Tautomerization in Synthetic Biology: Pathway Optimization
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tautomerization in Synthetic Biology: Background and Objectives
Tautomerization, a fundamental process in organic chemistry, has emerged as a crucial area of study in synthetic biology. This phenomenon involves the rapid interconversion between structural isomers, known as tautomers, which can significantly impact the properties and functions of biological molecules. The exploration of tautomerization in synthetic biology aims to harness this natural process for optimizing metabolic pathways and enhancing the production of valuable compounds.
The field of synthetic biology has witnessed remarkable growth over the past two decades, with researchers striving to engineer biological systems for various applications. As the complexity of engineered pathways increases, understanding and controlling tautomerization becomes increasingly important. This process can affect enzyme-substrate interactions, metabolic flux, and overall pathway efficiency, making it a critical factor in the design and optimization of synthetic biological systems.
The primary objective of researching tautomerization in synthetic biology is to develop strategies for manipulating this process to enhance the performance of engineered metabolic pathways. By gaining a deeper understanding of tautomerization mechanisms and their effects on biological systems, researchers aim to create more robust and efficient synthetic pathways for the production of pharmaceuticals, biofuels, and other high-value compounds.
One of the key goals is to identify and characterize tautomeric forms of important metabolic intermediates and products. This knowledge can be leveraged to design enzymes that preferentially stabilize desired tautomers, thereby improving reaction rates and yields. Additionally, researchers seek to develop computational models and tools that can predict tautomerization behavior in complex biological environments, enabling more accurate pathway design and optimization.
Another important objective is to explore the potential of tautomerization for creating novel biosynthetic routes. By strategically incorporating tautomerization steps into engineered pathways, it may be possible to access new chemical space and produce compounds that are challenging to synthesize through traditional methods. This approach could open up new avenues for drug discovery and the production of advanced materials.
As the field progresses, there is a growing emphasis on integrating tautomerization research with other cutting-edge technologies in synthetic biology. This includes combining tautomerization studies with protein engineering, metabolic engineering, and systems biology approaches to create more comprehensive strategies for pathway optimization. The ultimate aim is to develop a toolbox of techniques that allows synthetic biologists to precisely control tautomerization events within engineered biological systems, leading to more efficient and versatile bio-based production platforms.
The field of synthetic biology has witnessed remarkable growth over the past two decades, with researchers striving to engineer biological systems for various applications. As the complexity of engineered pathways increases, understanding and controlling tautomerization becomes increasingly important. This process can affect enzyme-substrate interactions, metabolic flux, and overall pathway efficiency, making it a critical factor in the design and optimization of synthetic biological systems.
The primary objective of researching tautomerization in synthetic biology is to develop strategies for manipulating this process to enhance the performance of engineered metabolic pathways. By gaining a deeper understanding of tautomerization mechanisms and their effects on biological systems, researchers aim to create more robust and efficient synthetic pathways for the production of pharmaceuticals, biofuels, and other high-value compounds.
One of the key goals is to identify and characterize tautomeric forms of important metabolic intermediates and products. This knowledge can be leveraged to design enzymes that preferentially stabilize desired tautomers, thereby improving reaction rates and yields. Additionally, researchers seek to develop computational models and tools that can predict tautomerization behavior in complex biological environments, enabling more accurate pathway design and optimization.
Another important objective is to explore the potential of tautomerization for creating novel biosynthetic routes. By strategically incorporating tautomerization steps into engineered pathways, it may be possible to access new chemical space and produce compounds that are challenging to synthesize through traditional methods. This approach could open up new avenues for drug discovery and the production of advanced materials.
As the field progresses, there is a growing emphasis on integrating tautomerization research with other cutting-edge technologies in synthetic biology. This includes combining tautomerization studies with protein engineering, metabolic engineering, and systems biology approaches to create more comprehensive strategies for pathway optimization. The ultimate aim is to develop a toolbox of techniques that allows synthetic biologists to precisely control tautomerization events within engineered biological systems, leading to more efficient and versatile bio-based production platforms.
