How Metal-Ligand Interactions Govern Tautomerization?
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal-Ligand Tautomerization Background
Metal-ligand interactions play a crucial role in governing tautomerization processes, which are fundamental to various chemical and biological systems. Tautomerization refers to the rapid interconversion between structural isomers, known as tautomers, that differ in the position of a proton and a π bond. This phenomenon is particularly significant in the context of metal-ligand complexes, where the presence of metal ions can significantly influence the tautomeric equilibrium.
The study of metal-ligand tautomerization has its roots in coordination chemistry and has evolved over the past several decades. Early investigations focused on simple organic ligands and their interactions with transition metals. As research progressed, the scope expanded to include more complex systems, such as biomolecules and synthetic catalysts, where tautomerization plays a critical role in function and reactivity.
The importance of metal-ligand tautomerization extends across multiple disciplines, including organic synthesis, catalysis, materials science, and biochemistry. In organic synthesis, understanding and controlling tautomerization can lead to more efficient and selective reactions. Catalytic processes often rely on the precise control of tautomeric forms to achieve desired transformations. In materials science, tautomerization can influence the electronic and optical properties of metal-organic frameworks and other functional materials.
From a biochemical perspective, metal-ligand tautomerization is essential in many enzymatic reactions and protein-ligand interactions. For example, the activity of metalloenzymes often depends on the specific tautomeric form of the bound substrate or cofactor. Additionally, tautomerization can affect the binding affinity and specificity of drugs to their target proteins, making it a crucial consideration in drug design and development.
The technological advancements in spectroscopic and computational methods have significantly enhanced our ability to study metal-ligand tautomerization. High-resolution NMR spectroscopy, X-ray crystallography, and advanced mass spectrometry techniques now allow researchers to observe and characterize tautomeric species with unprecedented detail. Complementing these experimental approaches, quantum mechanical calculations and molecular dynamics simulations provide valuable insights into the energetics and mechanisms of tautomerization processes.
As research in this field continues to evolve, several key objectives have emerged. These include developing predictive models for tautomeric preferences in metal-ligand systems, designing novel ligands that can control tautomerization through metal coordination, and exploiting metal-ligand tautomerization for the creation of responsive materials and smart catalysts. Furthermore, there is a growing interest in understanding how metal-ligand tautomerization contributes to the function of biological systems and how this knowledge can be applied to the development of new therapeutic strategies.
The study of metal-ligand tautomerization has its roots in coordination chemistry and has evolved over the past several decades. Early investigations focused on simple organic ligands and their interactions with transition metals. As research progressed, the scope expanded to include more complex systems, such as biomolecules and synthetic catalysts, where tautomerization plays a critical role in function and reactivity.
The importance of metal-ligand tautomerization extends across multiple disciplines, including organic synthesis, catalysis, materials science, and biochemistry. In organic synthesis, understanding and controlling tautomerization can lead to more efficient and selective reactions. Catalytic processes often rely on the precise control of tautomeric forms to achieve desired transformations. In materials science, tautomerization can influence the electronic and optical properties of metal-organic frameworks and other functional materials.
From a biochemical perspective, metal-ligand tautomerization is essential in many enzymatic reactions and protein-ligand interactions. For example, the activity of metalloenzymes often depends on the specific tautomeric form of the bound substrate or cofactor. Additionally, tautomerization can affect the binding affinity and specificity of drugs to their target proteins, making it a crucial consideration in drug design and development.
The technological advancements in spectroscopic and computational methods have significantly enhanced our ability to study metal-ligand tautomerization. High-resolution NMR spectroscopy, X-ray crystallography, and advanced mass spectrometry techniques now allow researchers to observe and characterize tautomeric species with unprecedented detail. Complementing these experimental approaches, quantum mechanical calculations and molecular dynamics simulations provide valuable insights into the energetics and mechanisms of tautomerization processes.
As research in this field continues to evolve, several key objectives have emerged. These include developing predictive models for tautomeric preferences in metal-ligand systems, designing novel ligands that can control tautomerization through metal coordination, and exploiting metal-ligand tautomerization for the creation of responsive materials and smart catalysts. Furthermore, there is a growing interest in understanding how metal-ligand tautomerization contributes to the function of biological systems and how this knowledge can be applied to the development of new therapeutic strategies.
Market Applications of Tautomerization
Tautomerization, a process involving the interconversion of structural isomers, has found significant applications across various industries. In the pharmaceutical sector, tautomerization plays a crucial role in drug design and development. Many drugs exhibit tautomeric forms, which can affect their solubility, bioavailability, and binding affinity to target molecules. Understanding and controlling tautomerization can lead to more effective drug formulations and improved therapeutic outcomes.
