How Spray Drying Alters Pharmaceutical Bioavailability
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Spray Drying Technology Evolution and Objectives
Spray drying technology has evolved significantly since its inception in the late 19th century. Initially developed for the dairy industry to produce milk powder, this technology has undergone remarkable transformations to become a cornerstone in pharmaceutical manufacturing. The 1940s marked the beginning of spray drying's application in pharmaceuticals, primarily for simple formulations. By the 1970s, advancements in atomization technology and drying chamber designs enabled more precise control over particle characteristics, expanding its pharmaceutical applications.
The 1990s witnessed a paradigm shift with the integration of computational fluid dynamics (CFD) modeling, allowing manufacturers to predict and optimize the spray drying process parameters. This period also saw the development of specialized nozzles capable of producing particles with tailored morphologies, crucial for controlling drug release profiles. The early 2000s brought miniaturized spray dryers designed specifically for pharmaceutical R&D, facilitating rapid prototyping and small-scale production of drug formulations.
Recent technological evolution has focused on continuous manufacturing capabilities, in-line process analytical technology (PAT), and the integration of quality-by-design (QbD) principles. These advancements have transformed spray drying from a simple dehydration method to a sophisticated technology capable of engineering particles with precise physicochemical properties that directly influence bioavailability.
The primary objective of spray drying in pharmaceutical applications is to overcome bioavailability challenges associated with poorly water-soluble drugs, which constitute approximately 40% of approved drugs and nearly 90% of developmental pipeline compounds. By converting crystalline drug substances into amorphous solid dispersions, spray drying aims to enhance dissolution rates and apparent solubility, thereby improving bioavailability.
Additional objectives include stabilizing thermolabile compounds through rapid drying, producing inhalable particles with optimal aerodynamic properties, and developing controlled-release formulations. The technology also seeks to improve manufacturing efficiency through continuous processing capabilities and reduced production steps compared to conventional methods like wet granulation followed by drying.
Current research objectives focus on expanding spray drying capabilities for biologics and vaccines, developing novel excipient systems for enhanced stability of amorphous dispersions, and creating advanced multi-component particles with programmable release profiles. There is also significant interest in developing predictive models that can accurately correlate spray drying parameters with resulting bioavailability, potentially reducing development timelines and costs while improving therapeutic outcomes.
The 1990s witnessed a paradigm shift with the integration of computational fluid dynamics (CFD) modeling, allowing manufacturers to predict and optimize the spray drying process parameters. This period also saw the development of specialized nozzles capable of producing particles with tailored morphologies, crucial for controlling drug release profiles. The early 2000s brought miniaturized spray dryers designed specifically for pharmaceutical R&D, facilitating rapid prototyping and small-scale production of drug formulations.
Recent technological evolution has focused on continuous manufacturing capabilities, in-line process analytical technology (PAT), and the integration of quality-by-design (QbD) principles. These advancements have transformed spray drying from a simple dehydration method to a sophisticated technology capable of engineering particles with precise physicochemical properties that directly influence bioavailability.
The primary objective of spray drying in pharmaceutical applications is to overcome bioavailability challenges associated with poorly water-soluble drugs, which constitute approximately 40% of approved drugs and nearly 90% of developmental pipeline compounds. By converting crystalline drug substances into amorphous solid dispersions, spray drying aims to enhance dissolution rates and apparent solubility, thereby improving bioavailability.
Additional objectives include stabilizing thermolabile compounds through rapid drying, producing inhalable particles with optimal aerodynamic properties, and developing controlled-release formulations. The technology also seeks to improve manufacturing efficiency through continuous processing capabilities and reduced production steps compared to conventional methods like wet granulation followed by drying.
Current research objectives focus on expanding spray drying capabilities for biologics and vaccines, developing novel excipient systems for enhanced stability of amorphous dispersions, and creating advanced multi-component particles with programmable release profiles. There is also significant interest in developing predictive models that can accurately correlate spray drying parameters with resulting bioavailability, potentially reducing development timelines and costs while improving therapeutic outcomes.