Market Analysis for Tautomerization Applications
The market for tautomerization applications in synthetic biology is experiencing significant growth, driven by the increasing demand for optimized biological pathways in various industries. Tautomerization, a process involving the interconversion of structural isomers, plays a crucial role in enhancing the efficiency and yield of biosynthetic processes. This has led to a surge in research and development activities focused on harnessing tautomerization for pathway optimization.
The pharmaceutical industry represents a major market segment for tautomerization applications. With the growing emphasis on developing novel drugs and improving existing formulations, pharmaceutical companies are increasingly turning to synthetic biology approaches that leverage tautomerization. This trend is particularly evident in the production of complex molecules and active pharmaceutical ingredients (APIs), where tautomerization can significantly impact the efficacy and stability of drug compounds.
In the biotechnology sector, tautomerization is gaining traction for its potential to enhance the production of high-value biochemicals and biofuels. Companies specializing in industrial biotechnology are exploring tautomerization-based strategies to optimize metabolic pathways, leading to improved yields and reduced production costs. This application is particularly relevant in the production of renewable chemicals and sustainable alternatives to petroleum-based products.
The agricultural industry is another key market for tautomerization applications, particularly in the development of crop protection products and genetically modified organisms (GMOs). Researchers are investigating how tautomerization can be utilized to enhance the efficacy of pesticides and herbicides, as well as to improve the nutritional content and stress resistance of crops.
The food and beverage industry is also showing interest in tautomerization applications, especially in the production of natural flavors and fragrances. By optimizing biosynthetic pathways through tautomerization, companies can achieve more efficient production of these high-value compounds, meeting the growing consumer demand for natural ingredients.
As the field of synthetic biology continues to advance, the market for tautomerization applications is expected to expand into new areas. Emerging applications include the development of biosensors, bioremediation technologies, and the production of novel biomaterials. These diverse applications underscore the versatility and potential of tautomerization in addressing various industrial and environmental challenges.
The global market for synthetic biology, which encompasses tautomerization applications, is projected to grow substantially in the coming years. This growth is fueled by increasing investments in research and development, favorable government policies promoting bio-based industries, and the rising adoption of synthetic biology approaches across multiple sectors.
The pharmaceutical industry represents a major market segment for tautomerization applications. With the growing emphasis on developing novel drugs and improving existing formulations, pharmaceutical companies are increasingly turning to synthetic biology approaches that leverage tautomerization. This trend is particularly evident in the production of complex molecules and active pharmaceutical ingredients (APIs), where tautomerization can significantly impact the efficacy and stability of drug compounds.
In the biotechnology sector, tautomerization is gaining traction for its potential to enhance the production of high-value biochemicals and biofuels. Companies specializing in industrial biotechnology are exploring tautomerization-based strategies to optimize metabolic pathways, leading to improved yields and reduced production costs. This application is particularly relevant in the production of renewable chemicals and sustainable alternatives to petroleum-based products.
The agricultural industry is another key market for tautomerization applications, particularly in the development of crop protection products and genetically modified organisms (GMOs). Researchers are investigating how tautomerization can be utilized to enhance the efficacy of pesticides and herbicides, as well as to improve the nutritional content and stress resistance of crops.
The food and beverage industry is also showing interest in tautomerization applications, especially in the production of natural flavors and fragrances. By optimizing biosynthetic pathways through tautomerization, companies can achieve more efficient production of these high-value compounds, meeting the growing consumer demand for natural ingredients.
As the field of synthetic biology continues to advance, the market for tautomerization applications is expected to expand into new areas. Emerging applications include the development of biosensors, bioremediation technologies, and the production of novel biomaterials. These diverse applications underscore the versatility and potential of tautomerization in addressing various industrial and environmental challenges.
The global market for synthetic biology, which encompasses tautomerization applications, is projected to grow substantially in the coming years. This growth is fueled by increasing investments in research and development, favorable government policies promoting bio-based industries, and the rising adoption of synthetic biology approaches across multiple sectors.
