Spray Drying of Active Pharmaceutical Ingredients: A Review
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
API Spray Drying Background and Objectives
Spray drying technology has evolved significantly over the past century, transforming from a simple dehydration method to a sophisticated particle engineering technique. Initially developed in the dairy industry in the 1870s, spray drying found its way into pharmaceutical applications in the mid-20th century. The technology's evolution has been characterized by continuous improvements in atomization techniques, drying chamber designs, and process control systems, enabling more precise manipulation of particle properties.
In recent decades, the pharmaceutical industry has witnessed a paradigm shift toward more complex active pharmaceutical ingredients (APIs) with challenging physicochemical properties, particularly poor water solubility. This trend has elevated spray drying from a mere drying technique to a strategic technology for enhancing drug bioavailability and stability. The ability to produce amorphous solid dispersions (ASDs) through spray drying has become particularly valuable for formulating poorly soluble drugs, which constitute approximately 70% of new chemical entities in development pipelines.
The global pharmaceutical spray drying market has experienced robust growth, with a compound annual growth rate exceeding 7% since 2015. This growth trajectory is expected to continue as pharmaceutical companies increasingly adopt spray drying to address formulation challenges and meet regulatory requirements for product quality and consistency. The technology's versatility in producing various dosage forms, including inhalable powders, has further expanded its application scope.
Current technical objectives in API spray drying focus on several key areas. Process optimization aims to enhance efficiency while maintaining product quality, addressing challenges such as thermal degradation of heat-sensitive compounds and achieving target particle characteristics. Scale-up methodologies seek to establish reliable correlations between laboratory-scale development and commercial production, ensuring consistent product attributes across different scales of operation.
Advanced process analytical technologies (PAT) are being integrated to enable real-time monitoring and control of critical process parameters, supporting quality-by-design approaches. Computational fluid dynamics (CFD) modeling and simulation tools are increasingly employed to predict spray drying behavior and optimize process parameters without extensive experimental trials.
The industry is also pursuing sustainable practices in spray drying operations, focusing on energy efficiency improvements, solvent recovery systems, and reduced environmental impact. Regulatory considerations remain paramount, with efforts directed toward establishing standardized approaches for validation, stability assessment, and quality control of spray-dried pharmaceutical products.
As pharmaceutical development continues to embrace complex molecular entities and personalized medicine approaches, spray drying technology is expected to play an increasingly critical role in enabling innovative drug delivery systems and improving therapeutic outcomes.
In recent decades, the pharmaceutical industry has witnessed a paradigm shift toward more complex active pharmaceutical ingredients (APIs) with challenging physicochemical properties, particularly poor water solubility. This trend has elevated spray drying from a mere drying technique to a strategic technology for enhancing drug bioavailability and stability. The ability to produce amorphous solid dispersions (ASDs) through spray drying has become particularly valuable for formulating poorly soluble drugs, which constitute approximately 70% of new chemical entities in development pipelines.
The global pharmaceutical spray drying market has experienced robust growth, with a compound annual growth rate exceeding 7% since 2015. This growth trajectory is expected to continue as pharmaceutical companies increasingly adopt spray drying to address formulation challenges and meet regulatory requirements for product quality and consistency. The technology's versatility in producing various dosage forms, including inhalable powders, has further expanded its application scope.
Current technical objectives in API spray drying focus on several key areas. Process optimization aims to enhance efficiency while maintaining product quality, addressing challenges such as thermal degradation of heat-sensitive compounds and achieving target particle characteristics. Scale-up methodologies seek to establish reliable correlations between laboratory-scale development and commercial production, ensuring consistent product attributes across different scales of operation.
Advanced process analytical technologies (PAT) are being integrated to enable real-time monitoring and control of critical process parameters, supporting quality-by-design approaches. Computational fluid dynamics (CFD) modeling and simulation tools are increasingly employed to predict spray drying behavior and optimize process parameters without extensive experimental trials.