Pharmaceutical Market Demand for Enhanced Bioavailability
The pharmaceutical industry is witnessing a significant shift towards enhanced bioavailability formulations, driven by the increasing complexity of new drug molecules. Approximately 70% of new chemical entities in development pipelines exhibit poor water solubility, presenting substantial challenges for effective drug delivery and therapeutic outcomes. This market need has catalyzed the adoption of advanced processing technologies like spray drying to overcome bioavailability limitations.
Global pharmaceutical companies are increasingly investing in bioavailability enhancement technologies, with the market for such solutions projected to reach $29.2 billion by 2027, growing at a CAGR of 10.8% from 2022. Spray drying technology specifically has seen adoption growth of 15.3% annually in pharmaceutical applications over the past five years, reflecting its critical role in addressing bioavailability challenges.
Patient-centric drug development trends are further amplifying demand for bioavailability-enhanced formulations. Healthcare providers and patients increasingly prefer oral solid dosage forms that deliver consistent therapeutic effects with reduced dosing frequency and minimized side effects. This preference has created a premium market segment for formulations that can demonstrate superior bioavailability profiles, with such products commanding price premiums of 30-40% compared to conventional formulations.
Regulatory agencies worldwide have also recognized the importance of bioavailability in drug approval processes. The FDA and EMA have implemented specific guidelines for demonstrating bioequivalence and bioavailability, creating regulatory incentives for pharmaceutical companies to invest in advanced formulation technologies like spray drying. This regulatory environment has accelerated market adoption of bioavailability enhancement technologies.
Generic drug manufacturers represent another significant market driver, as they seek competitive advantages through improved bioavailability formulations of off-patent drugs. The ability to reformulate existing active pharmaceutical ingredients with enhanced bioavailability profiles creates opportunities for product differentiation and extended lifecycle management, particularly valuable in crowded generic markets.
Emerging markets present substantial growth opportunities for bioavailability-enhanced pharmaceuticals. Countries like India, China, and Brazil are experiencing increased healthcare spending and growing demand for more effective medications. These markets are projected to account for 35% of global pharmaceutical growth through 2025, with bioavailability-enhanced formulations representing a key segment within this expansion.
Contract development and manufacturing organizations (CDMOs) have responded to this market demand by expanding their spray drying capabilities, with major players investing heavily in specialized equipment and expertise. This service sector growth indicates the strong industrial commitment to addressing bioavailability challenges through advanced processing technologies like spray drying.
Global pharmaceutical companies are increasingly investing in bioavailability enhancement technologies, with the market for such solutions projected to reach $29.2 billion by 2027, growing at a CAGR of 10.8% from 2022. Spray drying technology specifically has seen adoption growth of 15.3% annually in pharmaceutical applications over the past five years, reflecting its critical role in addressing bioavailability challenges.
Patient-centric drug development trends are further amplifying demand for bioavailability-enhanced formulations. Healthcare providers and patients increasingly prefer oral solid dosage forms that deliver consistent therapeutic effects with reduced dosing frequency and minimized side effects. This preference has created a premium market segment for formulations that can demonstrate superior bioavailability profiles, with such products commanding price premiums of 30-40% compared to conventional formulations.
Regulatory agencies worldwide have also recognized the importance of bioavailability in drug approval processes. The FDA and EMA have implemented specific guidelines for demonstrating bioequivalence and bioavailability, creating regulatory incentives for pharmaceutical companies to invest in advanced formulation technologies like spray drying. This regulatory environment has accelerated market adoption of bioavailability enhancement technologies.
Generic drug manufacturers represent another significant market driver, as they seek competitive advantages through improved bioavailability formulations of off-patent drugs. The ability to reformulate existing active pharmaceutical ingredients with enhanced bioavailability profiles creates opportunities for product differentiation and extended lifecycle management, particularly valuable in crowded generic markets.