The agrochemical industry also benefits from tautomerization research. Pesticides and herbicides often rely on tautomeric equilibria to enhance their efficacy and environmental stability. By manipulating tautomeric forms, agrochemical companies can develop products with improved pest control properties while minimizing environmental impact.
In the field of materials science, tautomerization has emerged as a valuable tool for creating smart materials with switchable properties. Researchers are exploring tautomeric systems for applications in sensors, optical switches, and data storage devices. These materials can change their physical or chemical properties in response to external stimuli, such as light, temperature, or pH, making them ideal for various technological applications.
The food and beverage industry has also found applications for tautomerization. Certain food additives and flavor compounds undergo tautomeric shifts, which can affect their taste, aroma, and stability. Understanding these processes allows for better control of food quality and the development of novel flavor profiles.
In the energy sector, tautomerization is being investigated for potential applications in hydrogen storage and fuel cell technologies. Certain tautomeric systems can reversibly bind and release hydrogen, offering promising avenues for clean energy storage and conversion.
The cosmetics industry is exploring tautomerization for the development of long-lasting and color-changing makeup products. Tautomeric pigments can shift their molecular structure in response to environmental factors, leading to dynamic color changes and improved product performance.
As research in metal-ligand interactions and their influence on tautomerization advances, new market applications are likely to emerge. The ability to fine-tune tautomeric equilibria through metal coordination opens up possibilities for creating responsive materials, catalysts, and molecular machines with unprecedented properties and functionalities.
The agrochemical industry also benefits from tautomerization research. Pesticides and herbicides often rely on tautomeric equilibria to enhance their efficacy and environmental stability. By manipulating tautomeric forms, agrochemical companies can develop products with improved pest control properties while minimizing environmental impact.
In the field of materials science, tautomerization has emerged as a valuable tool for creating smart materials with switchable properties. Researchers are exploring tautomeric systems for applications in sensors, optical switches, and data storage devices. These materials can change their physical or chemical properties in response to external stimuli, such as light, temperature, or pH, making them ideal for various technological applications.
The food and beverage industry has also found applications for tautomerization. Certain food additives and flavor compounds undergo tautomeric shifts, which can affect their taste, aroma, and stability. Understanding these processes allows for better control of food quality and the development of novel flavor profiles.
In the energy sector, tautomerization is being investigated for potential applications in hydrogen storage and fuel cell technologies. Certain tautomeric systems can reversibly bind and release hydrogen, offering promising avenues for clean energy storage and conversion.
The cosmetics industry is exploring tautomerization for the development of long-lasting and color-changing makeup products. Tautomeric pigments can shift their molecular structure in response to environmental factors, leading to dynamic color changes and improved product performance.
As research in metal-ligand interactions and their influence on tautomerization advances, new market applications are likely to emerge. The ability to fine-tune tautomeric equilibria through metal coordination opens up possibilities for creating responsive materials, catalysts, and molecular machines with unprecedented properties and functionalities.
Current Challenges in Metal-Ligand Interactions
The field of metal-ligand interactions in tautomerization faces several significant challenges that hinder our complete understanding and control of these processes. One of the primary obstacles is the complexity of the electronic structures involved in metal-ligand systems. The interplay between metal centers and ligands creates intricate electronic configurations that are difficult to predict and model accurately.
Another major challenge lies in the dynamic nature of tautomerization processes. The rapid interconversion between tautomeric forms often occurs on timescales that are challenging to capture experimentally. This temporal complexity makes it difficult to isolate and study individual tautomeric species, limiting our ability to fully characterize the role of metal-ligand interactions in these transformations.
The influence of environmental factors on metal-ligand interactions and tautomerization presents another significant hurdle. Factors such as solvent effects, pH, and temperature can dramatically alter the behavior of these systems. Developing a comprehensive understanding of how these external variables modulate metal-ligand interactions and, consequently, tautomerization processes remains a formidable task.
Furthermore, the multifaceted nature of metal-ligand interactions poses challenges in isolating and quantifying individual contributions. The interplay between various types of interactions, such as coordination bonds, π-backbonding, and electrostatic forces, creates a complex landscape that is difficult to deconvolute. This complexity makes it challenging to establish clear structure-property relationships and predict tautomerization behavior accurately.
