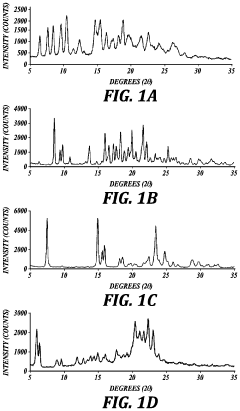
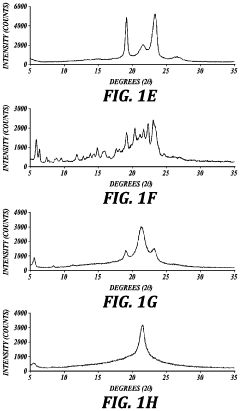
How Does Spray Drying Improve Active Ingredient Stability?
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Spray Drying Technology Evolution and Stability Goals
Spray drying technology has evolved significantly since its inception in the late 19th century. Initially developed for milk powder production, this technology has transformed into a sophisticated process widely utilized across pharmaceutical, food, and chemical industries. The evolution trajectory shows a clear shift from simple dehydration applications to complex formulation engineering aimed at enhancing active ingredient stability.
The 1950s marked a pivotal era when spray drying began to be systematically explored for pharmaceutical applications, primarily focusing on improving drug solubility. By the 1970s, researchers recognized its potential for creating amorphous solid dispersions, which represented a significant advancement in addressing stability challenges of crystalline materials.
The technological progression accelerated in the 1990s with the integration of computerized control systems and advanced atomization techniques. These innovations enabled precise manipulation of particle morphology, size distribution, and internal structure—critical parameters that directly influence active ingredient stability. The development of multi-stage drying chambers and modified atomization nozzles further expanded the capability to process heat-sensitive compounds without degradation.
Recent advancements have focused on nano-spray drying technologies, capable of producing submicron particles with enhanced surface properties. This miniaturization trend has opened new possibilities for stabilizing highly sensitive biomolecules, including proteins and peptides, by creating protective matrices that shield active ingredients from environmental stressors.
The stability goals driving spray drying innovation have evolved from simple moisture reduction to comprehensive protection against multiple degradation pathways. Modern spray drying aims to address oxidative degradation by incorporating antioxidants during the process, prevent hydrolytic breakdown through precise moisture control, and minimize thermal degradation via optimized temperature profiles and shorter residence times.
Another critical stability objective is the creation of amorphous solid dispersions that can maintain supersaturation of poorly soluble drugs while preventing recrystallization. This approach has become increasingly important as pharmaceutical pipelines feature more hydrophobic compounds with challenging stability profiles.
The industry now pursues spray drying processes that can simultaneously achieve multiple stability goals: extending shelf life, maintaining bioavailability, ensuring content uniformity, and preserving chemical integrity across diverse environmental conditions. These objectives have driven the development of specialized excipients and process parameters tailored to specific active ingredients.
Looking forward, the technology evolution continues toward more sustainable processes with reduced energy consumption, increased yield, and environmentally friendly formulations—all while maintaining or enhancing the fundamental stability benefits that have made spray drying an indispensable technology in modern formulation science.
The 1950s marked a pivotal era when spray drying began to be systematically explored for pharmaceutical applications, primarily focusing on improving drug solubility. By the 1970s, researchers recognized its potential for creating amorphous solid dispersions, which represented a significant advancement in addressing stability challenges of crystalline materials.
The technological progression accelerated in the 1990s with the integration of computerized control systems and advanced atomization techniques. These innovations enabled precise manipulation of particle morphology, size distribution, and internal structure—critical parameters that directly influence active ingredient stability. The development of multi-stage drying chambers and modified atomization nozzles further expanded the capability to process heat-sensitive compounds without degradation.
Recent advancements have focused on nano-spray drying technologies, capable of producing submicron particles with enhanced surface properties. This miniaturization trend has opened new possibilities for stabilizing highly sensitive biomolecules, including proteins and peptides, by creating protective matrices that shield active ingredients from environmental stressors.
The stability goals driving spray drying innovation have evolved from simple moisture reduction to comprehensive protection against multiple degradation pathways. Modern spray drying aims to address oxidative degradation by incorporating antioxidants during the process, prevent hydrolytic breakdown through precise moisture control, and minimize thermal degradation via optimized temperature profiles and shorter residence times.