Current Challenges in Tautomerization Control
Tautomerization control in synthetic biology presents several significant challenges that researchers and bioengineers must overcome to optimize pathways effectively. One of the primary obstacles is the inherent instability of tautomeric forms, which can rapidly interconvert under physiological conditions. This dynamic equilibrium makes it difficult to maintain a specific tautomer for desired biochemical reactions, potentially leading to reduced efficiency or unwanted side products in engineered pathways.
Another major challenge lies in the precise manipulation of tautomerization rates within cellular environments. The complex interplay between various factors such as pH, temperature, and the presence of specific enzymes or cofactors can significantly influence tautomeric equilibria. Controlling these parameters in vivo to favor a particular tautomeric form remains a formidable task, requiring sophisticated understanding and engineering of cellular microenvironments.
The lack of specific and efficient enzymes capable of catalyzing desired tautomerization reactions poses an additional hurdle. While nature has evolved enzymes that can control tautomerization in certain contexts, the repertoire of available biocatalysts for synthetic biology applications is limited. Developing or discovering new enzymes with high specificity and catalytic efficiency for targeted tautomerization reactions is crucial for pathway optimization but remains a significant challenge.
Furthermore, the prediction and modeling of tautomerization behavior in complex biological systems present substantial difficulties. Current computational models often struggle to accurately simulate the dynamic nature of tautomeric equilibria in the presence of multiple interacting biomolecules and varying cellular conditions. This limitation hampers the ability to design and optimize pathways that rely on controlled tautomerization steps.
The integration of tautomerization control strategies into larger metabolic networks adds another layer of complexity. Ensuring that manipulated tautomeric forms do not disrupt other cellular processes or lead to unintended metabolic consequences requires careful consideration and extensive testing. Balancing the desired tautomerization effects with overall cellular homeostasis and metabolic flux remains a significant challenge in pathway engineering.
Lastly, the development of robust analytical methods for real-time monitoring of tautomeric states in living cells presents ongoing difficulties. Current techniques often lack the sensitivity or specificity to accurately track tautomerization dynamics in situ, limiting our ability to fine-tune and optimize pathways based on real-time data. Overcoming these analytical challenges is crucial for advancing our understanding and control of tautomerization in synthetic biology applications.
Another major challenge lies in the precise manipulation of tautomerization rates within cellular environments. The complex interplay between various factors such as pH, temperature, and the presence of specific enzymes or cofactors can significantly influence tautomeric equilibria. Controlling these parameters in vivo to favor a particular tautomeric form remains a formidable task, requiring sophisticated understanding and engineering of cellular microenvironments.
The lack of specific and efficient enzymes capable of catalyzing desired tautomerization reactions poses an additional hurdle. While nature has evolved enzymes that can control tautomerization in certain contexts, the repertoire of available biocatalysts for synthetic biology applications is limited. Developing or discovering new enzymes with high specificity and catalytic efficiency for targeted tautomerization reactions is crucial for pathway optimization but remains a significant challenge.
Furthermore, the prediction and modeling of tautomerization behavior in complex biological systems present substantial difficulties. Current computational models often struggle to accurately simulate the dynamic nature of tautomeric equilibria in the presence of multiple interacting biomolecules and varying cellular conditions. This limitation hampers the ability to design and optimize pathways that rely on controlled tautomerization steps.
The integration of tautomerization control strategies into larger metabolic networks adds another layer of complexity. Ensuring that manipulated tautomeric forms do not disrupt other cellular processes or lead to unintended metabolic consequences requires careful consideration and extensive testing. Balancing the desired tautomerization effects with overall cellular homeostasis and metabolic flux remains a significant challenge in pathway engineering.
Lastly, the development of robust analytical methods for real-time monitoring of tautomeric states in living cells presents ongoing difficulties. Current techniques often lack the sensitivity or specificity to accurately track tautomerization dynamics in situ, limiting our ability to fine-tune and optimize pathways based on real-time data. Overcoming these analytical challenges is crucial for advancing our understanding and control of tautomerization in synthetic biology applications.
