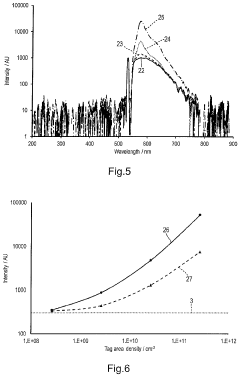
The Influence of Tautomerization on Fluorescent Quenching
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tautomerization and Fluorescence Quenching Background
Tautomerization is a fundamental chemical process involving the structural rearrangement of atoms within a molecule, resulting in the interconversion between two or more isomeric forms. This phenomenon plays a crucial role in various chemical and biological systems, including fluorescence quenching mechanisms. Fluorescence quenching, on the other hand, refers to the reduction in the intensity of fluorescence emission from a fluorophore due to various molecular interactions or processes.
The interplay between tautomerization and fluorescence quenching has been a subject of significant interest in the scientific community, particularly in the fields of photochemistry, molecular biology, and materials science. Tautomeric forms of a molecule can exhibit distinct photophysical properties, including differences in absorption and emission spectra, quantum yields, and excited-state lifetimes. These variations can profoundly impact the fluorescence behavior of the molecule and its susceptibility to quenching processes.
One of the primary mechanisms through which tautomerization influences fluorescence quenching is through excited-state intramolecular proton transfer (ESIPT). This process involves the transfer of a proton within the excited molecule, leading to the formation of a tautomeric species with altered electronic properties. ESIPT can result in significant Stokes shifts and dual emission, characteristics that are highly relevant to the design of fluorescent probes and sensors.
The influence of tautomerization on fluorescence quenching extends beyond intramolecular processes. Intermolecular interactions between fluorophores and quenchers can also be modulated by tautomeric equilibria. For instance, the hydrogen-bonding capabilities of different tautomeric forms can affect their interactions with surrounding molecules, potentially enhancing or inhibiting quenching processes.
In biological systems, tautomerization-induced fluorescence quenching plays a vital role in various processes, including DNA base pairing and enzyme catalysis. The ability of certain DNA bases to exist in multiple tautomeric forms can lead to mispairing and mutations, a phenomenon that has implications for genetic stability and evolution. Furthermore, the tautomeric states of amino acid residues in proteins can influence their fluorescence properties and their interactions with ligands or other biomolecules.
The study of tautomerization and its effects on fluorescence quenching has been greatly advanced by the development of sophisticated spectroscopic techniques and computational methods. Time-resolved spectroscopy, in particular, has provided valuable insights into the dynamics of tautomerization and its impact on excited-state processes. Quantum chemical calculations have also contributed significantly to our understanding of the energetics and kinetics of tautomeric transitions and their influence on electronic transitions.
The interplay between tautomerization and fluorescence quenching has been a subject of significant interest in the scientific community, particularly in the fields of photochemistry, molecular biology, and materials science. Tautomeric forms of a molecule can exhibit distinct photophysical properties, including differences in absorption and emission spectra, quantum yields, and excited-state lifetimes. These variations can profoundly impact the fluorescence behavior of the molecule and its susceptibility to quenching processes.
One of the primary mechanisms through which tautomerization influences fluorescence quenching is through excited-state intramolecular proton transfer (ESIPT). This process involves the transfer of a proton within the excited molecule, leading to the formation of a tautomeric species with altered electronic properties. ESIPT can result in significant Stokes shifts and dual emission, characteristics that are highly relevant to the design of fluorescent probes and sensors.
The influence of tautomerization on fluorescence quenching extends beyond intramolecular processes. Intermolecular interactions between fluorophores and quenchers can also be modulated by tautomeric equilibria. For instance, the hydrogen-bonding capabilities of different tautomeric forms can affect their interactions with surrounding molecules, potentially enhancing or inhibiting quenching processes.
In biological systems, tautomerization-induced fluorescence quenching plays a vital role in various processes, including DNA base pairing and enzyme catalysis. The ability of certain DNA bases to exist in multiple tautomeric forms can lead to mispairing and mutations, a phenomenon that has implications for genetic stability and evolution. Furthermore, the tautomeric states of amino acid residues in proteins can influence their fluorescence properties and their interactions with ligands or other biomolecules.