Another critical stability objective is the creation of amorphous solid dispersions that can maintain supersaturation of poorly soluble drugs while preventing recrystallization. This approach has become increasingly important as pharmaceutical pipelines feature more hydrophobic compounds with challenging stability profiles.
The industry now pursues spray drying processes that can simultaneously achieve multiple stability goals: extending shelf life, maintaining bioavailability, ensuring content uniformity, and preserving chemical integrity across diverse environmental conditions. These objectives have driven the development of specialized excipients and process parameters tailored to specific active ingredients.
Looking forward, the technology evolution continues toward more sustainable processes with reduced energy consumption, increased yield, and environmentally friendly formulations—all while maintaining or enhancing the fundamental stability benefits that have made spray drying an indispensable technology in modern formulation science.
Market Demand for Stable Active Ingredient Formulations
The global market for stable active ingredient formulations has witnessed substantial growth in recent years, driven primarily by the pharmaceutical, food and beverage, and agrochemical industries. This growth trajectory is expected to continue as manufacturers increasingly seek technologies that can extend product shelf life, maintain efficacy, and reduce degradation of sensitive compounds.
In the pharmaceutical sector, the demand for stable active pharmaceutical ingredients (APIs) has reached unprecedented levels, with the global pharmaceutical excipients market projected to grow at a CAGR of 6.0% through 2028. This surge is largely attributed to the rising prevalence of chronic diseases and the subsequent need for more effective drug delivery systems that can preserve therapeutic efficacy over extended periods.
Consumer preferences are significantly shaping market dynamics, with a growing demand for natural ingredients in food, beverages, and personal care products. These natural compounds often present stability challenges due to their sensitivity to environmental factors such as heat, light, and oxygen. Consequently, manufacturers are actively seeking advanced stabilization technologies to meet consumer expectations while maintaining product integrity.
The nutraceutical industry represents another significant market segment driving demand for stable formulations. With consumers increasingly turning to dietary supplements and functional foods for health benefits, manufacturers face the challenge of preserving bioactive compounds throughout the product lifecycle. This has created a substantial market opportunity for technologies that can effectively stabilize these sensitive ingredients.
Regulatory pressures also play a crucial role in market development. Stringent guidelines regarding product stability, shelf life, and efficacy claims have compelled manufacturers to invest in advanced formulation technologies. Spray drying has emerged as a preferred method due to its ability to meet these regulatory requirements while offering scalability for commercial production.
Geographically, North America and Europe currently dominate the market for stable active ingredient formulations, owing to their robust pharmaceutical and food processing industries. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by expanding healthcare infrastructure, increasing disposable incomes, and growing awareness about quality pharmaceutical and food products.
Economic factors further underscore the importance of stable formulations. Product recalls due to stability issues can cost manufacturers millions in direct losses and immeasurable damage to brand reputation. The investment in effective stabilization technologies like spray drying represents a strategic approach to risk mitigation and long-term market competitiveness.
In the pharmaceutical sector, the demand for stable active pharmaceutical ingredients (APIs) has reached unprecedented levels, with the global pharmaceutical excipients market projected to grow at a CAGR of 6.0% through 2028. This surge is largely attributed to the rising prevalence of chronic diseases and the subsequent need for more effective drug delivery systems that can preserve therapeutic efficacy over extended periods.
Consumer preferences are significantly shaping market dynamics, with a growing demand for natural ingredients in food, beverages, and personal care products. These natural compounds often present stability challenges due to their sensitivity to environmental factors such as heat, light, and oxygen. Consequently, manufacturers are actively seeking advanced stabilization technologies to meet consumer expectations while maintaining product integrity.
The nutraceutical industry represents another significant market segment driving demand for stable formulations. With consumers increasingly turning to dietary supplements and functional foods for health benefits, manufacturers face the challenge of preserving bioactive compounds throughout the product lifecycle. This has created a substantial market opportunity for technologies that can effectively stabilize these sensitive ingredients.
Regulatory pressures also play a crucial role in market development. Stringent guidelines regarding product stability, shelf life, and efficacy claims have compelled manufacturers to invest in advanced formulation technologies. Spray drying has emerged as a preferred method due to its ability to meet these regulatory requirements while offering scalability for commercial production.
