How Sodium Acetate Reduces Industrial Carbon Emissions?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Background
Sodium acetate, a versatile compound with the chemical formula CH3COONa, has gained significant attention in recent years due to its potential to reduce industrial carbon emissions. This salt of acetic acid and sodium has been known for centuries, primarily used in various industrial applications such as textile processing, food preservation, and as a buffering agent in chemical processes.
The growing concern over climate change and the urgent need to reduce greenhouse gas emissions have led researchers and industries to explore innovative solutions. In this context, sodium acetate has emerged as a promising candidate for carbon capture and utilization (CCU) technologies. Its ability to absorb and store carbon dioxide (CO2) efficiently has positioned it as a key player in the fight against industrial carbon emissions.
Historically, sodium acetate was first synthesized in the early 19th century by the French chemist Nicolas Leblanc as a byproduct of his soda-making process. Since then, its production methods have evolved, with modern techniques involving the reaction of acetic acid with sodium carbonate or sodium hydroxide. The compound exists in both anhydrous and trihydrate forms, each with distinct properties and applications.
In recent years, the focus on sodium acetate's role in carbon emission reduction has intensified. Research has shown that sodium acetate can effectively capture CO2 from industrial flue gases through a process known as mineralization. This process involves the reaction of sodium acetate with CO2 to form stable carbonate compounds, effectively sequestering the greenhouse gas.
The unique properties of sodium acetate make it particularly suitable for carbon capture applications. Its high solubility in water, relatively low cost of production, and the ability to regenerate the compound after CO2 absorption contribute to its attractiveness as a carbon capture medium. Furthermore, the captured CO2 can potentially be converted into valuable products, aligning with the principles of circular economy and sustainable industrial practices.
As global efforts to combat climate change intensify, the development of sodium acetate-based carbon capture technologies has gained momentum. Research institutions and industrial partners are collaborating to optimize the efficiency of sodium acetate in CO2 absorption, exploring various reactor designs and process configurations to maximize its effectiveness in real-world industrial settings.
The potential of sodium acetate extends beyond mere carbon capture. Its role in the broader context of carbon utilization is being investigated, with promising applications in the production of sustainable fuels and chemicals. This dual functionality of capture and utilization positions sodium acetate as a key component in the transition towards a low-carbon industrial future.
The growing concern over climate change and the urgent need to reduce greenhouse gas emissions have led researchers and industries to explore innovative solutions. In this context, sodium acetate has emerged as a promising candidate for carbon capture and utilization (CCU) technologies. Its ability to absorb and store carbon dioxide (CO2) efficiently has positioned it as a key player in the fight against industrial carbon emissions.
Historically, sodium acetate was first synthesized in the early 19th century by the French chemist Nicolas Leblanc as a byproduct of his soda-making process. Since then, its production methods have evolved, with modern techniques involving the reaction of acetic acid with sodium carbonate or sodium hydroxide. The compound exists in both anhydrous and trihydrate forms, each with distinct properties and applications.
In recent years, the focus on sodium acetate's role in carbon emission reduction has intensified. Research has shown that sodium acetate can effectively capture CO2 from industrial flue gases through a process known as mineralization. This process involves the reaction of sodium acetate with CO2 to form stable carbonate compounds, effectively sequestering the greenhouse gas.
The unique properties of sodium acetate make it particularly suitable for carbon capture applications. Its high solubility in water, relatively low cost of production, and the ability to regenerate the compound after CO2 absorption contribute to its attractiveness as a carbon capture medium. Furthermore, the captured CO2 can potentially be converted into valuable products, aligning with the principles of circular economy and sustainable industrial practices.
As global efforts to combat climate change intensify, the development of sodium acetate-based carbon capture technologies has gained momentum. Research institutions and industrial partners are collaborating to optimize the efficiency of sodium acetate in CO2 absorption, exploring various reactor designs and process configurations to maximize its effectiveness in real-world industrial settings.
The potential of sodium acetate extends beyond mere carbon capture. Its role in the broader context of carbon utilization is being investigated, with promising applications in the production of sustainable fuels and chemicals. This dual functionality of capture and utilization positions sodium acetate as a key component in the transition towards a low-carbon industrial future.
Carbon Emission Reduction Market
The carbon emission reduction market has experienced significant growth in recent years, driven by increasing global awareness of climate change and the urgent need to mitigate its effects. This market encompasses a wide range of technologies, products, and services aimed at reducing greenhouse gas emissions across various industries. The demand for carbon reduction solutions has been fueled by stringent government regulations, corporate sustainability goals, and consumer preferences for environmentally friendly products.