The study of tautomerization and its effects on fluorescence quenching has been greatly advanced by the development of sophisticated spectroscopic techniques and computational methods. Time-resolved spectroscopy, in particular, has provided valuable insights into the dynamics of tautomerization and its impact on excited-state processes. Quantum chemical calculations have also contributed significantly to our understanding of the energetics and kinetics of tautomeric transitions and their influence on electronic transitions.
Market Applications of Fluorescence-Based Technologies
Fluorescence-based technologies have found widespread applications across various market sectors, leveraging the unique properties of fluorescent molecules to enable advanced sensing, imaging, and analytical capabilities. In the biomedical field, fluorescence-based assays and imaging techniques have revolutionized diagnostics, drug discovery, and cellular research. These technologies allow for highly sensitive detection of biomolecules, real-time monitoring of cellular processes, and high-resolution imaging of biological structures.
The environmental monitoring sector has also embraced fluorescence-based technologies for rapid and sensitive detection of pollutants, contaminants, and harmful microorganisms in water, air, and soil samples. These methods offer advantages in terms of speed, sensitivity, and portability compared to traditional analytical techniques, making them valuable tools for on-site environmental assessments and regulatory compliance.
In the food and beverage industry, fluorescence-based technologies are employed for quality control, safety testing, and authentication purposes. They enable the detection of adulterants, toxins, and pathogens in food products, as well as the assessment of food freshness and composition. The non-destructive nature of fluorescence measurements makes them particularly suitable for in-line monitoring during food processing and packaging.
The materials science and manufacturing sectors utilize fluorescence-based technologies for quality control, defect detection, and process monitoring. These techniques can reveal structural defects, impurities, and material properties that are not visible to the naked eye or detectable by other means. In the semiconductor industry, for instance, fluorescence-based inspection systems are crucial for identifying nanoscale defects in chip manufacturing.
Forensic science and security applications benefit from fluorescence-based technologies in areas such as document authentication, crime scene investigation, and detection of explosives or illicit substances. The high sensitivity and specificity of fluorescence measurements allow for the detection of trace amounts of target compounds, enhancing the capabilities of law enforcement and security agencies.
The influence of tautomerization on fluorescent quenching is particularly relevant in these market applications, as it can affect the sensitivity, specificity, and reliability of fluorescence-based detection methods. Understanding and controlling tautomerization effects can lead to improved sensor designs, more accurate analytical techniques, and enhanced performance in various fluorescence-based technologies across different market sectors.
The environmental monitoring sector has also embraced fluorescence-based technologies for rapid and sensitive detection of pollutants, contaminants, and harmful microorganisms in water, air, and soil samples. These methods offer advantages in terms of speed, sensitivity, and portability compared to traditional analytical techniques, making them valuable tools for on-site environmental assessments and regulatory compliance.
In the food and beverage industry, fluorescence-based technologies are employed for quality control, safety testing, and authentication purposes. They enable the detection of adulterants, toxins, and pathogens in food products, as well as the assessment of food freshness and composition. The non-destructive nature of fluorescence measurements makes them particularly suitable for in-line monitoring during food processing and packaging.
The materials science and manufacturing sectors utilize fluorescence-based technologies for quality control, defect detection, and process monitoring. These techniques can reveal structural defects, impurities, and material properties that are not visible to the naked eye or detectable by other means. In the semiconductor industry, for instance, fluorescence-based inspection systems are crucial for identifying nanoscale defects in chip manufacturing.
Forensic science and security applications benefit from fluorescence-based technologies in areas such as document authentication, crime scene investigation, and detection of explosives or illicit substances. The high sensitivity and specificity of fluorescence measurements allow for the detection of trace amounts of target compounds, enhancing the capabilities of law enforcement and security agencies.
The influence of tautomerization on fluorescent quenching is particularly relevant in these market applications, as it can affect the sensitivity, specificity, and reliability of fluorescence-based detection methods. Understanding and controlling tautomerization effects can lead to improved sensor designs, more accurate analytical techniques, and enhanced performance in various fluorescence-based technologies across different market sectors.
Current Challenges in Tautomer-Induced Quenching
Tautomer-induced fluorescence quenching presents several significant challenges in current research and applications. One of the primary obstacles is the complexity of tautomeric equilibria in solution. The dynamic nature of tautomerization makes it difficult to predict and control the distribution of different tautomeric forms, which directly impacts the fluorescence quenching process. This unpredictability complicates the design of fluorescent sensors and probes that rely on tautomer-induced quenching mechanisms.