The industry is also pursuing sustainable practices in spray drying operations, focusing on energy efficiency improvements, solvent recovery systems, and reduced environmental impact. Regulatory considerations remain paramount, with efforts directed toward establishing standardized approaches for validation, stability assessment, and quality control of spray-dried pharmaceutical products.
As pharmaceutical development continues to embrace complex molecular entities and personalized medicine approaches, spray drying technology is expected to play an increasingly critical role in enabling innovative drug delivery systems and improving therapeutic outcomes.
Pharmaceutical Market Demand Analysis
The global pharmaceutical market has witnessed a significant shift towards more efficient drug delivery systems and manufacturing processes, with spray drying technology emerging as a critical enabler for modern pharmaceutical development. The market for spray-dried pharmaceutical ingredients is experiencing robust growth, driven by the increasing prevalence of chronic diseases, growing demand for biologics, and the push for improved bioavailability of poorly water-soluble drugs.
Current market analysis indicates that the global spray drying pharmaceutical market was valued at approximately $2.5 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 7.8% through 2028. This growth trajectory is supported by the rising demand for advanced drug formulations that can address complex therapeutic challenges while maintaining stability and efficacy.
The oncology segment represents the largest application area for spray-dried pharmaceuticals, accounting for nearly 30% of the market share. This is primarily due to the complex nature of cancer therapeutics and the need for precise drug delivery systems. Following closely is the respiratory disease segment, where spray-dried powders for inhalation have revolutionized treatment approaches for conditions like asthma and COPD.
Geographically, North America dominates the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the fastest growth is being observed in emerging markets across Asia-Pacific and Latin America, where expanding healthcare infrastructure and increasing healthcare expenditure are creating new opportunities for pharmaceutical manufacturing technologies.
The demand for spray-dried pharmaceuticals is further bolstered by the growing trend towards personalized medicine and targeted therapies. These advanced treatment approaches often require sophisticated drug delivery systems that can be effectively developed using spray drying technology. Additionally, the push for continuous manufacturing processes in the pharmaceutical industry aligns perfectly with the capabilities of spray drying technology.
Market research indicates that pharmaceutical companies are increasingly outsourcing spray drying operations to specialized contract development and manufacturing organizations (CDMOs), creating a parallel growth market for service providers with advanced spray drying capabilities. This trend is expected to continue as pharmaceutical companies focus on core research activities while leveraging external expertise for specialized manufacturing processes.
Consumer preferences are also shifting towards more convenient dosage forms, driving demand for orally disintegrating tablets and other patient-friendly formulations that can be efficiently produced using spray drying technology. This consumer-driven trend is expected to further accelerate market growth in the coming years.
Current market analysis indicates that the global spray drying pharmaceutical market was valued at approximately $2.5 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 7.8% through 2028. This growth trajectory is supported by the rising demand for advanced drug formulations that can address complex therapeutic challenges while maintaining stability and efficacy.
The oncology segment represents the largest application area for spray-dried pharmaceuticals, accounting for nearly 30% of the market share. This is primarily due to the complex nature of cancer therapeutics and the need for precise drug delivery systems. Following closely is the respiratory disease segment, where spray-dried powders for inhalation have revolutionized treatment approaches for conditions like asthma and COPD.
Geographically, North America dominates the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the fastest growth is being observed in emerging markets across Asia-Pacific and Latin America, where expanding healthcare infrastructure and increasing healthcare expenditure are creating new opportunities for pharmaceutical manufacturing technologies.
The demand for spray-dried pharmaceuticals is further bolstered by the growing trend towards personalized medicine and targeted therapies. These advanced treatment approaches often require sophisticated drug delivery systems that can be effectively developed using spray drying technology. Additionally, the push for continuous manufacturing processes in the pharmaceutical industry aligns perfectly with the capabilities of spray drying technology.