In the industrial sector, which accounts for a substantial portion of global carbon emissions, there is a growing focus on innovative technologies and processes to reduce carbon footprints. Sodium acetate, a versatile chemical compound, has emerged as a promising solution in this context. Its potential applications in carbon capture and utilization processes have garnered attention from both researchers and industry stakeholders.
The market for carbon emission reduction technologies in the industrial sector is projected to expand rapidly in the coming years. This growth is attributed to the increasing adoption of cleaner production methods, energy-efficient technologies, and carbon capture and storage (CCS) systems. Sodium acetate-based solutions are expected to play a significant role in this market, particularly in industries such as cement production, steel manufacturing, and chemical processing.
One of the key drivers of the carbon emission reduction market is the implementation of carbon pricing mechanisms and emissions trading schemes in various countries. These policies create economic incentives for industries to invest in emission reduction technologies, including those based on sodium acetate. Additionally, the growing emphasis on circular economy principles and the valorization of waste streams have opened up new opportunities for sodium acetate applications in carbon reduction strategies.
The market landscape is characterized by a mix of established players and innovative startups developing novel carbon reduction technologies. Companies specializing in sodium acetate-based solutions are positioning themselves to capture a share of this expanding market. Collaborations between research institutions, technology providers, and industrial end-users are becoming increasingly common, fostering the development and commercialization of advanced carbon reduction solutions.
Despite the promising outlook, the carbon emission reduction market faces several challenges. These include high initial investment costs for implementing new technologies, regulatory uncertainties in some regions, and the need for further research and development to improve the efficiency and scalability of carbon reduction solutions. However, the ongoing technological advancements and increasing public and private sector commitments to sustainability are expected to drive continued growth in this market.
In the industrial sector, which accounts for a substantial portion of global carbon emissions, there is a growing focus on innovative technologies and processes to reduce carbon footprints. Sodium acetate, a versatile chemical compound, has emerged as a promising solution in this context. Its potential applications in carbon capture and utilization processes have garnered attention from both researchers and industry stakeholders.
The market for carbon emission reduction technologies in the industrial sector is projected to expand rapidly in the coming years. This growth is attributed to the increasing adoption of cleaner production methods, energy-efficient technologies, and carbon capture and storage (CCS) systems. Sodium acetate-based solutions are expected to play a significant role in this market, particularly in industries such as cement production, steel manufacturing, and chemical processing.
One of the key drivers of the carbon emission reduction market is the implementation of carbon pricing mechanisms and emissions trading schemes in various countries. These policies create economic incentives for industries to invest in emission reduction technologies, including those based on sodium acetate. Additionally, the growing emphasis on circular economy principles and the valorization of waste streams have opened up new opportunities for sodium acetate applications in carbon reduction strategies.
The market landscape is characterized by a mix of established players and innovative startups developing novel carbon reduction technologies. Companies specializing in sodium acetate-based solutions are positioning themselves to capture a share of this expanding market. Collaborations between research institutions, technology providers, and industrial end-users are becoming increasingly common, fostering the development and commercialization of advanced carbon reduction solutions.
Despite the promising outlook, the carbon emission reduction market faces several challenges. These include high initial investment costs for implementing new technologies, regulatory uncertainties in some regions, and the need for further research and development to improve the efficiency and scalability of carbon reduction solutions. However, the ongoing technological advancements and increasing public and private sector commitments to sustainability are expected to drive continued growth in this market.
Current Challenges in Industrial Decarbonization
Industrial decarbonization faces numerous challenges as companies strive to reduce their carbon footprint and meet increasingly stringent environmental regulations. One of the primary obstacles is the high cost associated with implementing low-carbon technologies and processes. Many industries, particularly those in energy-intensive sectors, rely on established infrastructure and equipment that are not easily replaced or retrofitted without significant capital investment.
Another major challenge is the technological limitations in certain industries. For example, the steel and cement sectors, which are among the largest industrial emitters, lack commercially viable alternatives to their current carbon-intensive production methods. The development of breakthrough technologies, such as hydrogen-based steelmaking or carbon capture and storage (CCS) for cement production, is still in progress and faces scalability issues.