The development of suitable analytical techniques for studying metal-ligand interactions in tautomerization processes also remains a significant challenge. While advanced spectroscopic and computational methods have made significant strides, there is still a need for tools that can provide real-time, in situ observations of these dynamic processes at the molecular level.
Lastly, bridging the gap between fundamental research and practical applications presents ongoing challenges. Translating the insights gained from studying metal-ligand interactions in tautomerization into useful applications, such as catalyst design or drug development, requires overcoming numerous obstacles related to scalability, stability, and specificity.
Another major challenge lies in the dynamic nature of tautomerization processes. The rapid interconversion between tautomeric forms often occurs on timescales that are challenging to capture experimentally. This temporal complexity makes it difficult to isolate and study individual tautomeric species, limiting our ability to fully characterize the role of metal-ligand interactions in these transformations.
The influence of environmental factors on metal-ligand interactions and tautomerization presents another significant hurdle. Factors such as solvent effects, pH, and temperature can dramatically alter the behavior of these systems. Developing a comprehensive understanding of how these external variables modulate metal-ligand interactions and, consequently, tautomerization processes remains a formidable task.
Furthermore, the multifaceted nature of metal-ligand interactions poses challenges in isolating and quantifying individual contributions. The interplay between various types of interactions, such as coordination bonds, π-backbonding, and electrostatic forces, creates a complex landscape that is difficult to deconvolute. This complexity makes it challenging to establish clear structure-property relationships and predict tautomerization behavior accurately.
The development of suitable analytical techniques for studying metal-ligand interactions in tautomerization processes also remains a significant challenge. While advanced spectroscopic and computational methods have made significant strides, there is still a need for tools that can provide real-time, in situ observations of these dynamic processes at the molecular level.
Lastly, bridging the gap between fundamental research and practical applications presents ongoing challenges. Translating the insights gained from studying metal-ligand interactions in tautomerization into useful applications, such as catalyst design or drug development, requires overcoming numerous obstacles related to scalability, stability, and specificity.
Existing Metal-Ligand Interaction Models
01 Computational methods for studying metal-ligand interactions
Advanced computational techniques are employed to analyze and predict metal-ligand interactions and tautomerization processes. These methods include molecular dynamics simulations, density functional theory calculations, and machine learning algorithms to model complex chemical systems and their behavior.- Computational methods for studying metal-ligand interactions: Advanced computational techniques are employed to analyze and predict metal-ligand interactions and tautomerization processes. These methods include quantum mechanical calculations, molecular dynamics simulations, and machine learning approaches to model complex chemical systems and their behavior.
- Tautomerization in metal complexes: The study of tautomerization in metal complexes involves investigating the dynamic equilibrium between different structural isomers. This process can significantly affect the properties and reactivity of metal-ligand systems, influencing their applications in catalysis, materials science, and biological systems.
- Spectroscopic analysis of metal-ligand interactions: Various spectroscopic techniques are utilized to investigate metal-ligand interactions and tautomerization phenomena. These may include NMR, UV-Vis, IR, and X-ray spectroscopy, providing valuable insights into the electronic and structural properties of metal complexes.
- Design of novel ligands for metal complexation: Research focuses on developing new ligands with specific properties to control metal-ligand interactions and tautomerization processes. This involves the synthesis and characterization of organic compounds capable of forming stable complexes with various metal ions, with applications in catalysis, sensing, and materials science.
- Applications of metal-ligand tautomerization: The understanding and control of metal-ligand interactions and tautomerization processes have led to various practical applications. These include the development of new catalysts, molecular switches, sensors, and functional materials with tunable properties based on the interconversion between tautomeric forms.
02 Tautomerization in metal-organic frameworks
Metal-organic frameworks (MOFs) exhibit tautomerization phenomena influenced by metal-ligand interactions. This affects their structural properties, catalytic activity, and potential applications in gas storage and separation. Research focuses on understanding and controlling tautomerization in MOFs for improved performance.Expand Specific Solutions03 Ligand design for controlled metal-ligand interactions
Development of novel ligands with specific structural and electronic properties to fine-tune metal-ligand interactions and influence tautomerization processes. This approach is crucial in creating new catalysts, sensors, and materials with tailored functionalities.Expand Specific Solutions04 Spectroscopic studies of metal-ligand tautomerization
Advanced spectroscopic techniques, including NMR, UV-Vis, and X-ray absorption spectroscopy, are utilized to investigate metal-ligand interactions and tautomerization dynamics in real-time. These methods provide insights into reaction mechanisms and structural changes at the molecular level.Expand Specific Solutions05 Applications of metal-ligand tautomerization in catalysis
Exploitation of metal-ligand tautomerization phenomena in the design of efficient catalysts for various chemical transformations. This includes the development of switchable catalysts that can be controlled through external stimuli, leading to improved selectivity and yield in chemical reactions.Expand Specific Solutions
Key Players in Coordination Chemistry
The field of metal-ligand interactions governing tautomerization is in a developing stage, with growing market potential and increasing technological maturity. Research institutions and universities, such as The Regents of the University of California, Carnegie Mellon University, and Wuhan University, are at the forefront of advancing this technology. Industry players like BASF Corp. and ExxonMobil Chemical Patents, Inc. are also contributing to the field's development. The market size is expanding as applications in pharmaceuticals, materials science, and chemical engineering emerge. While the technology is not yet fully mature, significant progress has been made in understanding and controlling tautomerization processes through metal-ligand interactions, indicating a promising future for both academic research and industrial applications.