Existing Tautomerization Pathway Optimization Strategies
01 Computational methods for tautomerization pathway optimization
Advanced computational techniques are employed to model and optimize tautomerization pathways. These methods involve quantum mechanical calculations, molecular dynamics simulations, and machine learning algorithms to predict and analyze tautomeric equilibria, transition states, and reaction rates. By leveraging these computational tools, researchers can efficiently explore various tautomerization pathways and identify optimal conditions for desired transformations.- Computational methods for tautomerization pathway optimization: Advanced computational techniques are employed to model and optimize tautomerization pathways. These methods involve quantum mechanical calculations, molecular dynamics simulations, and machine learning algorithms to predict and analyze tautomeric equilibria, transition states, and reaction energetics. Such approaches enable researchers to identify the most favorable tautomerization routes and optimize reaction conditions.
- Experimental techniques for tautomerization analysis: Various experimental methods are utilized to study and optimize tautomerization pathways. These include spectroscopic techniques such as NMR, UV-Vis, and IR spectroscopy, as well as chromatographic methods. High-throughput screening approaches are also employed to rapidly assess tautomeric equilibria under different conditions, facilitating the optimization of reaction parameters and solvent systems.
- Solvent effects on tautomerization pathways: The choice of solvent plays a crucial role in tautomerization processes. Research focuses on understanding and manipulating solvent effects to optimize tautomerization pathways. This includes studying the impact of solvent polarity, hydrogen bonding capabilities, and pH on tautomeric equilibria and reaction kinetics. Optimizing solvent conditions can significantly influence the preferred tautomeric forms and reaction rates.
- Catalytic strategies for tautomerization control: Catalysts are developed and employed to control and optimize tautomerization pathways. This includes the use of metal catalysts, organocatalysts, and enzymes to selectively promote specific tautomeric forms or accelerate tautomerization rates. Research in this area aims to design highly selective and efficient catalytic systems for targeted tautomerization processes in various applications.
- Application of machine learning in tautomerization research: Machine learning algorithms are increasingly applied to predict and optimize tautomerization pathways. These approaches involve training models on large datasets of known tautomeric systems to predict equilibria, reaction rates, and optimal conditions for desired tautomeric forms. Machine learning techniques can significantly accelerate the discovery and optimization of tautomerization pathways in drug design, materials science, and other fields.
02 Experimental techniques for tautomerization studies
Various experimental methods are utilized to investigate and optimize tautomerization pathways. These include spectroscopic techniques such as NMR, UV-Vis, and IR spectroscopy, as well as chromatographic methods. Time-resolved spectroscopy and kinetic studies are employed to monitor tautomerization processes in real-time. Additionally, crystallography and mass spectrometry provide valuable insights into tautomeric structures and their interconversions.Expand Specific Solutions03 Solvent effects on tautomerization pathways
The choice of solvent plays a crucial role in tautomerization processes. Research focuses on understanding and manipulating solvent effects to optimize tautomerization pathways. This includes studying the impact of solvent polarity, hydrogen bonding capabilities, and pH on tautomeric equilibria and interconversion rates. Tailoring solvent conditions can shift equilibria towards desired tautomers or accelerate specific tautomerization pathways.Expand Specific Solutions04 Catalytic approaches for tautomerization control
Catalysts are employed to influence tautomerization pathways and enhance reaction rates. This includes the use of metal catalysts, organocatalysts, and enzymes to facilitate specific tautomeric transformations. Research in this area focuses on designing and optimizing catalytic systems that can selectively promote desired tautomerization pathways while suppressing unwanted ones, leading to improved yields and selectivities in chemical processes involving tautomers.Expand Specific Solutions05 Application of machine learning in tautomerization prediction
Machine learning algorithms are increasingly used to predict and optimize tautomerization pathways. These approaches involve training models on large datasets of known tautomeric systems to predict tautomeric propensities, equilibrium constants, and reaction rates for novel compounds. By integrating machine learning with quantum mechanical calculations and experimental data, researchers can rapidly screen and optimize tautomerization pathways for drug discovery, materials design, and other applications.Expand Specific Solutions
Key Players in Synthetic Biology and Tautomerization
The research on tautomerization in synthetic biology for pathway optimization is in an early developmental stage, with significant potential for growth. The market size is expanding as synthetic biology applications increase across various industries. While the technology is still evolving, it shows promise for enhancing biological processes. Leading institutions like MIT, National University of Singapore, and University of California are at the forefront of this research, leveraging their expertise in biotechnology and synthetic biology. Companies such as Galapagos NV and GreenLight Biosciences are also contributing to advancements in this field, indicating a competitive landscape with both academic and industrial players driving innovation.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced computational models for predicting tautomerization pathways in synthetic biology. Their approach combines machine learning algorithms with quantum mechanical calculations to accurately simulate tautomeric transitions[1]. This allows for rapid screening of potential synthetic pathways and identification of optimal reaction conditions. MIT researchers have also engineered novel enzymes capable of catalyzing specific tautomerization reactions with high efficiency and selectivity[3]. These engineered biocatalysts enable precise control over tautomeric equilibria in synthetic biological systems, facilitating pathway optimization.