Another challenge lies in the sensitivity of tautomerization to environmental factors. pH, temperature, solvent polarity, and the presence of specific ions can all influence the tautomeric equilibrium. This sensitivity makes it challenging to maintain consistent quenching effects across various experimental conditions or in different biological environments. Researchers must carefully consider and control these factors to achieve reproducible results and reliable sensor performance.
The speed of tautomerization poses an additional hurdle. In many cases, tautomerization occurs on a timescale faster than the fluorescence lifetime. This rapid interconversion between tautomeric forms can lead to complex photophysical behaviors that are difficult to interpret and model accurately. It also complicates the development of time-resolved fluorescence techniques for studying tautomer-induced quenching phenomena.
Furthermore, the structural similarity between tautomers often results in overlapping spectral features. This spectral overlap makes it challenging to distinguish and quantify the contributions of individual tautomeric species to the overall quenching effect. Advanced spectroscopic techniques and data analysis methods are required to deconvolute these overlapping signals and gain a clear understanding of the quenching mechanism.
The molecular-level understanding of tautomer-induced quenching mechanisms remains incomplete. While general principles are known, the specific electronic and vibrational interactions that lead to quenching in different molecular systems are not fully elucidated. This lack of detailed mechanistic insight hinders the rational design of new fluorescent probes that exploit tautomer-induced quenching for sensing applications.
Lastly, the integration of tautomer-induced quenching into practical sensing devices presents engineering challenges. Translating the molecular-level phenomena into robust, scalable, and user-friendly sensor platforms requires overcoming issues related to stability, sensitivity, and selectivity. Developing strategies to immobilize tautomeric molecules on surfaces or incorporate them into polymeric matrices while maintaining their quenching properties is an ongoing area of research and development.
Another challenge lies in the sensitivity of tautomerization to environmental factors. pH, temperature, solvent polarity, and the presence of specific ions can all influence the tautomeric equilibrium. This sensitivity makes it challenging to maintain consistent quenching effects across various experimental conditions or in different biological environments. Researchers must carefully consider and control these factors to achieve reproducible results and reliable sensor performance.
The speed of tautomerization poses an additional hurdle. In many cases, tautomerization occurs on a timescale faster than the fluorescence lifetime. This rapid interconversion between tautomeric forms can lead to complex photophysical behaviors that are difficult to interpret and model accurately. It also complicates the development of time-resolved fluorescence techniques for studying tautomer-induced quenching phenomena.
Furthermore, the structural similarity between tautomers often results in overlapping spectral features. This spectral overlap makes it challenging to distinguish and quantify the contributions of individual tautomeric species to the overall quenching effect. Advanced spectroscopic techniques and data analysis methods are required to deconvolute these overlapping signals and gain a clear understanding of the quenching mechanism.
The molecular-level understanding of tautomer-induced quenching mechanisms remains incomplete. While general principles are known, the specific electronic and vibrational interactions that lead to quenching in different molecular systems are not fully elucidated. This lack of detailed mechanistic insight hinders the rational design of new fluorescent probes that exploit tautomer-induced quenching for sensing applications.
Lastly, the integration of tautomer-induced quenching into practical sensing devices presents engineering challenges. Translating the molecular-level phenomena into robust, scalable, and user-friendly sensor platforms requires overcoming issues related to stability, sensitivity, and selectivity. Developing strategies to immobilize tautomeric molecules on surfaces or incorporate them into polymeric matrices while maintaining their quenching properties is an ongoing area of research and development.
Existing Methods for Mitigating Tautomeric Quenching
01 Tautomerization-based fluorescent probes
Fluorescent probes utilizing tautomerization mechanisms are developed for various sensing applications. These probes undergo structural changes upon interaction with specific analytes, resulting in fluorescence quenching or enhancement. The tautomeric shift alters the electronic properties of the molecule, affecting its fluorescence characteristics.- Tautomerization-induced fluorescence quenching mechanisms: Tautomerization can lead to fluorescence quenching by altering the electronic structure of fluorescent molecules. This process involves the rapid interconversion between tautomeric forms, which can disrupt the excited state and provide non-radiative decay pathways, effectively quenching fluorescence. Understanding these mechanisms is crucial for designing fluorescent probes and sensors.
- Fluorescent probes utilizing tautomerization for sensing: Researchers have developed fluorescent probes that exploit tautomerization-induced quenching for various sensing applications. These probes are designed to undergo tautomerization in response to specific analytes or environmental conditions, resulting in measurable changes in fluorescence intensity. This approach enables the detection and quantification of various targets, including ions, pH changes, and biomolecules.