The Regents of the University of California
Technical Solution: The University of California has developed advanced computational methods to study metal-ligand interactions in tautomerization processes. They utilize density functional theory (DFT) calculations to model the electronic structure of metal complexes and predict tautomeric equilibria[1]. Their approach combines ab initio molecular dynamics simulations with machine learning algorithms to explore the potential energy surfaces of tautomeric systems[3]. This allows for accurate prediction of tautomer stability and interconversion rates in the presence of various metal ions and ligands[5].
Strengths: Cutting-edge computational techniques, integration of machine learning. Weaknesses: High computational cost, potential limitations in modeling complex biological systems.
Carnegie Mellon University
Technical Solution: Carnegie Mellon University has pioneered the use of quantum mechanical/molecular mechanical (QM/MM) methods to investigate metal-ligand interactions in tautomerization. Their approach combines high-level quantum calculations for the metal center and immediate ligands with classical molecular mechanics for the surrounding environment[2]. This multi-scale modeling technique allows for accurate representation of electronic effects while accounting for the influence of the broader molecular context[4]. They have successfully applied this method to study tautomerization in metalloenzymes and organometallic catalysts[6].
Strengths: Balanced approach between accuracy and computational efficiency, applicable to large biomolecular systems. Weaknesses: Challenges in defining QM/MM boundaries, potential artifacts at the interface.
Core Innovations in Tautomerization Control
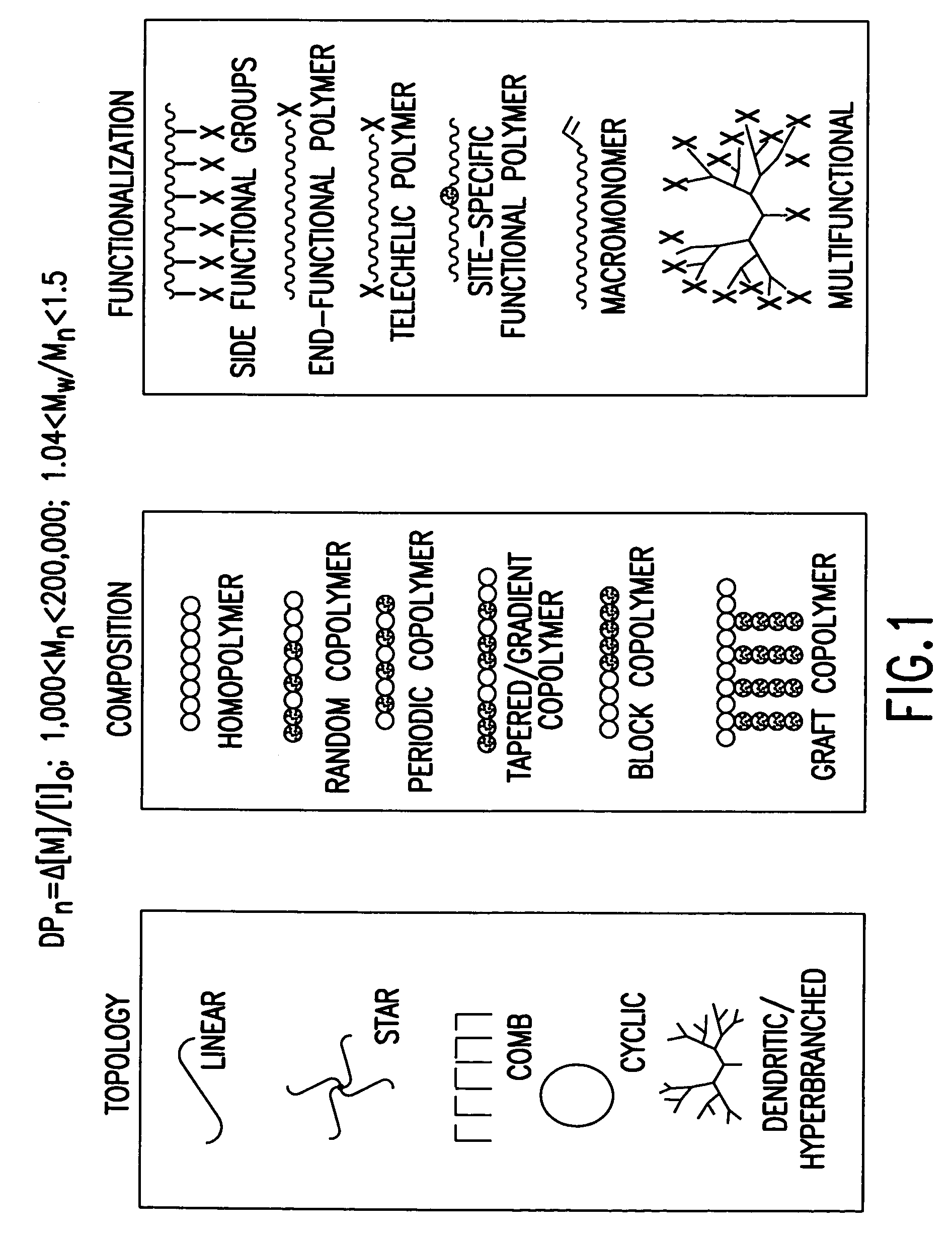
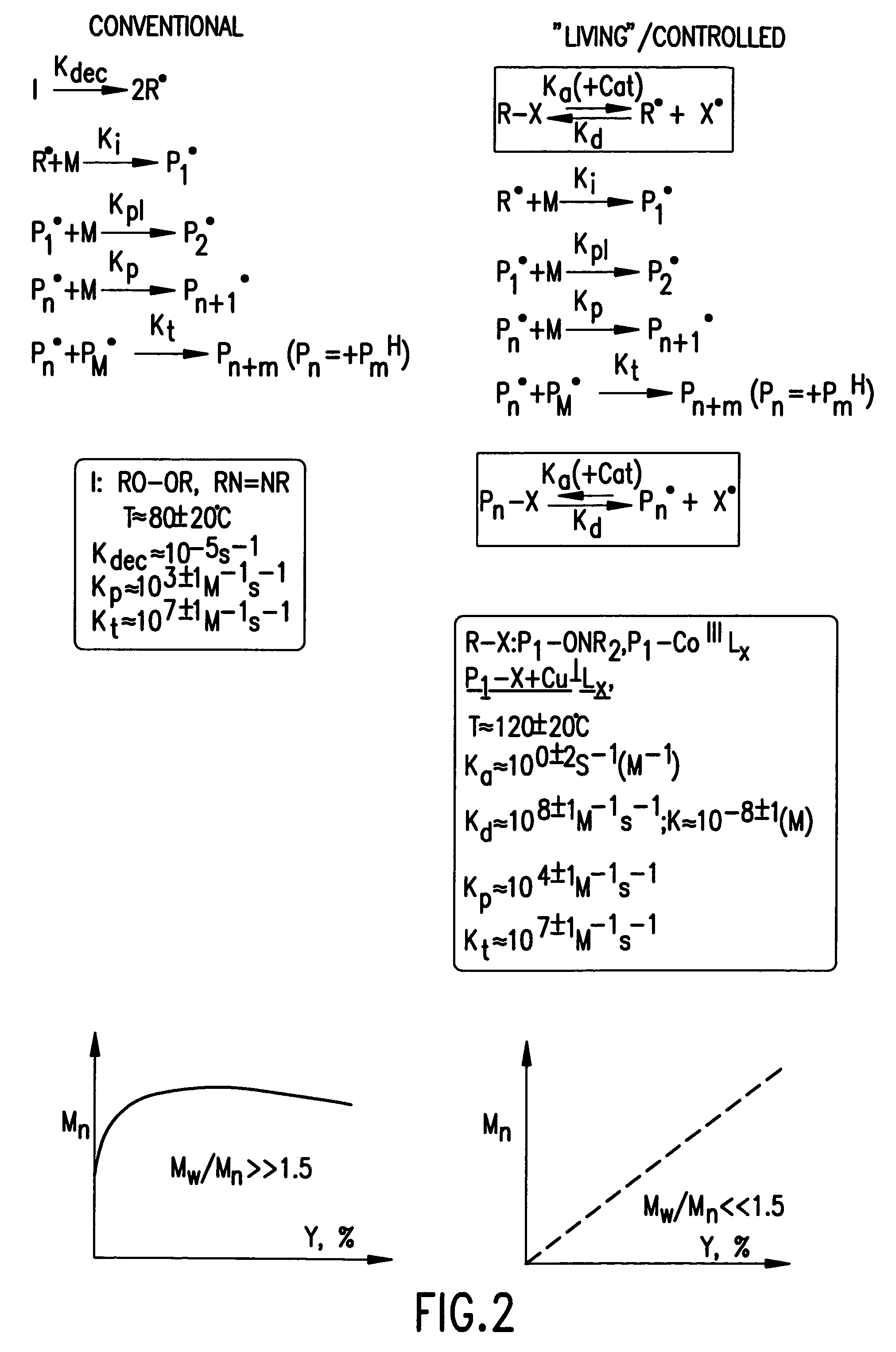
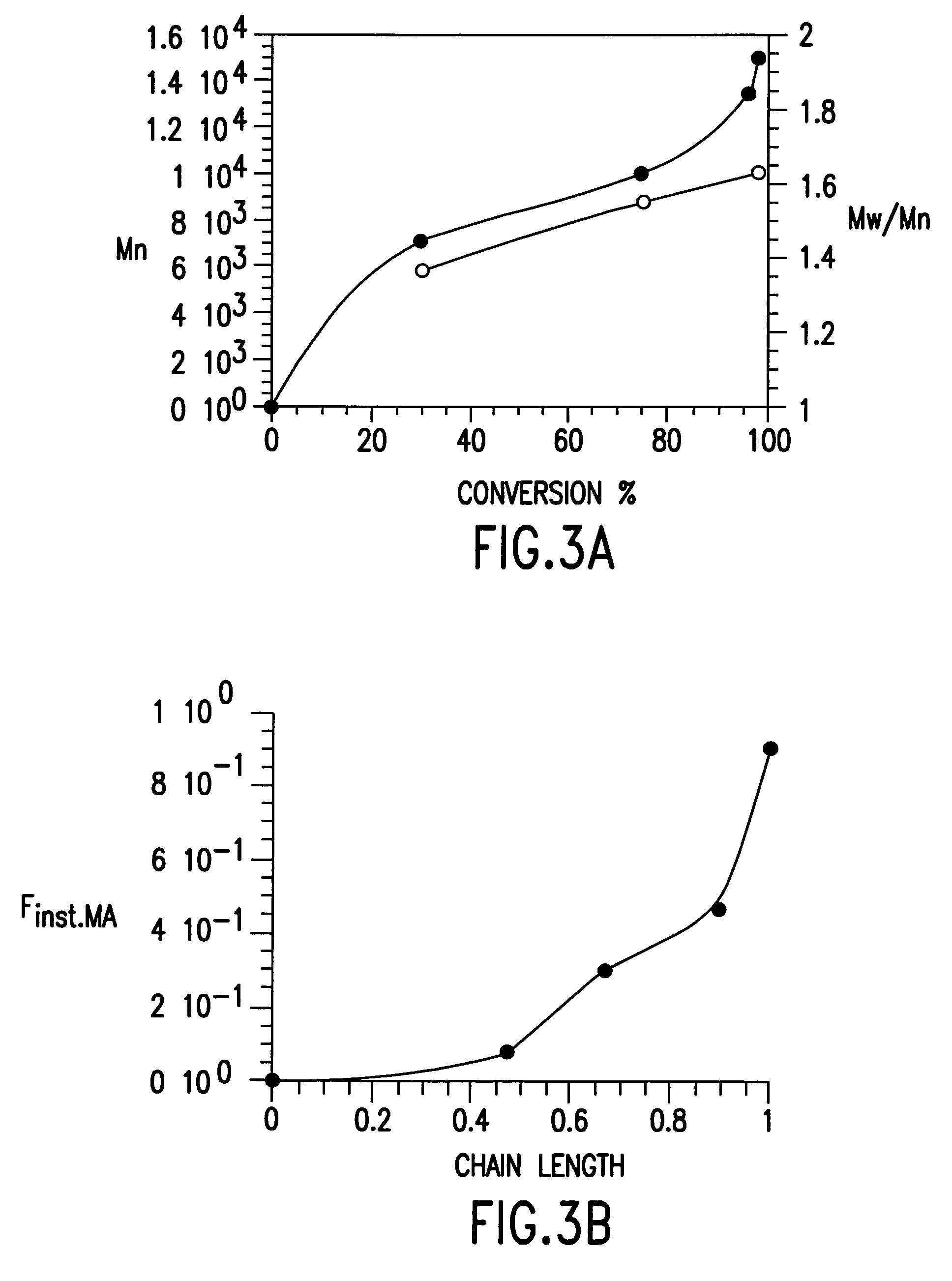
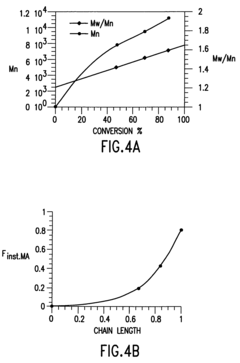
Processes based on atom (or group) transfer radical polymerization and novel (co)polymers having useful structures and properties
PatentInactiveUS7572874B2
Innovation
- The development of atom transfer radical polymerization (ATRP) using a transition metal compound in a reversible redox cycle with an initiator and a coordinating ligand to control the radical polymerization process, allowing for the production of polymers with predictable molecular weight and narrow polydispersity.