Market research indicates that pharmaceutical companies are increasingly outsourcing spray drying operations to specialized contract development and manufacturing organizations (CDMOs), creating a parallel growth market for service providers with advanced spray drying capabilities. This trend is expected to continue as pharmaceutical companies focus on core research activities while leveraging external expertise for specialized manufacturing processes.
Consumer preferences are also shifting towards more convenient dosage forms, driving demand for orally disintegrating tablets and other patient-friendly formulations that can be efficiently produced using spray drying technology. This consumer-driven trend is expected to further accelerate market growth in the coming years.
Current Spray Drying Technology Challenges
Despite significant advancements in spray drying technology for pharmaceutical applications, several critical challenges persist that limit its broader implementation and optimization. Process scalability remains a fundamental issue, as laboratory-scale parameters often fail to translate effectively to industrial production. The complex interplay between process variables creates significant hurdles in maintaining consistent product quality across different production scales, resulting in batch-to-batch variability that can compromise therapeutic efficacy.
Thermal sensitivity presents another major challenge, particularly for biologics and heat-labile Active Pharmaceutical Ingredients (APIs). The exposure to elevated temperatures during the drying process can lead to protein denaturation, loss of biological activity, and chemical degradation. While protective excipients offer some mitigation, finding the optimal balance between thermal protection and product functionality remains difficult.
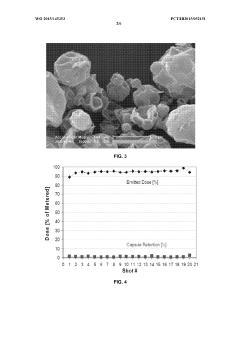
Particle engineering precision continues to be problematic despite technological advances. Achieving precise control over particle size distribution, morphology, and surface properties simultaneously is exceptionally challenging. These characteristics directly influence critical pharmaceutical properties including dissolution rate, bioavailability, and aerosolization behavior for pulmonary delivery systems.
Amorphous state stability represents a significant concern for spray-dried pharmaceuticals. Many APIs transition to amorphous forms during rapid drying, offering enhanced solubility but reduced physical stability. Preventing recrystallization during storage without compromising other product attributes requires sophisticated formulation strategies that are often product-specific and difficult to generalize.
Equipment limitations further constrain innovation in this field. Current atomization technologies struggle to consistently produce particles below 1 μm while maintaining narrow size distributions. Additionally, conventional cyclone collection systems demonstrate poor efficiency for fine particles, resulting in significant product loss and economic inefficiency.
Regulatory compliance adds complexity to implementation, with stringent requirements for process validation, reproducibility, and documentation. The multivariable nature of spray drying processes makes establishing clear design spaces challenging, complicating Quality by Design (QbD) approaches increasingly demanded by regulatory bodies.
Environmental and sustainability concerns have also emerged as significant challenges. The high energy consumption of conventional spray drying processes contributes substantially to pharmaceutical manufacturing's carbon footprint. Additionally, organic solvent usage in many formulations presents environmental hazards and requires sophisticated recovery systems to meet increasingly stringent emissions regulations.
Thermal sensitivity presents another major challenge, particularly for biologics and heat-labile Active Pharmaceutical Ingredients (APIs). The exposure to elevated temperatures during the drying process can lead to protein denaturation, loss of biological activity, and chemical degradation. While protective excipients offer some mitigation, finding the optimal balance between thermal protection and product functionality remains difficult.
Particle engineering precision continues to be problematic despite technological advances. Achieving precise control over particle size distribution, morphology, and surface properties simultaneously is exceptionally challenging. These characteristics directly influence critical pharmaceutical properties including dissolution rate, bioavailability, and aerosolization behavior for pulmonary delivery systems.
Amorphous state stability represents a significant concern for spray-dried pharmaceuticals. Many APIs transition to amorphous forms during rapid drying, offering enhanced solubility but reduced physical stability. Preventing recrystallization during storage without compromising other product attributes requires sophisticated formulation strategies that are often product-specific and difficult to generalize.