Geographically, North America and Europe currently dominate the market for stable active ingredient formulations, owing to their robust pharmaceutical and food processing industries. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by expanding healthcare infrastructure, increasing disposable incomes, and growing awareness about quality pharmaceutical and food products.
Economic factors further underscore the importance of stable formulations. Product recalls due to stability issues can cost manufacturers millions in direct losses and immeasurable damage to brand reputation. The investment in effective stabilization technologies like spray drying represents a strategic approach to risk mitigation and long-term market competitiveness.
Current Challenges in Active Ingredient Stabilization
Despite significant advancements in pharmaceutical and food industries, active ingredient stabilization remains a critical challenge. Many bioactive compounds exhibit inherent instability when exposed to environmental factors such as oxygen, light, moisture, and temperature fluctuations. These vulnerabilities often lead to degradation, reduced efficacy, and shortened shelf life of final products, creating substantial economic and quality control challenges for manufacturers.
Protein-based active ingredients present particularly complex stabilization issues due to their susceptibility to denaturation and aggregation. Even minor changes in environmental conditions can trigger conformational changes that compromise their biological activity. Similarly, lipophilic compounds face oxidation risks that can generate harmful byproducts and diminish therapeutic efficacy.
Conventional stabilization approaches often rely on chemical additives and preservatives, which increasingly face consumer resistance due to growing preferences for "clean label" products. This market shift has intensified the need for physical stabilization methods that maintain ingredient integrity without chemical intervention. Additionally, regulatory frameworks worldwide are becoming more stringent regarding stabilizing excipients, further complicating formulation strategies.
Scale-up challenges represent another significant hurdle in active ingredient stabilization. Laboratory-scale stabilization techniques frequently encounter difficulties when transferred to industrial production environments. The inconsistencies in heat transfer, mixing dynamics, and processing times between small and large-scale operations can lead to unpredictable stability profiles in final products.
Emerging active ingredients, particularly those derived from biotechnology and advanced extraction techniques, often exhibit unique stability profiles that resist conventional stabilization approaches. These novel compounds may require tailored stabilization strategies that current industry standards cannot adequately address.
Cost considerations further complicate stabilization efforts. While sophisticated encapsulation technologies show promise for enhancing stability, their implementation often involves substantial capital investment and increased production costs. This economic barrier particularly affects smaller manufacturers and limits widespread adoption of advanced stabilization techniques.
The pharmaceutical industry faces additional challenges with combination products, where multiple active ingredients with different stability requirements must coexist in a single formulation. These complex systems demand sophisticated stabilization approaches that can simultaneously address diverse degradation pathways without compromising the efficacy of any component.
Accelerated stability testing methodologies also present limitations in accurately predicting long-term stability under real-world conditions. The correlation between accelerated testing results and actual shelf-life performance remains imperfect, creating uncertainty in product development timelines and stability claims.
Protein-based active ingredients present particularly complex stabilization issues due to their susceptibility to denaturation and aggregation. Even minor changes in environmental conditions can trigger conformational changes that compromise their biological activity. Similarly, lipophilic compounds face oxidation risks that can generate harmful byproducts and diminish therapeutic efficacy.
Conventional stabilization approaches often rely on chemical additives and preservatives, which increasingly face consumer resistance due to growing preferences for "clean label" products. This market shift has intensified the need for physical stabilization methods that maintain ingredient integrity without chemical intervention. Additionally, regulatory frameworks worldwide are becoming more stringent regarding stabilizing excipients, further complicating formulation strategies.
Scale-up challenges represent another significant hurdle in active ingredient stabilization. Laboratory-scale stabilization techniques frequently encounter difficulties when transferred to industrial production environments. The inconsistencies in heat transfer, mixing dynamics, and processing times between small and large-scale operations can lead to unpredictable stability profiles in final products.
Emerging active ingredients, particularly those derived from biotechnology and advanced extraction techniques, often exhibit unique stability profiles that resist conventional stabilization approaches. These novel compounds may require tailored stabilization strategies that current industry standards cannot adequately address.