Emerging markets present substantial growth opportunities for bioavailability-enhanced pharmaceuticals. Countries like India, China, and Brazil are experiencing increased healthcare spending and growing demand for more effective medications. These markets are projected to account for 35% of global pharmaceutical growth through 2025, with bioavailability-enhanced formulations representing a key segment within this expansion.
Contract development and manufacturing organizations (CDMOs) have responded to this market demand by expanding their spray drying capabilities, with major players investing heavily in specialized equipment and expertise. This service sector growth indicates the strong industrial commitment to addressing bioavailability challenges through advanced processing technologies like spray drying.
Current Challenges in Spray Drying Pharmaceutical Applications
Despite the widespread adoption of spray drying in pharmaceutical manufacturing, several significant challenges continue to impede its optimal application for enhancing bioavailability. The primary technical hurdle remains the precise control of particle characteristics during the drying process. Variations in particle size distribution, morphology, and surface properties can dramatically affect dissolution rates and ultimately bioavailability, yet achieving consistent results across production batches remains difficult.
Thermal degradation presents another substantial challenge, particularly for heat-sensitive active pharmaceutical ingredients (APIs). The exposure to elevated temperatures during spray drying can trigger chemical decomposition or structural alterations that compromise therapeutic efficacy. While lower outlet temperatures might mitigate this risk, they often result in higher residual moisture content, creating stability concerns during storage.
Scale-up difficulties constitute a persistent obstacle in transitioning from laboratory development to commercial production. Parameters optimized at small scale frequently fail to yield identical results when implemented in industrial settings, necessitating extensive recalibration and validation. This challenge is exacerbated by the complex interplay between equipment design, process parameters, and formulation characteristics.
Amorphous state stability represents a double-edged sword in spray-dried pharmaceuticals. While the amorphous form often enhances dissolution and bioavailability, its inherent thermodynamic instability poses long-term storage challenges. Recrystallization during shelf life can negate the bioavailability advantages initially achieved, yet developing effective stabilization strategies remains challenging.
Excipient compatibility issues further complicate spray drying applications. The selection of appropriate carriers, stabilizers, and other functional excipients must balance processability requirements with their impact on the final product's bioavailability profile. Interactions between excipients and APIs during the drying process can sometimes lead to unexpected alterations in dissolution behavior or chemical stability.
Regulatory hurdles present non-technical but equally significant challenges. Demonstrating consistent quality attributes and establishing robust in-vitro/in-vivo correlations for spray-dried formulations often requires extensive documentation and validation studies. Regulatory agencies increasingly demand comprehensive understanding of process-property relationships, which necessitates sophisticated analytical approaches and predictive modeling capabilities.
Environmental and sustainability concerns are emerging as important considerations. The energy-intensive nature of spray drying operations and the frequent use of organic solvents raise questions about environmental impact and worker safety. Developing greener alternatives while maintaining product performance represents an ongoing challenge for pharmaceutical manufacturers.
Thermal degradation presents another substantial challenge, particularly for heat-sensitive active pharmaceutical ingredients (APIs). The exposure to elevated temperatures during spray drying can trigger chemical decomposition or structural alterations that compromise therapeutic efficacy. While lower outlet temperatures might mitigate this risk, they often result in higher residual moisture content, creating stability concerns during storage.
Scale-up difficulties constitute a persistent obstacle in transitioning from laboratory development to commercial production. Parameters optimized at small scale frequently fail to yield identical results when implemented in industrial settings, necessitating extensive recalibration and validation. This challenge is exacerbated by the complex interplay between equipment design, process parameters, and formulation characteristics.
Amorphous state stability represents a double-edged sword in spray-dried pharmaceuticals. While the amorphous form often enhances dissolution and bioavailability, its inherent thermodynamic instability poses long-term storage challenges. Recrystallization during shelf life can negate the bioavailability advantages initially achieved, yet developing effective stabilization strategies remains challenging.
Excipient compatibility issues further complicate spray drying applications. The selection of appropriate carriers, stabilizers, and other functional excipients must balance processability requirements with their impact on the final product's bioavailability profile. Interactions between excipients and APIs during the drying process can sometimes lead to unexpected alterations in dissolution behavior or chemical stability.