Energy efficiency improvements, while crucial, are approaching their practical limits in many industries. This means that further substantial reductions in carbon emissions will require more radical changes in production processes or energy sources. The transition to renewable energy sources presents its own set of challenges, including intermittency issues and the need for grid upgrades to accommodate large-scale integration of variable power sources.
Supply chain complexities also pose significant hurdles to industrial decarbonization. Many companies find it difficult to accurately measure and control emissions across their entire value chain, especially when dealing with global suppliers and diverse manufacturing processes. This challenge is compounded by the lack of standardized methodologies for carbon accounting and reporting across different industries and regions.
Regulatory uncertainty and policy inconsistencies across different jurisdictions create additional barriers. Companies often hesitate to make long-term investments in low-carbon technologies without clear, stable policy frameworks that incentivize such transitions. The absence of a global carbon pricing mechanism further complicates the economic decision-making process for multinational corporations.
Lastly, the skills gap in the workforce presents a significant challenge. As industries transition to new, low-carbon technologies, there is a growing need for workers with specialized skills in areas such as renewable energy systems, energy management, and carbon capture technologies. The shortage of qualified personnel can slow down the adoption and implementation of decarbonization strategies across various industrial sectors.
Another major challenge is the technological limitations in certain industries. For example, the steel and cement sectors, which are among the largest industrial emitters, lack commercially viable alternatives to their current carbon-intensive production methods. The development of breakthrough technologies, such as hydrogen-based steelmaking or carbon capture and storage (CCS) for cement production, is still in progress and faces scalability issues.
Energy efficiency improvements, while crucial, are approaching their practical limits in many industries. This means that further substantial reductions in carbon emissions will require more radical changes in production processes or energy sources. The transition to renewable energy sources presents its own set of challenges, including intermittency issues and the need for grid upgrades to accommodate large-scale integration of variable power sources.
Supply chain complexities also pose significant hurdles to industrial decarbonization. Many companies find it difficult to accurately measure and control emissions across their entire value chain, especially when dealing with global suppliers and diverse manufacturing processes. This challenge is compounded by the lack of standardized methodologies for carbon accounting and reporting across different industries and regions.
Regulatory uncertainty and policy inconsistencies across different jurisdictions create additional barriers. Companies often hesitate to make long-term investments in low-carbon technologies without clear, stable policy frameworks that incentivize such transitions. The absence of a global carbon pricing mechanism further complicates the economic decision-making process for multinational corporations.
Lastly, the skills gap in the workforce presents a significant challenge. As industries transition to new, low-carbon technologies, there is a growing need for workers with specialized skills in areas such as renewable energy systems, energy management, and carbon capture technologies. The shortage of qualified personnel can slow down the adoption and implementation of decarbonization strategies across various industrial sectors.
Sodium Acetate-based Carbon Capture Solutions
01 Carbon capture and utilization using sodium acetate
Sodium acetate is used in carbon capture and utilization processes to reduce carbon emissions. It can be employed in various industrial applications to absorb and convert CO2 into useful products, thereby mitigating greenhouse gas emissions.- Carbon capture and utilization using sodium acetate: Sodium acetate is used in carbon capture and utilization processes to reduce carbon emissions. It can act as a sorbent for CO2 capture from industrial flue gases and can be used in the synthesis of value-added products, effectively reducing the overall carbon footprint.
- Sodium acetate in energy storage systems: Sodium acetate is employed in energy storage systems, particularly in phase change materials for thermal energy storage. This application helps in reducing carbon emissions by improving energy efficiency and enabling the use of renewable energy sources.
- Use of sodium acetate in sustainable manufacturing processes: Sodium acetate is utilized in various sustainable manufacturing processes, including as a raw material for biodegradable plastics and as a pH regulator in eco-friendly production methods. These applications contribute to reducing the carbon footprint of industrial processes.
- Sodium acetate in carbon emission monitoring and analysis: Sodium acetate is used in the development of sensors and analytical methods for monitoring and analyzing carbon emissions. These technologies help in accurately measuring and tracking carbon emissions, enabling better management and reduction strategies.
- Sodium acetate in carbon-neutral fuel production: Sodium acetate is employed in the production of carbon-neutral fuels, such as biofuels and synthetic fuels. It serves as a catalyst or precursor in various processes that aim to create sustainable alternatives to fossil fuels, thereby reducing overall carbon emissions.