Computational Methods in Tautomerization
Computational methods have become indispensable tools in studying tautomerization processes, particularly in the context of metal-ligand interactions. These methods provide valuable insights into the energetics, kinetics, and mechanisms of tautomerization reactions, offering a complementary approach to experimental techniques.
Density Functional Theory (DFT) calculations are widely employed to investigate the electronic structure and energetics of tautomers and their metal complexes. DFT methods, such as B3LYP and M06-2X, coupled with appropriate basis sets, can accurately predict the relative stabilities of different tautomeric forms and their metal-bound counterparts. These calculations help elucidate how metal coordination affects the tautomeric equilibrium and the energetic barriers associated with tautomerization.
Ab initio molecular dynamics simulations offer a powerful means to explore the dynamic nature of tautomerization processes in metal-ligand systems. These simulations can capture the time-dependent behavior of tautomers, including the influence of solvent effects and temperature on the tautomerization rates and equilibria. By incorporating metal ions into these simulations, researchers can observe how metal-ligand interactions modulate the tautomerization landscape.
Quantum mechanical/molecular mechanical (QM/MM) methods provide a balanced approach to studying tautomerization in complex environments, such as enzymatic systems or heterogeneous catalysts. These hybrid methods allow for the accurate treatment of the tautomerizing moiety and its immediate metal coordination sphere using high-level quantum mechanical calculations, while the surrounding environment is modeled using more computationally efficient molecular mechanics.
Machine learning approaches are increasingly being applied to predict tautomerization propensities and to identify key structural features that influence metal-ligand interactions in tautomeric systems. These methods can rapidly screen large datasets of metal-ligand complexes to identify promising candidates for further experimental or computational investigation.
Transition state theory calculations, combined with electronic structure methods, enable the determination of activation energies and rate constants for tautomerization reactions in the presence of metal ions. These calculations provide crucial information about the kinetic aspects of metal-mediated tautomerization processes, helping to elucidate the catalytic role of metal centers in facilitating proton transfer reactions.
Density Functional Theory (DFT) calculations are widely employed to investigate the electronic structure and energetics of tautomers and their metal complexes. DFT methods, such as B3LYP and M06-2X, coupled with appropriate basis sets, can accurately predict the relative stabilities of different tautomeric forms and their metal-bound counterparts. These calculations help elucidate how metal coordination affects the tautomeric equilibrium and the energetic barriers associated with tautomerization.
Ab initio molecular dynamics simulations offer a powerful means to explore the dynamic nature of tautomerization processes in metal-ligand systems. These simulations can capture the time-dependent behavior of tautomers, including the influence of solvent effects and temperature on the tautomerization rates and equilibria. By incorporating metal ions into these simulations, researchers can observe how metal-ligand interactions modulate the tautomerization landscape.
Quantum mechanical/molecular mechanical (QM/MM) methods provide a balanced approach to studying tautomerization in complex environments, such as enzymatic systems or heterogeneous catalysts. These hybrid methods allow for the accurate treatment of the tautomerizing moiety and its immediate metal coordination sphere using high-level quantum mechanical calculations, while the surrounding environment is modeled using more computationally efficient molecular mechanics.
Machine learning approaches are increasingly being applied to predict tautomerization propensities and to identify key structural features that influence metal-ligand interactions in tautomeric systems. These methods can rapidly screen large datasets of metal-ligand complexes to identify promising candidates for further experimental or computational investigation.