Regulatory hurdles present non-technical but equally significant challenges. Demonstrating consistent quality attributes and establishing robust in-vitro/in-vivo correlations for spray-dried formulations often requires extensive documentation and validation studies. Regulatory agencies increasingly demand comprehensive understanding of process-property relationships, which necessitates sophisticated analytical approaches and predictive modeling capabilities.
Environmental and sustainability concerns are emerging as important considerations. The energy-intensive nature of spray drying operations and the frequent use of organic solvents raise questions about environmental impact and worker safety. Developing greener alternatives while maintaining product performance represents an ongoing challenge for pharmaceutical manufacturers.
Established Spray Drying Techniques for Bioavailability Enhancement
01 Spray drying techniques for enhancing bioavailability of pharmaceuticals
Spray drying technology can significantly enhance the bioavailability of pharmaceutical compounds by creating amorphous solid dispersions. This process involves atomizing a solution containing the active ingredient and carrier materials, then rapidly drying the droplets to form particles with improved dissolution properties. The resulting amorphous state of the drug increases solubility and dissolution rate, leading to enhanced bioavailability, especially for poorly water-soluble compounds.- Spray drying techniques for enhancing bioavailability of pharmaceuticals: Spray drying can significantly improve the bioavailability of pharmaceutical compounds by creating amorphous solid dispersions. This process reduces particle size and increases surface area, leading to enhanced dissolution rates and improved absorption in the gastrointestinal tract. The technique is particularly valuable for poorly water-soluble drugs, as it can transform crystalline structures into more soluble amorphous forms.
- Carrier selection and formulation optimization for spray-dried products: The selection of appropriate carriers and excipients plays a crucial role in optimizing spray-dried formulations for enhanced bioavailability. Carriers such as cyclodextrins, polymers, and surfactants can improve solubility and stability of the active ingredients. Formulation parameters including carrier-to-drug ratio, solvent selection, and additive incorporation can be optimized to achieve desired physicochemical properties and maximize bioavailability of the final product.
- Process parameters affecting bioavailability in spray drying: Critical process parameters in spray drying significantly impact the bioavailability of the final product. These parameters include inlet/outlet temperature, atomization pressure, feed concentration, and drying gas flow rate. Optimizing these variables can control particle morphology, size distribution, residual moisture content, and amorphous character of the spray-dried material, all of which directly influence dissolution rate and bioavailability of the active ingredients.
- Novel spray drying technologies for bioavailability enhancement: Advanced spray drying technologies have been developed specifically to address bioavailability challenges. These include nano spray drying, spray freeze drying, and co-processing techniques that can produce particles with tailored characteristics. Such innovations enable the creation of nanoparticles, porous structures, and composite materials that demonstrate superior dissolution profiles and enhanced bioavailability compared to conventional formulations.
- Stability and characterization of spray-dried formulations for bioavailability: The stability of spray-dried formulations is essential for maintaining enhanced bioavailability throughout the product lifecycle. Characterization techniques such as differential scanning calorimetry, X-ray diffraction, and spectroscopic methods are employed to assess the physical state, crystallinity, and molecular interactions within spray-dried products. Stabilization strategies including the use of specific polymers, antioxidants, and controlled storage conditions help preserve the amorphous state and prevent recrystallization, thereby maintaining improved bioavailability over time.