Equipment limitations further constrain innovation in this field. Current atomization technologies struggle to consistently produce particles below 1 μm while maintaining narrow size distributions. Additionally, conventional cyclone collection systems demonstrate poor efficiency for fine particles, resulting in significant product loss and economic inefficiency.
Regulatory compliance adds complexity to implementation, with stringent requirements for process validation, reproducibility, and documentation. The multivariable nature of spray drying processes makes establishing clear design spaces challenging, complicating Quality by Design (QbD) approaches increasingly demanded by regulatory bodies.
Environmental and sustainability concerns have also emerged as significant challenges. The high energy consumption of conventional spray drying processes contributes substantially to pharmaceutical manufacturing's carbon footprint. Additionally, organic solvent usage in many formulations presents environmental hazards and requires sophisticated recovery systems to meet increasingly stringent emissions regulations.
Current API Spray Drying Methodologies
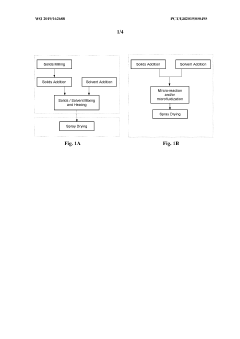
01 Process parameters for spray drying pharmaceuticals
Specific process parameters are critical for effective spray drying of active pharmaceutical ingredients. These parameters include inlet/outlet temperature, atomization pressure, feed rate, and drying gas flow rate. Optimizing these variables helps control particle size, morphology, and moisture content, which directly impact the stability and bioavailability of the final pharmaceutical product. Proper parameter selection can prevent degradation of heat-sensitive compounds while ensuring consistent product quality.- Process parameters for spray drying pharmaceuticals: Specific process parameters are critical for successful spray drying of active pharmaceutical ingredients. These include optimizing inlet/outlet temperatures, feed rate, atomization pressure, and drying gas flow rate. Proper control of these parameters ensures consistent particle size distribution, morphology, and stability of the final product while minimizing degradation of heat-sensitive compounds. The selection of appropriate process conditions depends on the physicochemical properties of the active ingredient and desired product characteristics.
- Excipients and carriers for spray-dried formulations: Selection of suitable excipients and carriers plays a crucial role in spray drying of pharmaceuticals. Carriers such as lactose, mannitol, and various polymers help improve flowability, stability, and dissolution properties of the final product. Protective excipients like trehalose and cyclodextrins can shield active ingredients from thermal degradation during the spray drying process. The choice of excipients significantly impacts the physical and chemical stability of the spray-dried pharmaceutical formulation and its subsequent performance in drug delivery systems.
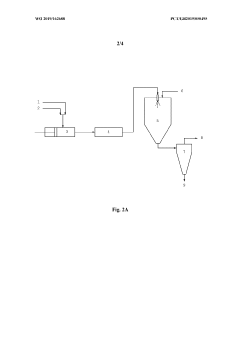
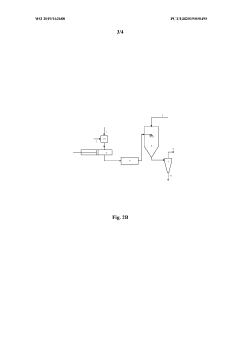
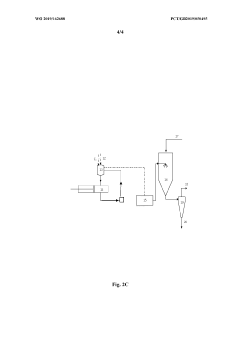
- Equipment design and optimization for pharmaceutical spray drying: Specialized equipment design is essential for efficient spray drying of pharmaceutical ingredients. This includes optimized spray nozzle configurations, drying chamber geometries, and collection systems tailored for pharmaceutical applications. Advanced designs incorporate features for aseptic processing, containment of potent compounds, and minimization of product loss. Innovations in equipment design focus on improving thermal efficiency, reducing residence time for heat-sensitive materials, and enhancing particle engineering capabilities to achieve desired product characteristics.