Transition state theory calculations, combined with electronic structure methods, enable the determination of activation energies and rate constants for tautomerization reactions in the presence of metal ions. These calculations provide crucial information about the kinetic aspects of metal-mediated tautomerization processes, helping to elucidate the catalytic role of metal centers in facilitating proton transfer reactions.
Environmental Factors Affecting Tautomerization
Environmental factors play a crucial role in governing tautomerization processes, particularly in the context of metal-ligand interactions. Temperature is one of the most significant factors influencing tautomerization rates and equilibria. Higher temperatures generally accelerate tautomerization by providing the necessary energy to overcome activation barriers. This effect is particularly pronounced in metal-ligand systems, where thermal energy can facilitate the breaking and reforming of coordination bonds.
Solvent polarity is another critical environmental factor affecting tautomerization in metal-ligand complexes. Polar solvents can stabilize charged or highly polarized tautomeric forms, potentially shifting the equilibrium towards these species. Conversely, non-polar solvents may favor neutral tautomers. The solvent's ability to form hydrogen bonds can also impact tautomerization by stabilizing specific tautomeric forms through intermolecular interactions.
pH is a key environmental factor that can dramatically influence tautomerization processes involving metal-ligand interactions. In acidic conditions, protonation of ligands or metal centers can occur, potentially altering the tautomeric equilibrium. Conversely, basic conditions may lead to deprotonation, favoring different tautomeric forms. The pH-dependent behavior of metal-ligand systems can be exploited in various applications, such as pH-responsive materials and sensors.
The presence of specific ions or other chemical species in the environment can also affect tautomerization. For instance, certain metal ions may preferentially coordinate with one tautomeric form over another, effectively shifting the equilibrium. Similarly, the presence of competing ligands can influence the stability of different tautomers by altering the overall coordination environment of the metal center.
Pressure is another environmental factor that can impact tautomerization, albeit to a lesser extent than temperature or solvent effects. High pressures can potentially favor tautomeric forms with smaller molecular volumes, although this effect is generally more subtle in metal-ligand systems compared to purely organic compounds.
Light exposure can also play a role in tautomerization processes, particularly in photosensitive metal-ligand complexes. Photoinduced tautomerization can occur when specific wavelengths of light provide the energy necessary to overcome tautomerization barriers or induce electronic transitions that facilitate the process.
Understanding these environmental factors and their interplay is crucial for predicting and controlling tautomerization in metal-ligand systems. This knowledge can be applied in various fields, including catalysis, materials science, and drug design, where precise control over tautomeric equilibria can lead to enhanced performance and novel functionalities.
Solvent polarity is another critical environmental factor affecting tautomerization in metal-ligand complexes. Polar solvents can stabilize charged or highly polarized tautomeric forms, potentially shifting the equilibrium towards these species. Conversely, non-polar solvents may favor neutral tautomers. The solvent's ability to form hydrogen bonds can also impact tautomerization by stabilizing specific tautomeric forms through intermolecular interactions.
pH is a key environmental factor that can dramatically influence tautomerization processes involving metal-ligand interactions. In acidic conditions, protonation of ligands or metal centers can occur, potentially altering the tautomeric equilibrium. Conversely, basic conditions may lead to deprotonation, favoring different tautomeric forms. The pH-dependent behavior of metal-ligand systems can be exploited in various applications, such as pH-responsive materials and sensors.
The presence of specific ions or other chemical species in the environment can also affect tautomerization. For instance, certain metal ions may preferentially coordinate with one tautomeric form over another, effectively shifting the equilibrium. Similarly, the presence of competing ligands can influence the stability of different tautomers by altering the overall coordination environment of the metal center.
Pressure is another environmental factor that can impact tautomerization, albeit to a lesser extent than temperature or solvent effects. High pressures can potentially favor tautomeric forms with smaller molecular volumes, although this effect is generally more subtle in metal-ligand systems compared to purely organic compounds.
Light exposure can also play a role in tautomerization processes, particularly in photosensitive metal-ligand complexes. Photoinduced tautomerization can occur when specific wavelengths of light provide the energy necessary to overcome tautomerization barriers or induce electronic transitions that facilitate the process.
Understanding these environmental factors and their interplay is crucial for predicting and controlling tautomerization in metal-ligand systems. This knowledge can be applied in various fields, including catalysis, materials science, and drug design, where precise control over tautomeric equilibria can lead to enhanced performance and novel functionalities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!