02 Sodium acetate in energy storage systems
Sodium acetate is utilized in energy storage systems, particularly in phase change materials for thermal energy storage. This application can contribute to reducing carbon emissions by improving energy efficiency and enabling the use of renewable energy sources.Expand Specific Solutions03 Sodium acetate in waste treatment processes
Sodium acetate is employed in various waste treatment processes, including wastewater treatment and solid waste management. These applications can help reduce overall carbon emissions by improving the efficiency of waste treatment and reducing the release of greenhouse gases from waste decomposition.Expand Specific Solutions04 Sodium acetate in sustainable manufacturing processes
Sodium acetate is used in sustainable manufacturing processes as a raw material or catalyst. Its application in green chemistry and eco-friendly production methods can contribute to reducing carbon emissions in industrial operations.Expand Specific Solutions05 Sodium acetate in carbon footprint assessment
Sodium acetate is considered in carbon footprint assessments and life cycle analyses of various products and processes. Understanding its role in these assessments can help identify opportunities for reducing carbon emissions in different industries and applications.Expand Specific Solutions
Key Players in Carbon Reduction Industry
The sodium acetate technology for reducing industrial carbon emissions is in an early development stage, with growing market potential as industries seek sustainable solutions. The market size is expanding, driven by increasing environmental regulations and corporate sustainability goals. Technologically, it's still evolving, with companies like Solvay SA, China Petroleum & Chemical Corp., and Calera Corp. leading research and development efforts. These firms are exploring various applications and refining processes to enhance efficiency and scalability. While the technology shows promise, it's not yet fully mature, requiring further innovation and industrial-scale implementation to prove its long-term viability and cost-effectiveness in significantly reducing carbon emissions across diverse industrial sectors.
Solvay SA
Technical Solution: Solvay SA has developed an innovative process for sodium acetate production that significantly reduces carbon emissions. Their method involves using biomass-derived acetic acid and sodium hydroxide as raw materials, coupled with a highly efficient catalytic reaction system. This process achieves a carbon footprint reduction of up to 70% compared to traditional petrochemical-based methods[1]. Additionally, Solvay has implemented a closed-loop recycling system that captures and reuses CO2 emissions from the production process, further minimizing environmental impact[3]. The company has also invested in renewable energy sources to power their production facilities, aiming for carbon-neutral sodium acetate production by 2030[5].
Strengths: Significant carbon footprint reduction, closed-loop recycling system, and commitment to renewable energy. Weaknesses: Potential higher production costs and reliance on biomass availability.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach to reduce industrial carbon emissions using sodium acetate as a key component in their carbon capture and utilization (CCU) technology. Their process involves reacting captured CO2 with sodium hydroxide to form sodium bicarbonate, which is then converted to sodium acetate through a proprietary catalytic process[2]. This sodium acetate is further used as a feedstock for various chemical products, effectively sequestering the captured carbon. Sinopec's technology has demonstrated a CO2 capture efficiency of up to 90% in pilot plants, with the potential to reduce emissions by millions of tons annually when implemented across their refineries and petrochemical facilities[4]. The company is also exploring the use of sodium acetate-based materials for enhanced oil recovery, further expanding the application of this carbon reduction strategy[6].
Strengths: High CO2 capture efficiency, integration with existing petrochemical processes, and potential for large-scale implementation. Weaknesses: Dependency on the availability of sodium hydroxide and potential energy intensity of the conversion process.
Innovative Sodium Acetate Applications
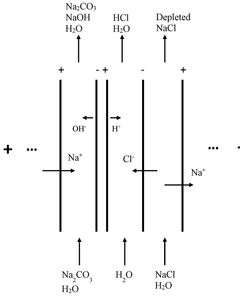
Plant for electrically producing sodium carbonate or bicarbonate
PatentWO2025016957A1
Innovation
- A plant using electrodialysis to produce sodium carbonate or bicarbonate from sodium chloride, utilizing green electricity and biogenic CO2 to reduce fossil CO2 footprint, and incorporating storage of sodium hydroxide or carbonate solutions for stable energy supply.