- Particle engineering through spray drying: Spray drying enables precise particle engineering of pharmaceutical ingredients through control of process parameters and formulation composition. This technique allows for the production of particles with specific size distributions, densities, and morphologies optimized for various delivery routes including pulmonary, oral, and parenteral administration. Advanced particle engineering approaches can create composite particles, hollow structures, or encapsulated systems with enhanced stability, bioavailability, and controlled release properties. The ability to tailor particle characteristics makes spray drying valuable for developing innovative drug delivery systems.
- Stability enhancement of spray-dried pharmaceuticals: Various strategies are employed to enhance the stability of spray-dried pharmaceutical formulations. These include the use of stabilizing additives, optimization of drying conditions to minimize thermal stress, and post-processing treatments such as secondary drying or packaging under controlled atmospheres. Amorphous solid dispersions created through spray drying can improve the stability and solubility of poorly water-soluble drugs. Understanding the relationship between process conditions, formulation composition, and storage stability is crucial for developing robust spray-dried pharmaceutical products with acceptable shelf life.
02 Excipient selection for spray-dried pharmaceutical formulations
The choice of excipients plays a crucial role in spray drying of active pharmaceutical ingredients. Carriers such as lactose, mannitol, and various polymers can enhance stability, improve flow properties, and protect APIs during the drying process. Certain excipients can modify the glass transition temperature of the formulation, preventing crystallization and maintaining amorphous state. Additionally, surfactants may be incorporated to improve wettability and dissolution rate of the final spray-dried powder.Expand Specific Solutions03 Equipment design and modifications for pharmaceutical spray drying
Specialized equipment designs and modifications are essential for efficient spray drying of pharmaceutical ingredients. These include customized atomization nozzles, cyclone separators with improved collection efficiency, and integrated filtration systems to capture fine particles. Advanced designs incorporate closed-loop systems to maintain sterility and prevent cross-contamination. Some equipment features allow for aseptic processing of sensitive compounds and continuous manufacturing capabilities to enhance production efficiency and consistency.Expand Specific Solutions04 Stabilization techniques for spray-dried APIs
Various stabilization techniques can be employed during spray drying to preserve the integrity and activity of pharmaceutical ingredients. These include the use of antioxidants to prevent oxidative degradation, pH modifiers to maintain optimal chemical stability, and cryoprotectants to protect proteins and peptides. Encapsulation methods using lipids or polymers can shield sensitive compounds from environmental factors. Some techniques involve co-processing with stabilizing agents that form protective matrices around the active ingredients.Expand Specific Solutions05 Particle engineering for enhanced drug delivery
Spray drying enables sophisticated particle engineering for pharmaceutical applications. By controlling process conditions and formulation components, manufacturers can produce particles with specific size distributions, density, porosity, and surface characteristics. These engineered particles can enhance drug delivery by improving lung deposition for inhalation products, increasing dissolution rates for poorly soluble drugs, or providing controlled release profiles. Advanced techniques allow for the creation of composite particles with multiple functionalities or co-processed ingredients with synergistic effects.Expand Specific Solutions
Leading Pharmaceutical Spray Drying Equipment Manufacturers
The spray drying of active pharmaceutical ingredients (APIs) is currently in a growth phase, with the global market expanding due to increasing demand for advanced drug delivery systems. The technology has reached moderate maturity, with established players like Novartis AG, Eli Lilly & Co., and Merck Sharp & Dohme leading commercial applications, while specialized equipment manufacturers such as GEA Systems and Tofflon Science & Technology provide technological infrastructure. Companies including Lonza, Hovione Scientia, and Boehringer Ingelheim have developed significant expertise in contract manufacturing of spray-dried APIs. Research institutions collaborate with pharmaceutical companies to advance the technology, addressing challenges in process optimization, stability enhancement, and scalability, particularly for complex biologics and poorly soluble compounds.