- Tautomerization in nucleic acid base pairs and its impact on fluorescence: Tautomerization plays a significant role in the fluorescence properties of nucleic acid base pairs. The interconversion between different tautomeric forms of nucleobases can affect their hydrogen bonding patterns and electronic structures, leading to changes in fluorescence emission. This phenomenon has implications for DNA/RNA-based fluorescent probes and the study of nucleic acid dynamics.
- Photoinduced tautomerization and its effect on fluorescence quenching: Light-induced tautomerization can significantly impact fluorescence properties. Upon photoexcitation, some molecules undergo rapid tautomerization, leading to excited-state intramolecular proton transfer (ESIPT). This process can result in fluorescence quenching or spectral shifts, which can be harnessed for the development of photoswitchable fluorescent probes and materials.
- Applications of tautomerization-based fluorescence quenching in biosensing: Tautomerization-induced fluorescence quenching has found numerous applications in biosensing. Researchers have developed fluorescent probes that undergo tautomerization in response to specific biological targets or processes, resulting in measurable changes in fluorescence. These probes have been used for detecting enzymes, monitoring cellular processes, and studying protein-ligand interactions, offering high sensitivity and selectivity in biological systems.
02 Intramolecular charge transfer (ICT) in tautomeric systems
Intramolecular charge transfer processes in tautomeric systems can lead to fluorescence quenching. The tautomerization-induced ICT alters the electronic distribution within the molecule, potentially resulting in non-radiative decay pathways and reduced fluorescence intensity.Expand Specific Solutions03 pH-dependent tautomerization and fluorescence
The pH of the environment can influence tautomerization processes, leading to changes in fluorescence properties. Certain fluorescent compounds exhibit pH-dependent tautomerization, which can be exploited for sensing applications or pH-responsive fluorescent materials.Expand Specific Solutions04 Excited-state intramolecular proton transfer (ESIPT)
ESIPT is a specific type of tautomerization that occurs in the excited state, often leading to fluorescence quenching or spectral shifts. This process involves the transfer of a proton within the molecule upon excitation, resulting in altered fluorescence properties.Expand Specific Solutions05 Tautomerization-induced aggregation and quenching
Tautomerization can induce molecular aggregation in some systems, leading to fluorescence quenching through aggregate-induced quenching mechanisms. The formation of non-fluorescent aggregates or excimers due to tautomeric shifts can result in decreased overall fluorescence intensity.Expand Specific Solutions
Key Players in Fluorescence Research and Industry
The field of fluorescent quenching influenced by tautomerization is in a developing stage, with growing market potential and increasing technological maturity. The competitive landscape is characterized by a mix of established chemical companies, academic institutions, and emerging biotech firms. Key players like Solvay Specialty Polymers, DAIKIN INDUSTRIES, and AGC Inc. are leveraging their expertise in fluorinated polymers and materials to advance this technology. Universities such as California, South China University of Technology, and Maryland Baltimore County are contributing significant research. The involvement of pharmaceutical companies like F. Hoffmann-La Roche and Sunshine Lake Pharma indicates potential applications in drug discovery and development. As the technology matures, we can expect increased collaboration between industry and academia to drive innovation and commercialization in this field.
The Regents of the University of California
Technical Solution: The University of California has developed advanced fluorescence quenching techniques to study tautomerization. They utilize time-resolved fluorescence spectroscopy to observe the dynamic interconversion between tautomers[1]. Their approach involves synthesizing novel fluorescent probes that exhibit tautomerism, allowing for real-time monitoring of tautomeric shifts in various chemical environments[3]. The research team has also implemented computational modeling to predict tautomeric equilibria and their effects on fluorescence quenching, enhancing the accuracy of their experimental results[5].
Strengths: Cutting-edge spectroscopic techniques, interdisciplinary approach combining synthesis, spectroscopy, and computational modeling. Weaknesses: Potential limitations in applying findings to complex biological systems.
Applied Biosystems LLC
Technical Solution: Applied Biosystems has developed a proprietary fluorescence quenching technology that accounts for tautomerization effects in nucleic acid detection assays. Their approach utilizes specially designed fluorescent probes that can distinguish between tautomeric forms of nucleobases[2]. The company has integrated this technology into their real-time PCR systems, allowing for more accurate quantification of DNA and RNA[4]. They have also created software algorithms that compensate for tautomerization-induced variations in fluorescence quenching, improving the reliability of their genetic analysis platforms[6].