02 Excipient selection for spray-dried formulations
The choice of excipients plays a crucial role in spray-dried formulations for bioavailability enhancement. Polymers such as hydroxypropyl methylcellulose (HPMC), polyvinylpyrrolidone (PVP), and cyclodextrins can stabilize the amorphous state of drugs and prevent recrystallization. Surfactants may be incorporated to improve wettability and dissolution. The optimal selection and ratio of these excipients can significantly impact the physical stability and dissolution performance of the final spray-dried product.Expand Specific Solutions03 Process parameters optimization for spray drying
Critical process parameters in spray drying significantly affect the bioavailability of the final product. These parameters include inlet/outlet temperature, atomization pressure, feed concentration, feed rate, and drying gas flow rate. Optimizing these variables can control particle size, morphology, residual moisture content, and degree of crystallinity, all of which influence dissolution rate and bioavailability. Advanced process analytical technology can be used to monitor and adjust these parameters in real-time to achieve consistent product quality.Expand Specific Solutions04 Spray drying of biologics and sensitive compounds
Spray drying offers advantages for improving the bioavailability of biologics and thermally sensitive compounds. The process can be modified with lower temperatures, specialized atomization techniques, and protective excipients to preserve the activity of proteins, peptides, and enzymes. This approach creates stable, dry powder formulations with enhanced shelf-life and bioavailability compared to liquid formulations. The technique is particularly valuable for pulmonary delivery systems where local and systemic bioavailability can be optimized through particle engineering.Expand Specific Solutions05 Scale-up and industrial applications of spray drying for bioavailability enhancement
Scaling up spray drying processes from laboratory to industrial production presents challenges in maintaining consistent bioavailability enhancement. Considerations include equipment design differences, heat and mass transfer variations, and residence time distributions. Quality by Design (QbD) approaches help identify critical quality attributes and process parameters that affect bioavailability. Continuous manufacturing systems with integrated spray drying can improve efficiency and consistency in producing high-bioavailability formulations at commercial scale.Expand Specific Solutions
Leading Companies and Research Institutions in Spray Drying
The pharmaceutical spray drying market is currently in a growth phase, with increasing adoption across major pharmaceutical companies due to its ability to enhance bioavailability of poorly soluble drugs. The global market size for pharmaceutical spray drying technology is expanding rapidly, driven by the growing pipeline of complex drug formulations. Technologically, the field shows varying maturity levels, with companies like Vertex Pharmaceuticals, Novartis AG, and Boehringer Ingelheim leading innovation through advanced applications in bioavailability enhancement. Mid-tier players including Hovione Scientia and Synthon BV are developing specialized expertise, while emerging companies like Bioinicia SL are introducing novel approaches. Research institutions such as Ghent University and University of Washington are contributing fundamental advancements, creating a competitive landscape where collaboration between industry and academia is accelerating technological maturity in pharmaceutical spray drying applications.
Boehringer Ingelheim Pharma GmbH & Co., KG
Technical Solution: Boehringer Ingelheim has developed a comprehensive spray drying platform focused on enhancing bioavailability of poorly soluble compounds. Their technology employs a systematic approach to amorphous solid dispersion (ASD) development, utilizing a proprietary polymer selection framework that matches carrier properties to specific API characteristics. Boehringer's spray drying process incorporates advanced atomization systems with adjustable parameters that can be optimized for different formulation types. Their technology features sophisticated feed preparation systems that ensure complete API dissolution and homogeneity prior to atomization, critical for consistent amorphous dispersion formation. Boehringer has developed specialized in-line process analytical technology (PAT) tools that monitor critical quality attributes during processing, enabling real-time adjustments to maintain optimal conditions. Their approach includes integrated stability prediction models that assess the long-term performance of spray-dried dispersions under various storage conditions. Additionally, Boehringer has pioneered scale-up methodologies that maintain consistent product quality from development to commercial manufacturing scales[9][10].
Strengths: Extensive experience in translating spray-dried formulations from development to commercial manufacturing with robust quality systems. Their integrated approach combines formulation expertise with process engineering capabilities. Weaknesses: Conservative approach may limit application to more challenging compounds, and relatively higher resource requirements for development compared to simpler formulation approaches.
Hovione Scientia Ltd.
Technical Solution: Hovione has developed advanced spray drying technologies specifically designed to enhance pharmaceutical bioavailability of poorly water-soluble drugs. Their approach centers on amorphous solid dispersion (ASD) technology, where the active pharmaceutical ingredient (API) is molecularly dispersed within a polymer matrix. This process converts crystalline drug structures into amorphous forms with significantly higher dissolution rates. Hovione's proprietary spray drying process utilizes specialized atomization nozzles that create uniform droplet sizes, ensuring consistent particle morphology and size distribution. Their technology incorporates real-time process analytical technology (PAT) monitoring systems that adjust critical process parameters during operation to maintain optimal conditions for amorphous state formation. Additionally, Hovione has developed specialized polymer selection algorithms that match carrier polymers to specific API properties, maximizing physical stability and dissolution enhancement[1][2].