Cost considerations further complicate stabilization efforts. While sophisticated encapsulation technologies show promise for enhancing stability, their implementation often involves substantial capital investment and increased production costs. This economic barrier particularly affects smaller manufacturers and limits widespread adoption of advanced stabilization techniques.
The pharmaceutical industry faces additional challenges with combination products, where multiple active ingredients with different stability requirements must coexist in a single formulation. These complex systems demand sophisticated stabilization approaches that can simultaneously address diverse degradation pathways without compromising the efficacy of any component.
Accelerated stability testing methodologies also present limitations in accurately predicting long-term stability under real-world conditions. The correlation between accelerated testing results and actual shelf-life performance remains imperfect, creating uncertainty in product development timelines and stability claims.
Current Spray Drying Stabilization Mechanisms
01 Process parameters for spray drying stability
Controlling process parameters such as temperature, pressure, and flow rate is crucial for achieving stability in spray drying operations. Optimal temperature settings prevent degradation of heat-sensitive compounds while ensuring proper drying. Pressure control affects droplet size and distribution, which impacts product uniformity. Flow rate adjustments can minimize agglomeration and ensure consistent particle size distribution, all contributing to enhanced stability of the final dried product.- Process parameters for spray drying stability: Controlling process parameters is crucial for achieving stability in spray drying operations. These parameters include inlet/outlet temperature, atomization pressure, feed rate, and drying air flow rate. Optimizing these variables helps maintain consistent particle size distribution, moisture content, and physical properties of the dried product, resulting in improved stability of the final powder.
- Stabilizing additives for spray dried formulations: Various additives can be incorporated into formulations prior to spray drying to enhance stability. These include surfactants, polymers, antioxidants, and carbohydrates that function as protective agents. These stabilizers prevent degradation during the drying process, reduce moisture absorption, improve flowability, and extend shelf life of the spray dried products.
- Equipment design for improved spray drying stability: Specialized equipment designs can significantly improve spray drying stability. Features such as optimized spray nozzle configurations, chamber geometry, air flow patterns, and collection systems help maintain consistent drying conditions. Advanced equipment may include integrated monitoring systems that allow real-time adjustments to maintain process stability and product quality.
- Post-processing techniques for spray dried product stability: After spray drying, various post-processing techniques can be employed to enhance product stability. These include controlled cooling, secondary drying, surface coating, and specialized packaging methods. These approaches help maintain the physical and chemical integrity of spray dried products during storage and transportation, preventing agglomeration and degradation.
- Formulation composition for enhanced spray drying stability: The composition of the formulation being spray dried significantly impacts stability. Optimizing the ratio of active ingredients to excipients, selecting compatible components, adjusting pH, and controlling solids content can improve drying efficiency and product stability. Properly designed formulations resist degradation during the high-temperature drying process and maintain their intended properties in the final dried state.
02 Formulation additives for improved stability
Various additives can be incorporated into spray drying formulations to enhance stability. These include stabilizers that prevent degradation during the drying process, carriers that improve flowability and reduce stickiness, and protective agents that shield active ingredients from heat and oxidation. The selection of appropriate excipients can significantly impact the physical and chemical stability of spray-dried products, resulting in extended shelf life and maintained efficacy.Expand Specific Solutions03 Equipment design for stability enhancement
The design of spray drying equipment plays a significant role in ensuring product stability. Features such as specialized atomization nozzles, optimized drying chamber geometry, and efficient air flow patterns can minimize thermal stress and residence time. Advanced collection systems reduce product loss and prevent degradation. Innovations in equipment design focus on maintaining consistent drying conditions and minimizing hot spots that could compromise product stability.Expand Specific Solutions04 Stabilization techniques for sensitive materials
Special techniques have been developed to stabilize heat-sensitive materials during spray drying. These include microencapsulation to create protective barriers around active ingredients, co-drying with protective polymers, and the use of antioxidants to prevent oxidative degradation. Low-temperature spray drying methods and the incorporation of cryoprotectants can preserve the integrity of proteins, enzymes, and other biological materials that are particularly susceptible to denaturation during conventional drying processes.Expand Specific Solutions05 Post-processing methods for stability maintenance
Various post-processing methods can be employed to maintain the stability of spray-dried products. These include controlled packaging under inert atmospheres to prevent moisture uptake and oxidation, secondary drying steps to achieve optimal residual moisture content, and surface modification techniques to reduce hygroscopicity. Storage condition optimization and the application of protective coatings can further extend product stability by minimizing degradation reactions and preserving physical characteristics over time.Expand Specific Solutions
Leading Companies in Spray Drying Technology
Spray drying technology for active ingredient stability is currently in a mature growth phase, with the global market estimated at $7-9 billion and expanding at 5-7% annually. The competitive landscape features established pharmaceutical and flavor companies alongside specialized technology providers. Leading players like Lonza, Novartis, and Vertex Pharmaceuticals have developed advanced stabilization techniques for pharmaceutical applications, while Firmenich, Symrise, and ZoomEssence have pioneered flavor encapsulation innovations. Chinese companies including Sinopec and Harbin Kanglong are rapidly expanding capabilities, particularly in cost-effective manufacturing. Technical maturity varies by application, with pharmaceutical implementations reaching higher sophistication levels through proprietary technologies that enhance bioavailability and extend shelf life of sensitive compounds.
