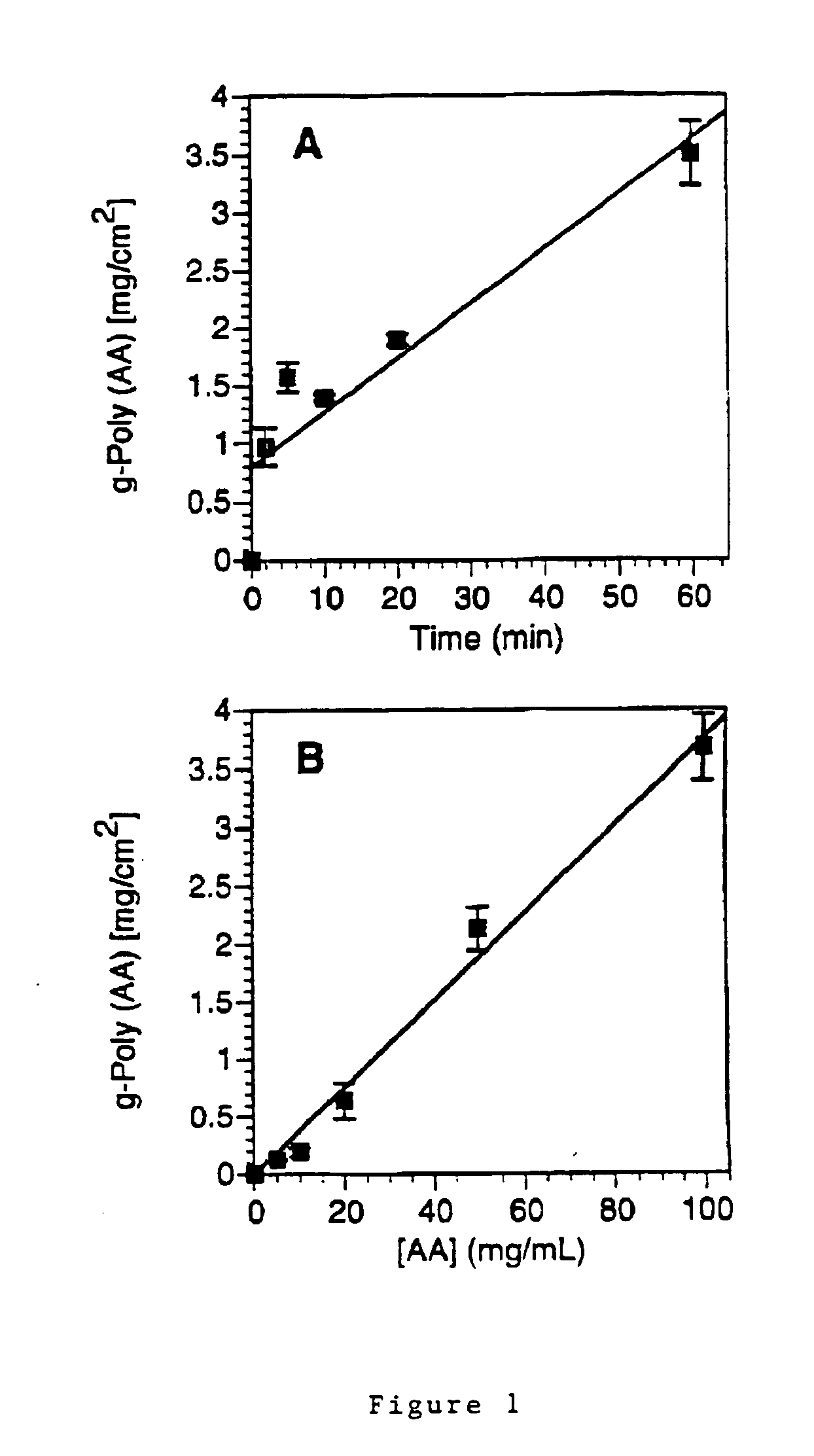