Strengths: Industry-leading expertise in amorphous solid dispersions with demonstrated commercial scale capabilities. Their integrated approach from formulation to manufacturing provides end-to-end solutions. Weaknesses: Higher production costs compared to conventional manufacturing methods, and potential stability challenges with certain highly crystalline compounds requiring specialized storage conditions.
Critical Patents and Innovations in Pharmaceutical Spray Drying
Pharmaceutical compositions
PatentInactiveUS20090247468A1
Innovation
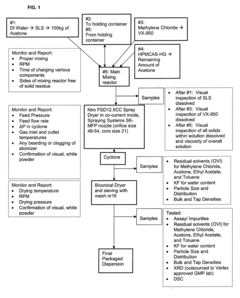
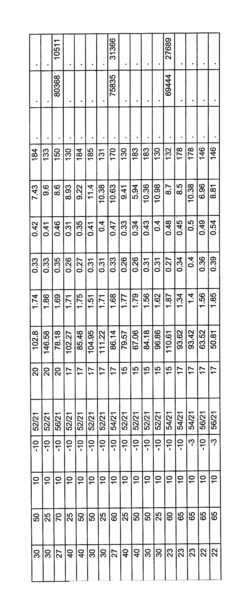
- The use of spray drying with a solvent mixture that includes a non-volatile or high boiling solvent to produce amorphous solid dispersions of drugs, such as VX-950, which enhances particle size, density, and flowability, and includes polymers like HPMC and HPMCAS to improve solubility and stability.
Fluidized spray drying
PatentWO2008080167A2
Innovation
- Fluidized spray drying (FSD) process that involves atomizing a feed solution containing VX-950 and a polymer like HPMCAS in a solvent, allowing for the separation of larger particles and reintroduction of fines to form agglomerates, resulting in a product with reduced fines, improved flowability, and increased stability, enabling direct compression into oral dosage forms.
Regulatory Considerations for Spray-Dried Pharmaceuticals
Regulatory frameworks governing spray-dried pharmaceuticals have evolved significantly to address the unique characteristics and challenges associated with this manufacturing technique. The FDA, EMA, and other global regulatory bodies have established specific guidelines for spray-dried formulations, recognizing their distinct impact on bioavailability and drug performance. These regulations typically focus on process validation, quality control parameters, and stability testing protocols tailored to spray-dried products.
Manufacturers must demonstrate robust control over critical process parameters including inlet/outlet temperatures, feed rate, atomization pressure, and drying gas flow rate. These parameters directly influence particle morphology, residual moisture content, and ultimately drug bioavailability. Regulatory submissions require comprehensive documentation of the relationship between these process parameters and final product characteristics through Quality by Design (QbD) approaches.
Stability testing requirements for spray-dried pharmaceuticals often exceed standard protocols, with particular emphasis on monitoring physical stability, amorphous content retention, and potential recrystallization phenomena. Accelerated stability studies under various humidity conditions are typically mandated to ensure the maintenance of enhanced bioavailability throughout the product lifecycle.
Scale-up considerations present unique regulatory challenges for spray-dried pharmaceuticals. Authorities require manufacturers to establish clear correlations between laboratory, pilot, and commercial-scale production, with particular focus on maintaining consistent particle characteristics and dissolution profiles across scales. Process Analytical Technology (PAT) implementation is increasingly expected to demonstrate real-time quality assurance.
Pharmacopoeial standards have been adapted to address the specific properties of spray-dried formulations, including specialized dissolution testing methodologies that account for rapid dissolution kinetics and supersaturation phenomena. Regulatory bodies increasingly require biorelevant dissolution testing that better predicts in vivo performance of spray-dried formulations.