Strengths: Cutting-edge computational modeling and enzyme engineering capabilities. Weaknesses: May require significant computational resources and specialized expertise to implement.
The Regents of the University of California
Technical Solution: The University of California has pioneered the use of directed evolution techniques to optimize tautomerization pathways in synthetic biology. Their approach involves creating large libraries of enzyme variants and subjecting them to high-throughput screening to identify mutants with enhanced tautomerization activity[2]. This method has successfully produced enzymes capable of catalyzing challenging tautomerization reactions with improved efficiency and selectivity. Additionally, UC researchers have developed novel biosensors that can detect and quantify specific tautomeric forms in real-time, enabling precise monitoring and control of tautomerization processes in living cells[4]. These tools have been instrumental in optimizing synthetic pathways involving tautomerization steps.
Strengths: Powerful directed evolution platform and innovative biosensor technology. Weaknesses: Time-consuming process of library generation and screening.
Innovative Approaches in Tautomerization Control
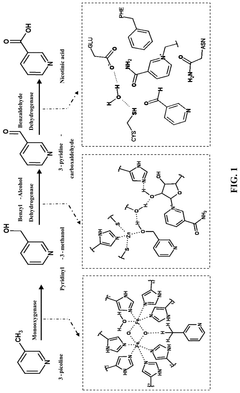
Synthetic biology approach to synthesize nicotinic acid from 3-picoline
PatentPendingUS20250101476A1
Innovation
- The development of a biosynthetic method using microbial biotransformation, involving the integration of monooxygenase, electron transfer component, benzyl alcohol dehydrogenase, and benzaldehyde dehydrogenase enzymes, optimized through gene isolation, enzyme engineering, and structural insights, to achieve a high conversion rate of 90% or higher from 3-picoline to nicotinic acid.
Microbial engineering for the production of chemical and pharmaceutical products from the isoprenoid pathway
PatentWO2011060057A1
Innovation
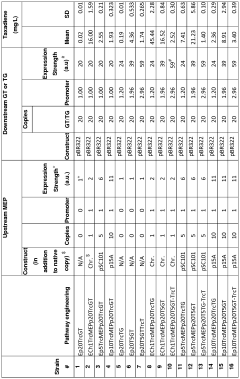
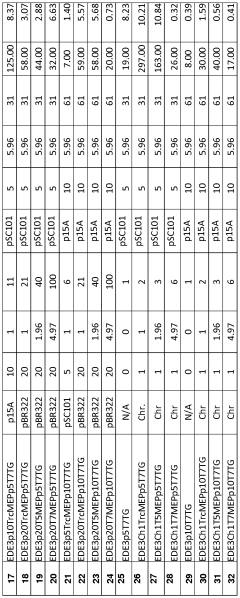
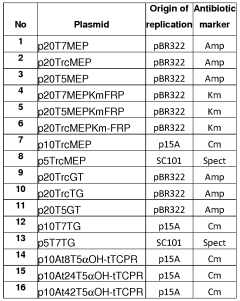
- A multivariate-modular approach is employed to optimize the upstream and downstream pathways in E. coli, balancing gene expression and plasmid copy numbers to achieve a 15,000-fold increase in taxadiene production, and engineering P450-based oxidation chemistry for enhanced conversion to taxadien-5a-ol, allowing for large-scale microbial production of Taxol and its derivatives.