Strengths: Integration of tautomerization considerations into commercial genetic analysis platforms, improving accuracy in nucleic acid detection. Weaknesses: Technology may be limited to specific applications in molecular biology and diagnostics.
Innovative Approaches to Tautomer-Resistant Fluorophores
Autofluorescence quenching assay and device
PatentInactiveUS20210208075A1
Innovation
- A method and device that utilize the autofluorescence of a substrate material, such as nitrocellulose, without added fluorophores, where a quenching substance forms an analyte-quenching complex that decreases the autofluorescence signal to determine analyte presence or concentration, using a lateral flow immunoassay device with photodetectors to measure the autofluorescence and absorbance changes.
Computational Modeling of Tautomeric Transitions
Computational modeling of tautomeric transitions has become an essential tool in understanding the influence of tautomerization on fluorescent quenching. These models provide valuable insights into the complex interplay between molecular structure, electronic properties, and photophysical processes. Advanced quantum chemical methods, such as density functional theory (DFT) and time-dependent DFT (TD-DFT), are commonly employed to simulate the ground and excited state properties of tautomeric species.
One of the primary challenges in modeling tautomeric transitions is accurately representing the potential energy surfaces of different tautomeric forms. This requires careful consideration of both intramolecular and intermolecular interactions, including hydrogen bonding and solvent effects. Hybrid quantum mechanics/molecular mechanics (QM/MM) approaches have proven particularly useful in addressing these challenges, allowing for the explicit treatment of the tautomeric molecule and its immediate environment.
Molecular dynamics simulations play a crucial role in exploring the conformational space of tautomeric species and capturing the dynamic nature of tautomerization processes. These simulations can reveal the timescales and mechanisms of tautomeric interconversions, which are often critical in determining the overall fluorescence quenching behavior. Advanced sampling techniques, such as metadynamics and replica exchange, are frequently employed to overcome energy barriers and explore rare events in tautomeric transitions.
Machine learning approaches have recently emerged as powerful tools for modeling tautomeric transitions and predicting their impact on fluorescent quenching. Neural network potentials trained on high-level quantum chemical data can provide accurate and computationally efficient representations of potential energy surfaces, enabling large-scale simulations of tautomeric systems. These methods are particularly promising for screening and designing new fluorescent probes with tailored tautomeric properties.
The integration of computational modeling with experimental techniques, such as ultrafast spectroscopy and single-molecule fluorescence measurements, has greatly enhanced our understanding of tautomerization-induced fluorescence quenching. By combining theoretical predictions with experimental observations, researchers can validate and refine computational models, leading to more accurate and predictive simulations of tautomeric transitions and their photophysical consequences.
One of the primary challenges in modeling tautomeric transitions is accurately representing the potential energy surfaces of different tautomeric forms. This requires careful consideration of both intramolecular and intermolecular interactions, including hydrogen bonding and solvent effects. Hybrid quantum mechanics/molecular mechanics (QM/MM) approaches have proven particularly useful in addressing these challenges, allowing for the explicit treatment of the tautomeric molecule and its immediate environment.
Molecular dynamics simulations play a crucial role in exploring the conformational space of tautomeric species and capturing the dynamic nature of tautomerization processes. These simulations can reveal the timescales and mechanisms of tautomeric interconversions, which are often critical in determining the overall fluorescence quenching behavior. Advanced sampling techniques, such as metadynamics and replica exchange, are frequently employed to overcome energy barriers and explore rare events in tautomeric transitions.
Machine learning approaches have recently emerged as powerful tools for modeling tautomeric transitions and predicting their impact on fluorescent quenching. Neural network potentials trained on high-level quantum chemical data can provide accurate and computationally efficient representations of potential energy surfaces, enabling large-scale simulations of tautomeric systems. These methods are particularly promising for screening and designing new fluorescent probes with tailored tautomeric properties.
The integration of computational modeling with experimental techniques, such as ultrafast spectroscopy and single-molecule fluorescence measurements, has greatly enhanced our understanding of tautomerization-induced fluorescence quenching. By combining theoretical predictions with experimental observations, researchers can validate and refine computational models, leading to more accurate and predictive simulations of tautomeric transitions and their photophysical consequences.