Zoomessence, Inc.
Technical Solution: ZoomEssence has developed a proprietary low-temperature spray drying technology called Zooming® that significantly enhances active ingredient stability. Unlike conventional spray drying that typically operates at 100-200°C inlet temperatures, their technology utilizes a controlled-flow, low-temperature process (typically below 40°C) that minimizes thermal degradation of sensitive compounds. The system employs specialized atomization techniques and a unique drying chamber design that creates optimal particle formation conditions without excessive heat exposure. ZoomEssence's technology incorporates proprietary encapsulation matrices that form protective barriers around active ingredients, shielding them from oxygen, moisture, and other environmental factors that accelerate degradation. Their process allows for the incorporation of multiple stabilizing agents in precise ratios, creating customized stability profiles for specific active ingredients. The resulting particles feature optimized microstructures that enhance both chemical stability and controlled release properties.
Strengths: Superior preservation of heat-sensitive compounds, enhanced flavor/fragrance retention, and reduced energy consumption. Weaknesses: Potentially higher production costs for certain applications and more complex equipment requirements compared to conventional spray dryers.
Vectura Ltd.
Technical Solution: Vectura has developed specialized spray drying technology focused on enhancing the stability of inhaled pharmaceutical formulations. Their PowderHale® and PowderMax® platforms utilize precise control of spray drying parameters to create engineered particles with optimized aerodynamic properties while maintaining active ingredient stability. Vectura's technology incorporates advanced atomization systems that produce uniform droplet sizes, ensuring consistent particle morphology critical for both stability and inhalation performance. Their process employs carefully controlled temperature profiles that minimize thermal stress while achieving optimal moisture removal. Vectura has pioneered the co-spray drying of active ingredients with specialized excipients that form protective matrices, shielding sensitive compounds from degradation factors like oxygen and moisture. Their technology includes specialized collection systems designed to minimize mechanical stress during powder recovery, preserving particle integrity and stability. Additionally, Vectura implements PAT (Process Analytical Technology) tools for real-time monitoring of critical quality attributes throughout the spray drying process.
Strengths: Specialized expertise in respiratory formulations, ability to create particles with both stability and optimal aerodynamic properties, and extensive regulatory experience. Weaknesses: Primarily focused on inhalation applications rather than broader pharmaceutical or food applications, and potentially higher development costs for complex formulations.
Key Patents in Spray Drying Stabilization Methods
Combination pharmaceutical compositions and methods thereof
PatentPendingUS20220096503A1
Innovation
- A combination pharmaceutical composition is developed by dissolving hydrophobic and hydrophilic therapeutic agents with lipid excipients in an alcoholic solvent, followed by controlled solvent removal through spray drying, creating a stable powder with a unified multi-drug motif structure that maintains long-range order and extended plasma drug concentrations.
Stable non-aqueous single phase viscous vehicles and formulations utilizing such vehicles
PatentInactiveUS20110308061A1
Innovation
- Development of stable non-aqueous single phase biocompatible viscous vehicles comprising polymers, surfactants, and solvents, which uniformly suspend proteins and maintain stability at body temperature for extended periods, allowing for low flow rate delivery through implantable devices.