Boehringer Ingelheim Pharma GmbH & Co., KG
Technical Solution: Boehringer Ingelheim has developed a comprehensive spray drying platform called "SoluBIlity" specifically for enhancing bioavailability of poorly soluble compounds. Their technology focuses on creating stable amorphous solid dispersions through optimized spray drying processes. The company employs a systematic approach to formulation development, utilizing computational modeling to predict polymer-drug interactions and stability. Their spray drying facilities feature customized atomization systems that can achieve precise droplet formation and rapid evaporation kinetics. Boehringer's technology incorporates in-line process analytical technology (PAT) for real-time monitoring of critical quality attributes including residual solvent levels, particle size distribution, and amorphous content. The company has successfully applied this technology to multiple commercial products, achieving up to 40-fold improvement in dissolution rates for challenging molecules while maintaining physical stability throughout the product shelf life.
Strengths: Vertically integrated capabilities from early development to commercial manufacturing; proprietary predictive modeling tools for formulation optimization; extensive experience with regulatory submissions for spray-dried products. Weaknesses: Technology primarily optimized for their internal pipeline compounds; potentially higher production costs compared to conventional solid dosage manufacturing approaches.
Hovione Scientia Ltd.
Technical Solution: Hovione has developed advanced spray drying technology platforms specifically designed for pharmaceutical applications, focusing on enhancing bioavailability of poorly water-soluble drugs. Their approach involves creating amorphous solid dispersions (ASDs) through spray drying, where the API is molecularly dispersed within a polymer matrix. Hovione's technology enables precise control over particle engineering parameters including particle size, density, and morphology. Their spray drying facilities operate under cGMP conditions with scale flexibility from lab development (grams) to commercial manufacturing (tons). Hovione has pioneered continuous manufacturing approaches for spray dried dispersions, integrating real-time process analytical technology (PAT) for monitoring critical quality attributes during production. Their technology has demonstrated success in improving dissolution rates by up to 50-fold for BCS Class II compounds compared to crystalline forms.
Strengths: Industry-leading expertise in spray drying with dedicated facilities across multiple scales; proprietary formulation approaches for challenging molecules; integrated analytical capabilities for solid-state characterization. Weaknesses: Higher processing costs compared to conventional manufacturing methods; potential challenges with stability of amorphous systems requiring specialized packaging solutions.
Critical Patents in Pharmaceutical Spray Drying
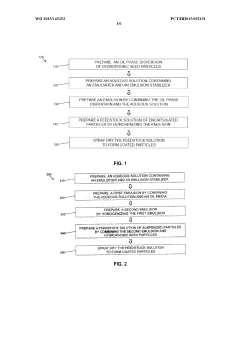
Spray-dried solid-in-oil-in-water dispersions for inhalation of active pharmaceutical ingredients
PatentWO2015145353A1
Innovation
- The development of stable solid-in-oil-in-water dispersions using hydrophobic drugs dispersed in liquid perfluorocarbons, stabilized by long-chain phospholipids, which are then spray-dried to form particles with a porous shell, maintaining the biochemical integrity and activity of the API while preventing aggregation.
A spray drying process with continuous preparation of spray solution
PatentWO2019162688A1
Innovation
- A continuous spray drying process that uses microfluidization to micronize and homogeneously mix active pharmaceutical ingredients and excipients with solvents in a micro-reaction chamber, eliminating the need for additional batch operations and reducing hold times, thereby enhancing solubility and stability.
Regulatory Compliance for Spray-Dried Pharmaceuticals
Regulatory compliance represents a critical aspect of pharmaceutical spray drying processes, requiring manufacturers to navigate complex frameworks established by global regulatory bodies. The FDA in the United States, EMA in Europe, and other international authorities have developed comprehensive guidelines specifically addressing spray-dried pharmaceutical products, with particular emphasis on quality control, process validation, and contamination prevention.