Biosafety and Regulatory Considerations
The integration of tautomerization in synthetic biology pathways necessitates careful consideration of biosafety and regulatory aspects. Tautomers, being structural isomers that readily interconvert, pose unique challenges in terms of safety assessment and regulatory compliance. The dynamic nature of tautomeric compounds can lead to unexpected biological interactions, potentially affecting the safety profile of engineered organisms or their products.
From a biosafety perspective, the introduction of tautomerization-based pathways requires thorough risk assessment. The ability of tautomers to switch between different forms may alter their interactions with cellular components, potentially leading to unintended metabolic consequences or toxicity. Researchers must conduct comprehensive studies to evaluate the potential impacts on host organisms, non-target species, and the environment. This includes assessing the stability of tautomeric compounds under various conditions and their potential to accumulate or persist in biological systems.
Regulatory frameworks for synthetic biology applications involving tautomerization are still evolving. Current regulations may not fully address the unique properties of tautomeric compounds, necessitating case-by-case evaluations. Regulatory bodies will likely require detailed characterization of tautomeric pathways, including the kinetics of interconversion, potential intermediates, and any byproducts formed during the process. Demonstrating containment strategies and fail-safe mechanisms to prevent uncontrolled release of engineered organisms or tautomeric compounds will be crucial for regulatory approval.
The development of standardized protocols for safety testing and risk assessment of tautomerization-based synthetic biology applications is essential. This may include in vitro and in vivo studies to evaluate the potential for genotoxicity, mutagenicity, and ecological impacts. Additionally, long-term monitoring strategies may be required to assess the stability and behavior of engineered organisms and their tautomeric products in various environmental conditions.
Transparency and open communication with regulatory agencies and the public are vital for the responsible development of tautomerization-based synthetic biology applications. Researchers and companies working in this field should proactively engage with stakeholders to address concerns and establish trust. This may involve developing clear guidelines for the safe handling, storage, and disposal of tautomeric compounds and engineered organisms.
As the field advances, it is likely that regulatory frameworks will need to be updated to specifically address the unique challenges posed by tautomerization in synthetic biology. This may include the development of new risk assessment models, safety guidelines, and containment protocols tailored to the dynamic nature of tautomeric systems. International collaboration and harmonization of regulatory approaches will be crucial to ensure consistent safety standards and facilitate the global development and application of this technology.
From a biosafety perspective, the introduction of tautomerization-based pathways requires thorough risk assessment. The ability of tautomers to switch between different forms may alter their interactions with cellular components, potentially leading to unintended metabolic consequences or toxicity. Researchers must conduct comprehensive studies to evaluate the potential impacts on host organisms, non-target species, and the environment. This includes assessing the stability of tautomeric compounds under various conditions and their potential to accumulate or persist in biological systems.
Regulatory frameworks for synthetic biology applications involving tautomerization are still evolving. Current regulations may not fully address the unique properties of tautomeric compounds, necessitating case-by-case evaluations. Regulatory bodies will likely require detailed characterization of tautomeric pathways, including the kinetics of interconversion, potential intermediates, and any byproducts formed during the process. Demonstrating containment strategies and fail-safe mechanisms to prevent uncontrolled release of engineered organisms or tautomeric compounds will be crucial for regulatory approval.
The development of standardized protocols for safety testing and risk assessment of tautomerization-based synthetic biology applications is essential. This may include in vitro and in vivo studies to evaluate the potential for genotoxicity, mutagenicity, and ecological impacts. Additionally, long-term monitoring strategies may be required to assess the stability and behavior of engineered organisms and their tautomeric products in various environmental conditions.
Transparency and open communication with regulatory agencies and the public are vital for the responsible development of tautomerization-based synthetic biology applications. Researchers and companies working in this field should proactively engage with stakeholders to address concerns and establish trust. This may involve developing clear guidelines for the safe handling, storage, and disposal of tautomeric compounds and engineered organisms.