Regulatory Compliance for Spray-Dried Products
Regulatory compliance represents a critical aspect of spray-dried pharmaceutical and food products, requiring manufacturers to navigate complex frameworks established by various international regulatory bodies. The FDA in the United States, EMA in Europe, and similar authorities worldwide have established specific guidelines governing spray-dried products, particularly those containing active pharmaceutical ingredients (APIs). These regulations focus primarily on ensuring product safety, consistency, and stability throughout the product lifecycle.
Manufacturing facilities utilizing spray drying technology must adhere to Good Manufacturing Practice (GMP) standards, which include specific requirements for equipment validation, process parameters documentation, and quality control measures. The spray drying process itself must be validated according to regulatory expectations, demonstrating that it consistently produces products meeting predetermined specifications for stability and potency.
Quality by Design (QbD) principles have become increasingly important in regulatory compliance for spray-dried products. Manufacturers must identify critical quality attributes (CQAs) and critical process parameters (CPPs) that affect the stability of active ingredients. This includes establishing acceptable ranges for inlet/outlet temperatures, feed rates, atomization parameters, and other factors that directly impact the physical and chemical stability of the final product.
Stability testing protocols for spray-dried products must comply with ICH guidelines (particularly ICH Q1A-Q1F), which outline requirements for stability studies under various storage conditions. For spray-dried pharmaceuticals, manufacturers must demonstrate that the enhanced stability achieved through spray drying persists throughout the claimed shelf life under specified storage conditions. This typically involves accelerated and long-term stability studies examining parameters such as moisture content, particle size distribution, dissolution rates, and chemical degradation.
Documentation requirements present another significant regulatory consideration. Manufacturers must maintain comprehensive records of process development, validation studies, and stability data. For novel spray-dried formulations, regulatory submissions often require detailed information about how the spray drying process specifically contributes to active ingredient stability, supported by comparative stability data against conventional formulations.
Regulatory bodies increasingly focus on the control of residual solvents in spray-dried products, particularly when organic solvents are used in the feed solution. Compliance with ICH Q3C guidelines for residual solvent limits is mandatory, requiring validated analytical methods to demonstrate that spray-dried products meet established safety thresholds. Additionally, manufacturers must address potential concerns regarding the formation of degradation products during the spray drying process, implementing appropriate controls and analytical methods to identify and quantify these impurities.
Manufacturing facilities utilizing spray drying technology must adhere to Good Manufacturing Practice (GMP) standards, which include specific requirements for equipment validation, process parameters documentation, and quality control measures. The spray drying process itself must be validated according to regulatory expectations, demonstrating that it consistently produces products meeting predetermined specifications for stability and potency.
Quality by Design (QbD) principles have become increasingly important in regulatory compliance for spray-dried products. Manufacturers must identify critical quality attributes (CQAs) and critical process parameters (CPPs) that affect the stability of active ingredients. This includes establishing acceptable ranges for inlet/outlet temperatures, feed rates, atomization parameters, and other factors that directly impact the physical and chemical stability of the final product.
Stability testing protocols for spray-dried products must comply with ICH guidelines (particularly ICH Q1A-Q1F), which outline requirements for stability studies under various storage conditions. For spray-dried pharmaceuticals, manufacturers must demonstrate that the enhanced stability achieved through spray drying persists throughout the claimed shelf life under specified storage conditions. This typically involves accelerated and long-term stability studies examining parameters such as moisture content, particle size distribution, dissolution rates, and chemical degradation.
Documentation requirements present another significant regulatory consideration. Manufacturers must maintain comprehensive records of process development, validation studies, and stability data. For novel spray-dried formulations, regulatory submissions often require detailed information about how the spray drying process specifically contributes to active ingredient stability, supported by comparative stability data against conventional formulations.
Regulatory bodies increasingly focus on the control of residual solvents in spray-dried products, particularly when organic solvents are used in the feed solution. Compliance with ICH Q3C guidelines for residual solvent limits is mandatory, requiring validated analytical methods to demonstrate that spray-dried products meet established safety thresholds. Additionally, manufacturers must address potential concerns regarding the formation of degradation products during the spray drying process, implementing appropriate controls and analytical methods to identify and quantify these impurities.