Environmental Factors Affecting Tautomerization Rates
Tautomerization rates are significantly influenced by various environmental factors, which play a crucial role in the dynamics of fluorescent quenching processes. Temperature is one of the primary factors affecting tautomerization rates. Higher temperatures generally lead to increased molecular motion and energy, facilitating the interconversion between tautomeric forms. This temperature dependence can be described by the Arrhenius equation, which relates the rate constant to the activation energy and temperature.
Solvent polarity is another critical environmental factor impacting tautomerization rates. Polar solvents can stabilize charged or highly polar tautomeric forms, potentially altering the equilibrium between different tautomers. The dielectric constant of the solvent medium can affect the energy barriers for tautomerization, thus influencing the rate of interconversion. Additionally, specific solvent-solute interactions, such as hydrogen bonding, can either promote or hinder tautomerization processes.
pH plays a significant role in tautomerization rates, particularly for molecules containing acidic or basic functional groups. Changes in pH can alter the protonation state of these groups, leading to shifts in tautomeric equilibria. In some cases, pH-induced tautomerization can result in dramatic changes in fluorescence properties, making it a valuable tool for pH sensing applications.
The presence of metal ions in the environment can also affect tautomerization rates. Metal ions can coordinate with specific functional groups in fluorescent molecules, potentially stabilizing certain tautomeric forms or catalyzing tautomerization processes. This metal ion-induced tautomerization can lead to significant changes in fluorescence behavior, forming the basis for metal ion sensing applications.
Pressure is another environmental factor that can influence tautomerization rates, albeit to a lesser extent than temperature or solvent effects. High pressures can alter the volume of activation for tautomerization processes, potentially affecting the rate of interconversion between tautomeric forms. This pressure dependence can be particularly relevant in high-pressure spectroscopic studies or in understanding tautomerization processes in extreme environments.
Light exposure can also impact tautomerization rates, especially for photochromic compounds. Certain wavelengths of light can induce photochemical reactions that facilitate tautomerization, leading to changes in molecular structure and, consequently, fluorescence properties. This photo-induced tautomerization forms the basis for various light-responsive materials and molecular switches.
Understanding these environmental factors and their effects on tautomerization rates is crucial for optimizing fluorescent quenching processes and developing more efficient and responsive fluorescent sensors and materials. By manipulating these environmental conditions, researchers can fine-tune the tautomerization behavior of fluorescent molecules, leading to enhanced performance in various applications, from biological imaging to environmental monitoring.
Solvent polarity is another critical environmental factor impacting tautomerization rates. Polar solvents can stabilize charged or highly polar tautomeric forms, potentially altering the equilibrium between different tautomers. The dielectric constant of the solvent medium can affect the energy barriers for tautomerization, thus influencing the rate of interconversion. Additionally, specific solvent-solute interactions, such as hydrogen bonding, can either promote or hinder tautomerization processes.
pH plays a significant role in tautomerization rates, particularly for molecules containing acidic or basic functional groups. Changes in pH can alter the protonation state of these groups, leading to shifts in tautomeric equilibria. In some cases, pH-induced tautomerization can result in dramatic changes in fluorescence properties, making it a valuable tool for pH sensing applications.
The presence of metal ions in the environment can also affect tautomerization rates. Metal ions can coordinate with specific functional groups in fluorescent molecules, potentially stabilizing certain tautomeric forms or catalyzing tautomerization processes. This metal ion-induced tautomerization can lead to significant changes in fluorescence behavior, forming the basis for metal ion sensing applications.
Pressure is another environmental factor that can influence tautomerization rates, albeit to a lesser extent than temperature or solvent effects. High pressures can alter the volume of activation for tautomerization processes, potentially affecting the rate of interconversion between tautomeric forms. This pressure dependence can be particularly relevant in high-pressure spectroscopic studies or in understanding tautomerization processes in extreme environments.
Light exposure can also impact tautomerization rates, especially for photochromic compounds. Certain wavelengths of light can induce photochemical reactions that facilitate tautomerization, leading to changes in molecular structure and, consequently, fluorescence properties. This photo-induced tautomerization forms the basis for various light-responsive materials and molecular switches.
Understanding these environmental factors and their effects on tautomerization rates is crucial for optimizing fluorescent quenching processes and developing more efficient and responsive fluorescent sensors and materials. By manipulating these environmental conditions, researchers can fine-tune the tautomerization behavior of fluorescent molecules, leading to enhanced performance in various applications, from biological imaging to environmental monitoring.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!