International harmonization efforts are underway to standardize regulatory approaches to spray-dried pharmaceuticals, though significant regional variations persist. The ICH has incorporated considerations for spray-dried products within broader quality guidelines, while industry consortia are developing standardized characterization methods specifically for spray-dried systems to facilitate regulatory acceptance.
Emerging regulatory trends include increased scrutiny of excipient selection for spray-dried formulations, particularly regarding their functional roles in maintaining supersaturation and inhibiting precipitation in vivo. Additionally, regulatory expectations are evolving toward more comprehensive biopharmaceutical characterization, including advanced imaging techniques and molecular-level analysis of drug-excipient interactions in the solid state.
Manufacturers must demonstrate robust control over critical process parameters including inlet/outlet temperatures, feed rate, atomization pressure, and drying gas flow rate. These parameters directly influence particle morphology, residual moisture content, and ultimately drug bioavailability. Regulatory submissions require comprehensive documentation of the relationship between these process parameters and final product characteristics through Quality by Design (QbD) approaches.
Stability testing requirements for spray-dried pharmaceuticals often exceed standard protocols, with particular emphasis on monitoring physical stability, amorphous content retention, and potential recrystallization phenomena. Accelerated stability studies under various humidity conditions are typically mandated to ensure the maintenance of enhanced bioavailability throughout the product lifecycle.
Scale-up considerations present unique regulatory challenges for spray-dried pharmaceuticals. Authorities require manufacturers to establish clear correlations between laboratory, pilot, and commercial-scale production, with particular focus on maintaining consistent particle characteristics and dissolution profiles across scales. Process Analytical Technology (PAT) implementation is increasingly expected to demonstrate real-time quality assurance.
Pharmacopoeial standards have been adapted to address the specific properties of spray-dried formulations, including specialized dissolution testing methodologies that account for rapid dissolution kinetics and supersaturation phenomena. Regulatory bodies increasingly require biorelevant dissolution testing that better predicts in vivo performance of spray-dried formulations.
International harmonization efforts are underway to standardize regulatory approaches to spray-dried pharmaceuticals, though significant regional variations persist. The ICH has incorporated considerations for spray-dried products within broader quality guidelines, while industry consortia are developing standardized characterization methods specifically for spray-dried systems to facilitate regulatory acceptance.
Emerging regulatory trends include increased scrutiny of excipient selection for spray-dried formulations, particularly regarding their functional roles in maintaining supersaturation and inhibiting precipitation in vivo. Additionally, regulatory expectations are evolving toward more comprehensive biopharmaceutical characterization, including advanced imaging techniques and molecular-level analysis of drug-excipient interactions in the solid state.
Scale-up Challenges and Manufacturing Optimization
Scaling up spray drying processes from laboratory to commercial production presents significant challenges that can impact pharmaceutical bioavailability. The transition requires careful consideration of equipment differences, process parameters, and quality control measures. Laboratory-scale spray dryers typically operate with feed rates of 1-100 mL/min, while commercial units handle 10-1000 L/hr, necessitating substantial parameter adjustments that can alter particle characteristics and dissolution profiles.
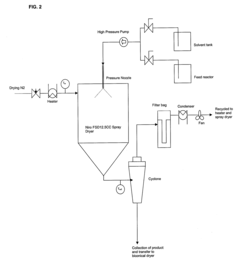
Heat and mass transfer dynamics change dramatically with scale, affecting drying kinetics and potentially modifying the amorphous state or crystallinity of the final product. These modifications directly influence bioavailability, as changes in solid-state properties can alter dissolution rates and drug release profiles. Studies have shown that scale-up can lead to 15-30% variations in particle size distribution, potentially compromising therapeutic efficacy.
Process parameter optimization becomes critical during scale-up. Inlet temperature, atomization pressure, feed concentration, and feed rate must be systematically adjusted to maintain consistent product quality. Quality by Design (QbD) approaches have emerged as essential methodologies, employing Design of Experiments (DoE) to establish robust design spaces that ensure bioavailability remains within acceptable ranges despite scale changes.