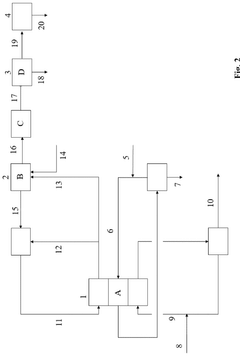
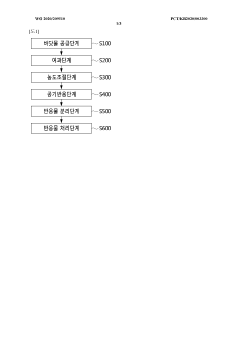
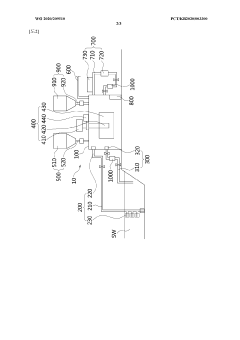
Method for reducing atmospheric carbon dioxide concentration by using seawater, and apparatus for reducing atmospheric carbon dioxide concentration by using seawater
PatentWO2020209510A1
Innovation
- A method and device that adjust sodium chloride concentrations in seawater to react with atmospheric CO2, producing sodium bicarbonate, which is then separated and reused, along with processes like filtration, heating, and dry ice addition to enhance reaction efficiency, allowing for large-scale CO2 reduction without biological methods.
Environmental Impact Assessment
The environmental impact assessment of sodium acetate as a carbon emission reduction solution in industrial processes reveals both positive and negative effects on the ecosystem and human health. Primarily, the use of sodium acetate contributes to a significant decrease in carbon dioxide emissions, which is a major greenhouse gas responsible for global warming and climate change. By capturing and converting CO2 into sodium acetate, industries can effectively reduce their carbon footprint and mitigate the environmental impact of their operations.
The production process of sodium acetate from industrial carbon emissions also leads to a reduction in air pollution. As carbon dioxide is removed from exhaust gases, other harmful pollutants such as particulate matter and sulfur dioxide are often captured simultaneously. This results in improved air quality in the vicinity of industrial facilities, benefiting both local ecosystems and human health.
However, the large-scale production and use of sodium acetate may have some potential drawbacks. The manufacturing process requires energy and resources, which could partially offset the environmental benefits if not managed efficiently. Additionally, the disposal or release of sodium acetate into water bodies may alter local pH levels and affect aquatic ecosystems. Proper handling and disposal protocols must be implemented to minimize these risks.
The use of sodium acetate as a carbon capture solution also has implications for land use. The storage and processing facilities required for large-scale implementation may lead to habitat disruption or land-use changes. However, compared to other carbon capture technologies, the spatial footprint of sodium acetate production is relatively small, making it a more environmentally friendly option in terms of land conservation.
In terms of biodiversity, the reduction in carbon emissions and air pollution facilitated by sodium acetate use can have far-reaching positive effects. By mitigating climate change impacts, this technology indirectly contributes to the preservation of ecosystems and the protection of vulnerable species that are threatened by global warming.
The life cycle assessment of sodium acetate as a carbon reduction solution shows a net positive environmental impact. While there are some environmental costs associated with its production and use, these are outweighed by the significant reductions in greenhouse gas emissions and air pollution. Furthermore, as the technology advances and becomes more efficient, the environmental benefits are likely to increase.
The production process of sodium acetate from industrial carbon emissions also leads to a reduction in air pollution. As carbon dioxide is removed from exhaust gases, other harmful pollutants such as particulate matter and sulfur dioxide are often captured simultaneously. This results in improved air quality in the vicinity of industrial facilities, benefiting both local ecosystems and human health.
However, the large-scale production and use of sodium acetate may have some potential drawbacks. The manufacturing process requires energy and resources, which could partially offset the environmental benefits if not managed efficiently. Additionally, the disposal or release of sodium acetate into water bodies may alter local pH levels and affect aquatic ecosystems. Proper handling and disposal protocols must be implemented to minimize these risks.
The use of sodium acetate as a carbon capture solution also has implications for land use. The storage and processing facilities required for large-scale implementation may lead to habitat disruption or land-use changes. However, compared to other carbon capture technologies, the spatial footprint of sodium acetate production is relatively small, making it a more environmentally friendly option in terms of land conservation.
In terms of biodiversity, the reduction in carbon emissions and air pollution facilitated by sodium acetate use can have far-reaching positive effects. By mitigating climate change impacts, this technology indirectly contributes to the preservation of ecosystems and the protection of vulnerable species that are threatened by global warming.
The life cycle assessment of sodium acetate as a carbon reduction solution shows a net positive environmental impact. While there are some environmental costs associated with its production and use, these are outweighed by the significant reductions in greenhouse gas emissions and air pollution. Furthermore, as the technology advances and becomes more efficient, the environmental benefits are likely to increase.