These regulatory frameworks mandate rigorous documentation of spray drying parameters, including inlet/outlet temperatures, feed rates, atomization settings, and drying gas properties. Such documentation must demonstrate consistent production of pharmaceutical products meeting predetermined quality specifications across multiple batches, establishing what regulators term as a "state of control."
Quality by Design (QbD) principles have become increasingly central to regulatory compliance for spray-dried pharmaceuticals. This approach requires manufacturers to identify Critical Quality Attributes (CQAs) and Critical Process Parameters (CPPs) that significantly impact product quality. For spray-dried APIs, these typically include particle size distribution, residual solvent levels, moisture content, and physical stability characteristics.
Process Analytical Technology (PAT) implementation represents another regulatory expectation, enabling real-time monitoring of spray drying operations. Near-infrared spectroscopy, laser diffraction, and other advanced analytical techniques allow manufacturers to detect process deviations before they result in quality issues, aligning with regulatory preferences for proactive quality management.
Stability testing protocols for spray-dried pharmaceuticals present unique regulatory challenges. Regulatory bodies require comprehensive data demonstrating that the amorphous or partially crystalline states often produced through spray drying maintain their intended properties throughout the product's shelf life. This necessitates specialized stability protocols beyond those applied to conventionally processed APIs.
Cross-contamination prevention measures receive heightened regulatory scrutiny for spray drying operations. Dedicated equipment, validated cleaning procedures, and environmental monitoring systems are essential components of regulatory compliance, particularly for contract manufacturing organizations processing multiple products in shared facilities.
International harmonization efforts, including ICH guidelines Q8, Q9, and Q10, have established more consistent global expectations for spray-dried pharmaceutical manufacturing. However, significant regional variations persist, requiring manufacturers to develop compliance strategies addressing specific requirements in target markets while maintaining core quality standards.
These regulatory frameworks mandate rigorous documentation of spray drying parameters, including inlet/outlet temperatures, feed rates, atomization settings, and drying gas properties. Such documentation must demonstrate consistent production of pharmaceutical products meeting predetermined quality specifications across multiple batches, establishing what regulators term as a "state of control."
Quality by Design (QbD) principles have become increasingly central to regulatory compliance for spray-dried pharmaceuticals. This approach requires manufacturers to identify Critical Quality Attributes (CQAs) and Critical Process Parameters (CPPs) that significantly impact product quality. For spray-dried APIs, these typically include particle size distribution, residual solvent levels, moisture content, and physical stability characteristics.
Process Analytical Technology (PAT) implementation represents another regulatory expectation, enabling real-time monitoring of spray drying operations. Near-infrared spectroscopy, laser diffraction, and other advanced analytical techniques allow manufacturers to detect process deviations before they result in quality issues, aligning with regulatory preferences for proactive quality management.
Stability testing protocols for spray-dried pharmaceuticals present unique regulatory challenges. Regulatory bodies require comprehensive data demonstrating that the amorphous or partially crystalline states often produced through spray drying maintain their intended properties throughout the product's shelf life. This necessitates specialized stability protocols beyond those applied to conventionally processed APIs.
Cross-contamination prevention measures receive heightened regulatory scrutiny for spray drying operations. Dedicated equipment, validated cleaning procedures, and environmental monitoring systems are essential components of regulatory compliance, particularly for contract manufacturing organizations processing multiple products in shared facilities.
International harmonization efforts, including ICH guidelines Q8, Q9, and Q10, have established more consistent global expectations for spray-dried pharmaceutical manufacturing. However, significant regional variations persist, requiring manufacturers to develop compliance strategies addressing specific requirements in target markets while maintaining core quality standards.
Scale-up Considerations for Industrial API Spray Drying
Scaling up spray drying processes from laboratory to industrial scale presents significant challenges that must be addressed systematically to ensure consistent product quality and process efficiency. The transition requires careful consideration of equipment design differences, as industrial spray dryers typically feature more complex atomization systems, larger drying chambers, and sophisticated powder collection mechanisms compared to their laboratory counterparts.
