturers must demonstrate how the relationship between critical process parameters and critical quality attributes is maintained across scales. This requires establishing acceptable ranges for parameters like atomization gas flow rate, liquid feed rate, and inlet/outlet temperatures that may need adjustment between scales while still ensuring equivalent product stability profiles. Statistical approaches such as Design of Experiments (DoE) are increasingly utilized to develop robust scale-up models that predict stability outcomes.
Material handling considerations also change dramatically with scale. Larger batches involve extended processing times, potentially exposing thermolabile compounds to degradative conditions for longer periods. Additionally, the increased mechanical stress in industrial spray dryers can induce structural changes in sensitive molecules. Validation protocols must therefore include accelerated and long-term stability studies specifically designed to detect scale-dependent degradation pathways, with particular attention to oxidative, thermal, and moisture-induced instability mechanisms that may be amplified during commercial production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!