As the field advances, it is likely that regulatory frameworks will need to be updated to specifically address the unique challenges posed by tautomerization in synthetic biology. This may include the development of new risk assessment models, safety guidelines, and containment protocols tailored to the dynamic nature of tautomeric systems. International collaboration and harmonization of regulatory approaches will be crucial to ensure consistent safety standards and facilitate the global development and application of this technology.
Computational Tools for Tautomerization Prediction
Computational tools for tautomerization prediction have become increasingly sophisticated and essential in synthetic biology research. These tools leverage advanced algorithms and machine learning techniques to model and predict tautomeric equilibria, which is crucial for optimizing synthetic pathways involving tautomerizable compounds. One of the most widely used computational approaches is quantum mechanical (QM) calculations, which provide highly accurate predictions of tautomer stability and interconversion barriers. Density Functional Theory (DFT) methods, in particular, offer a good balance between accuracy and computational cost for tautomer predictions in biological systems.
Machine learning models have also emerged as powerful tools for tautomerization prediction. These models are trained on large datasets of experimentally determined tautomeric equilibria and can rapidly predict tautomerization propensities for novel compounds. Graph neural networks (GNNs) have shown particular promise in this area, as they can effectively capture the structural features that influence tautomerization. Some advanced GNN models can even predict pH-dependent tautomeric distributions, which is crucial for understanding tautomerization in biological contexts.
Molecular dynamics (MD) simulations provide another valuable approach for studying tautomerization in complex biological environments. Enhanced sampling techniques, such as metadynamics and umbrella sampling, allow researchers to explore the free energy landscapes of tautomeric transitions and estimate interconversion rates. These methods are particularly useful for understanding how the cellular environment influences tautomeric equilibria.
Cheminformatics tools have also been developed to rapidly enumerate and evaluate tautomers for large compound libraries. These tools often employ rule-based approaches combined with empirical or machine learning-based scoring functions to predict the most stable tautomers and their relative populations. Such high-throughput methods are invaluable for virtual screening and pathway design in synthetic biology applications.
Integration of these computational tools with experimental techniques has led to the development of hybrid approaches for tautomerization prediction. For example, QM/MM (Quantum Mechanics/Molecular Mechanics) methods combine the accuracy of quantum calculations with the efficiency of molecular mechanics simulations, allowing for the study of tautomerization in large biomolecular systems. Similarly, machine learning models trained on both experimental data and high-level quantum chemical calculations can provide rapid and accurate predictions for a wide range of compounds and conditions.
Machine learning models have also emerged as powerful tools for tautomerization prediction. These models are trained on large datasets of experimentally determined tautomeric equilibria and can rapidly predict tautomerization propensities for novel compounds. Graph neural networks (GNNs) have shown particular promise in this area, as they can effectively capture the structural features that influence tautomerization. Some advanced GNN models can even predict pH-dependent tautomeric distributions, which is crucial for understanding tautomerization in biological contexts.
Molecular dynamics (MD) simulations provide another valuable approach for studying tautomerization in complex biological environments. Enhanced sampling techniques, such as metadynamics and umbrella sampling, allow researchers to explore the free energy landscapes of tautomeric transitions and estimate interconversion rates. These methods are particularly useful for understanding how the cellular environment influences tautomeric equilibria.
Cheminformatics tools have also been developed to rapidly enumerate and evaluate tautomers for large compound libraries. These tools often employ rule-based approaches combined with empirical or machine learning-based scoring functions to predict the most stable tautomers and their relative populations. Such high-throughput methods are invaluable for virtual screening and pathway design in synthetic biology applications.
Integration of these computational tools with experimental techniques has led to the development of hybrid approaches for tautomerization prediction. For example, QM/MM (Quantum Mechanics/Molecular Mechanics) methods combine the accuracy of quantum calculations with the efficiency of molecular mechanics simulations, allowing for the study of tautomerization in large biomolecular systems. Similarly, machine learning models trained on both experimental data and high-level quantum chemical calculations can provide rapid and accurate predictions for a wide range of compounds and conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!