Process parameters must be meticulously adjusted during scale-up. The atomization conditions, including nozzle type, pressure, and feed rate, need recalibration to maintain similar droplet size distributions. Thermal conditions also require adjustment, as heat transfer dynamics change substantially with increased chamber dimensions, potentially affecting API stability and morphology.
Residence time variations between scales can significantly impact product characteristics. Industrial dryers typically have longer residence times due to larger chamber volumes, necessitating adjustments to inlet temperatures and feed concentrations to achieve comparable moisture content and particle properties. Mathematical modeling and computational fluid dynamics (CFD) simulations have become essential tools for predicting these scale-dependent behaviors.
Quality control strategies must evolve during scale-up to accommodate larger batch sizes and continuous processing requirements. Online monitoring technologies, including particle size analyzers, moisture sensors, and spectroscopic methods, enable real-time process adjustments to maintain critical quality attributes. Implementing Quality by Design (QbD) principles helps identify critical process parameters and establish appropriate control strategies.
Regulatory considerations become increasingly important at industrial scale. Process validation protocols must demonstrate that the scaled-up process consistently produces API powder with the required specifications. This includes establishing acceptable ranges for critical process parameters and demonstrating robust control strategies that ensure batch-to-batch consistency.
Economic factors significantly influence scale-up decisions. Capital investment for industrial spray dryers is substantial, requiring careful evaluation of throughput requirements, energy efficiency, and operational costs. Advanced energy recovery systems, such as closed-loop configurations with heat exchangers, can improve efficiency but add complexity to the scale-up process.
Safety considerations also intensify with scale. The handling of larger volumes of organic solvents increases explosion risks, necessitating robust safety systems including oxygen monitoring, explosion suppression mechanisms, and proper electrical classification. Additionally, containment strategies for highly potent APIs must be scaled appropriately to maintain operator safety while preserving process efficiency.
Process parameters must be meticulously adjusted during scale-up. The atomization conditions, including nozzle type, pressure, and feed rate, need recalibration to maintain similar droplet size distributions. Thermal conditions also require adjustment, as heat transfer dynamics change substantially with increased chamber dimensions, potentially affecting API stability and morphology.
Residence time variations between scales can significantly impact product characteristics. Industrial dryers typically have longer residence times due to larger chamber volumes, necessitating adjustments to inlet temperatures and feed concentrations to achieve comparable moisture content and particle properties. Mathematical modeling and computational fluid dynamics (CFD) simulations have become essential tools for predicting these scale-dependent behaviors.
Quality control strategies must evolve during scale-up to accommodate larger batch sizes and continuous processing requirements. Online monitoring technologies, including particle size analyzers, moisture sensors, and spectroscopic methods, enable real-time process adjustments to maintain critical quality attributes. Implementing Quality by Design (QbD) principles helps identify critical process parameters and establish appropriate control strategies.
Regulatory considerations become increasingly important at industrial scale. Process validation protocols must demonstrate that the scaled-up process consistently produces API powder with the required specifications. This includes establishing acceptable ranges for critical process parameters and demonstrating robust control strategies that ensure batch-to-batch consistency.
Economic factors significantly influence scale-up decisions. Capital investment for industrial spray dryers is substantial, requiring careful evaluation of throughput requirements, energy efficiency, and operational costs. Advanced energy recovery systems, such as closed-loop configurations with heat exchangers, can improve efficiency but add complexity to the scale-up process.
Safety considerations also intensify with scale. The handling of larger volumes of organic solvents increases explosion risks, necessitating robust safety systems including oxygen monitoring, explosion suppression mechanisms, and proper electrical classification. Additionally, containment strategies for highly potent APIs must be scaled appropriately to maintain operator safety while preserving process efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!