Manufacturing optimization strategies include implementing Process Analytical Technology (PAT) tools for real-time monitoring of critical quality attributes. Near-infrared spectroscopy and Raman spectroscopy enable continuous assessment of moisture content and solid-state characteristics during production, allowing for immediate process adjustments to maintain bioavailability specifications. These technologies have demonstrated capability to reduce batch-to-batch variability by up to 40%.
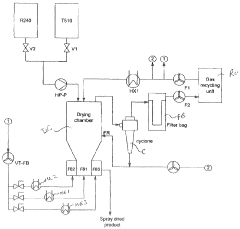
Computational fluid dynamics (CFD) modeling has revolutionized scale-up approaches by simulating drying chamber conditions across different equipment sizes. These models predict particle trajectories, temperature profiles, and residence times, enabling manufacturers to anticipate bioavailability challenges before physical implementation. Recent advances in CFD modeling have improved prediction accuracy to within 10% of experimental results for key parameters affecting bioavailability.
Equipment design considerations also play a crucial role in successful scale-up. Modern spray dryers incorporate features like multi-nozzle configurations, optimized chamber geometries, and advanced cyclone separators to maintain consistent particle formation conditions. These design elements help preserve the intended solid dispersion characteristics that enhance bioavailability, particularly for BCS Class II and IV compounds where solubility improvements are critical.
Regulatory considerations add another layer of complexity to scale-up operations. Manufacturers must demonstrate that bioavailability remains consistent throughout scale-up through comparative dissolution studies, in vitro-in vivo correlation (IVIVC) models, and sometimes bioequivalence studies. The FDA and EMA increasingly expect robust scale-up strategies with clearly defined control strategies to ensure therapeutic performance remains unchanged.
Heat and mass transfer dynamics change dramatically with scale, affecting drying kinetics and potentially modifying the amorphous state or crystallinity of the final product. These modifications directly influence bioavailability, as changes in solid-state properties can alter dissolution rates and drug release profiles. Studies have shown that scale-up can lead to 15-30% variations in particle size distribution, potentially compromising therapeutic efficacy.
Process parameter optimization becomes critical during scale-up. Inlet temperature, atomization pressure, feed concentration, and feed rate must be systematically adjusted to maintain consistent product quality. Quality by Design (QbD) approaches have emerged as essential methodologies, employing Design of Experiments (DoE) to establish robust design spaces that ensure bioavailability remains within acceptable ranges despite scale changes.
Manufacturing optimization strategies include implementing Process Analytical Technology (PAT) tools for real-time monitoring of critical quality attributes. Near-infrared spectroscopy and Raman spectroscopy enable continuous assessment of moisture content and solid-state characteristics during production, allowing for immediate process adjustments to maintain bioavailability specifications. These technologies have demonstrated capability to reduce batch-to-batch variability by up to 40%.
Computational fluid dynamics (CFD) modeling has revolutionized scale-up approaches by simulating drying chamber conditions across different equipment sizes. These models predict particle trajectories, temperature profiles, and residence times, enabling manufacturers to anticipate bioavailability challenges before physical implementation. Recent advances in CFD modeling have improved prediction accuracy to within 10% of experimental results for key parameters affecting bioavailability.
Equipment design considerations also play a crucial role in successful scale-up. Modern spray dryers incorporate features like multi-nozzle configurations, optimized chamber geometries, and advanced cyclone separators to maintain consistent particle formation conditions. These design elements help preserve the intended solid dispersion characteristics that enhance bioavailability, particularly for BCS Class II and IV compounds where solubility improvements are critical.
Regulatory considerations add another layer of complexity to scale-up operations. Manufacturers must demonstrate that bioavailability remains consistent throughout scale-up through comparative dissolution studies, in vitro-in vivo correlation (IVIVC) models, and sometimes bioequivalence studies. The FDA and EMA increasingly expect robust scale-up strategies with clearly defined control strategies to ensure therapeutic performance remains unchanged.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!