Scale-up Considerations for Industrial Applications
Scaling up spray drying operations from laboratory to industrial scale presents significant engineering challenges that must be addressed to maintain product quality and process efficiency. The transition requires careful consideration of equipment design, process parameters, and quality control measures to ensure consistent active ingredient stability across production volumes.
Equipment selection becomes a critical factor in industrial applications, with specialized spray dryers designed to handle larger throughput while maintaining optimal drying conditions. Industrial-scale spray dryers typically feature more sophisticated atomization systems, enhanced heat transfer capabilities, and advanced control systems that must be properly calibrated to replicate laboratory-scale stability benefits.
Process parameters require systematic adjustment during scale-up to maintain the thermodynamic environment that protects active ingredients. Feed rate, inlet/outlet temperatures, and atomization pressure must be recalibrated proportionally to equipment size while preserving the rapid drying kinetics that minimize thermal exposure. Computational fluid dynamics modeling has emerged as a valuable tool for predicting airflow patterns and temperature distributions within larger drying chambers.
Residence time distribution represents another critical consideration, as larger equipment dimensions can alter the time active ingredients spend in various temperature zones. Engineers must design spray paths and air flow patterns that minimize hot spots and ensure uniform drying conditions throughout the chamber, preventing localized degradation of sensitive compounds.
Atomization technology scaling presents unique challenges, as nozzle performance characteristics change with increased throughput requirements. Multi-nozzle configurations, pressure nozzles, or rotary atomizers may be employed depending on production volume and formulation properties, each requiring specific optimization to maintain the desired particle size distribution that contributes to stability.
Quality assurance protocols must evolve with scale-up, implementing in-process analytical technologies that monitor critical parameters in real-time. Continuous monitoring of moisture content, particle size, and temperature profiles enables immediate adjustments to maintain stability-enhancing conditions throughout production runs.
Economic considerations also influence scale-up decisions, with energy efficiency becoming increasingly important at industrial scales. Heat recovery systems, optimized air handling, and process integration strategies can significantly reduce operational costs while maintaining the controlled drying environment necessary for active ingredient stability.
Regulatory compliance adds another dimension to scale-up considerations, requiring thorough documentation of process equivalence between development and commercial scales. Validation protocols must demonstrate that the stability benefits observed in laboratory studies are consistently achieved in full-scale production.
Equipment selection becomes a critical factor in industrial applications, with specialized spray dryers designed to handle larger throughput while maintaining optimal drying conditions. Industrial-scale spray dryers typically feature more sophisticated atomization systems, enhanced heat transfer capabilities, and advanced control systems that must be properly calibrated to replicate laboratory-scale stability benefits.
Process parameters require systematic adjustment during scale-up to maintain the thermodynamic environment that protects active ingredients. Feed rate, inlet/outlet temperatures, and atomization pressure must be recalibrated proportionally to equipment size while preserving the rapid drying kinetics that minimize thermal exposure. Computational fluid dynamics modeling has emerged as a valuable tool for predicting airflow patterns and temperature distributions within larger drying chambers.
Residence time distribution represents another critical consideration, as larger equipment dimensions can alter the time active ingredients spend in various temperature zones. Engineers must design spray paths and air flow patterns that minimize hot spots and ensure uniform drying conditions throughout the chamber, preventing localized degradation of sensitive compounds.
Atomization technology scaling presents unique challenges, as nozzle performance characteristics change with increased throughput requirements. Multi-nozzle configurations, pressure nozzles, or rotary atomizers may be employed depending on production volume and formulation properties, each requiring specific optimization to maintain the desired particle size distribution that contributes to stability.
Quality assurance protocols must evolve with scale-up, implementing in-process analytical technologies that monitor critical parameters in real-time. Continuous monitoring of moisture content, particle size, and temperature profiles enables immediate adjustments to maintain stability-enhancing conditions throughout production runs.
Economic considerations also influence scale-up decisions, with energy efficiency becoming increasingly important at industrial scales. Heat recovery systems, optimized air handling, and process integration strategies can significantly reduce operational costs while maintaining the controlled drying environment necessary for active ingredient stability.
Regulatory compliance adds another dimension to scale-up considerations, requiring thorough documentation of process equivalence between development and commercial scales. Validation protocols must demonstrate that the stability benefits observed in laboratory studies are consistently achieved in full-scale production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!