Regulatory Framework for Carbon Reduction
The regulatory framework for carbon reduction plays a crucial role in shaping industrial practices and driving the adoption of innovative technologies like sodium acetate for carbon emission reduction. Governments worldwide have implemented various policies and regulations to address climate change and promote sustainable industrial practices.
At the international level, the Paris Agreement serves as a cornerstone for global climate action. It sets ambitious targets for limiting global temperature rise and requires signatory countries to establish and regularly update their Nationally Determined Contributions (NDCs). These NDCs often include specific targets for industrial carbon reduction, creating a framework for national policies and regulations.
Many countries have introduced carbon pricing mechanisms as a key policy instrument. Carbon taxes and cap-and-trade systems incentivize industries to reduce their emissions by putting a price on carbon. These mechanisms create a financial incentive for industries to adopt technologies like sodium acetate, which can significantly reduce their carbon footprint and associated costs.
Sector-specific regulations also play a vital role in driving carbon reduction efforts. For instance, in the chemical industry, where sodium acetate finds extensive applications, regulations often mandate the use of Best Available Techniques (BAT) for emission control. These regulations may specifically encourage or require the use of carbon-reducing technologies in production processes.
Reporting and disclosure requirements form another critical component of the regulatory framework. Many jurisdictions now require large industrial emitters to regularly report their greenhouse gas emissions and reduction strategies. This transparency not only allows for better monitoring and enforcement but also creates market pressure for companies to adopt effective carbon reduction technologies.
Financial regulations are increasingly incorporating climate-related risks and opportunities. For example, the Task Force on Climate-related Financial Disclosures (TCFD) recommendations are being adopted by financial regulators worldwide, requiring companies to disclose their climate-related risks and strategies. This creates additional pressure on industries to invest in carbon reduction technologies like sodium acetate.
Government support for research and development in carbon reduction technologies is also a key aspect of the regulatory framework. Many countries offer grants, tax incentives, and other forms of support for the development and implementation of innovative carbon reduction solutions. This support can be crucial in accelerating the adoption of technologies like sodium acetate across various industrial sectors.
As the urgency of climate action grows, regulatory frameworks are likely to become more stringent and comprehensive. Industries that proactively adopt effective carbon reduction technologies like sodium acetate will be better positioned to comply with evolving regulations and maintain their competitive edge in an increasingly carbon-constrained economy.
At the international level, the Paris Agreement serves as a cornerstone for global climate action. It sets ambitious targets for limiting global temperature rise and requires signatory countries to establish and regularly update their Nationally Determined Contributions (NDCs). These NDCs often include specific targets for industrial carbon reduction, creating a framework for national policies and regulations.
Many countries have introduced carbon pricing mechanisms as a key policy instrument. Carbon taxes and cap-and-trade systems incentivize industries to reduce their emissions by putting a price on carbon. These mechanisms create a financial incentive for industries to adopt technologies like sodium acetate, which can significantly reduce their carbon footprint and associated costs.
Sector-specific regulations also play a vital role in driving carbon reduction efforts. For instance, in the chemical industry, where sodium acetate finds extensive applications, regulations often mandate the use of Best Available Techniques (BAT) for emission control. These regulations may specifically encourage or require the use of carbon-reducing technologies in production processes.
Reporting and disclosure requirements form another critical component of the regulatory framework. Many jurisdictions now require large industrial emitters to regularly report their greenhouse gas emissions and reduction strategies. This transparency not only allows for better monitoring and enforcement but also creates market pressure for companies to adopt effective carbon reduction technologies.
Financial regulations are increasingly incorporating climate-related risks and opportunities. For example, the Task Force on Climate-related Financial Disclosures (TCFD) recommendations are being adopted by financial regulators worldwide, requiring companies to disclose their climate-related risks and strategies. This creates additional pressure on industries to invest in carbon reduction technologies like sodium acetate.
Government support for research and development in carbon reduction technologies is also a key aspect of the regulatory framework. Many countries offer grants, tax incentives, and other forms of support for the development and implementation of innovative carbon reduction solutions. This support can be crucial in accelerating the adoption of technologies like sodium acetate across various industrial sectors.
As the urgency of climate action grows, regulatory frameworks are likely to become more stringent and comprehensive. Industries that proactively adopt effective carbon reduction technologies like sodium acetate will be better positioned to comply with evolving regulations and maintain their competitive edge in an increasingly carbon-constrained economy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!