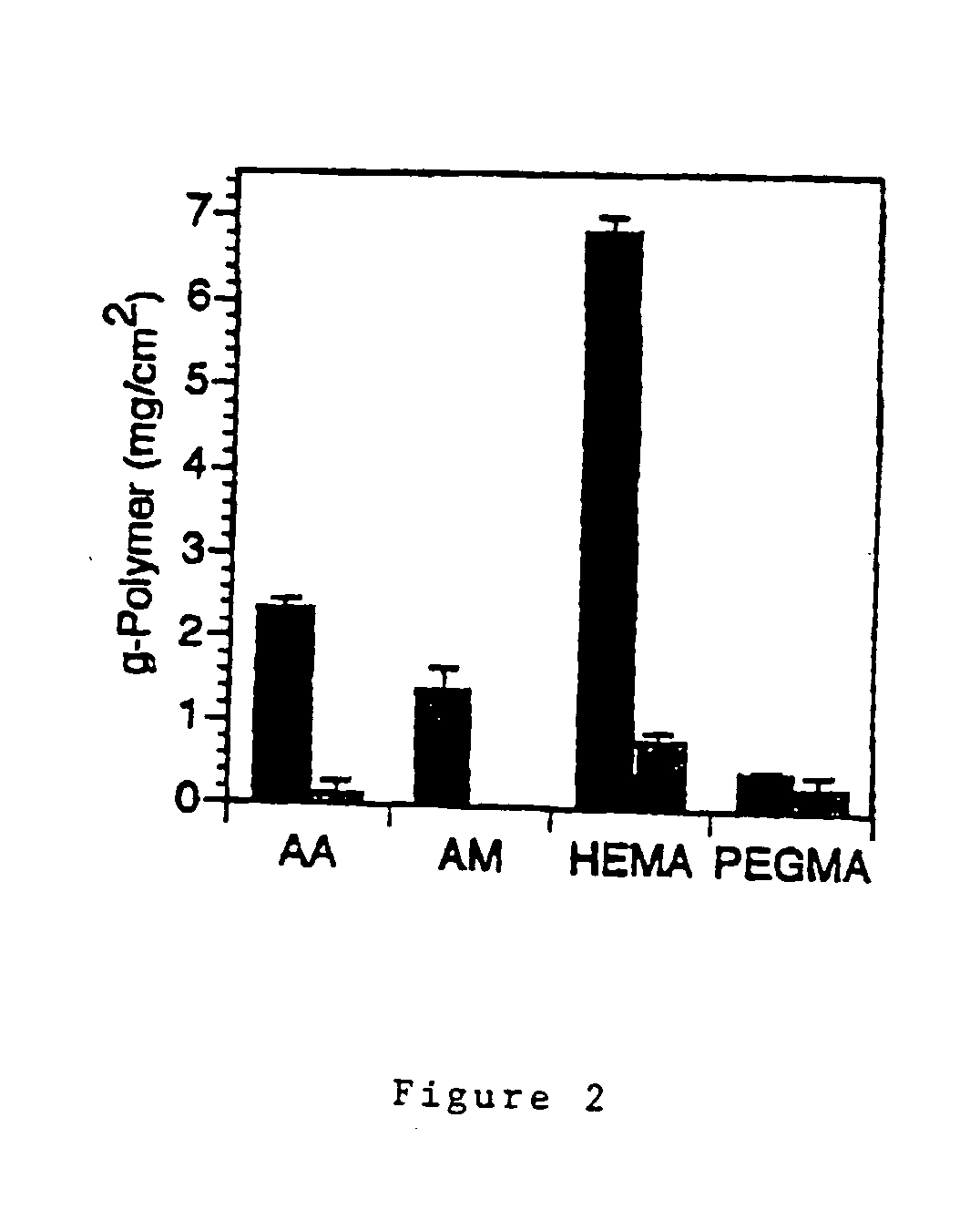
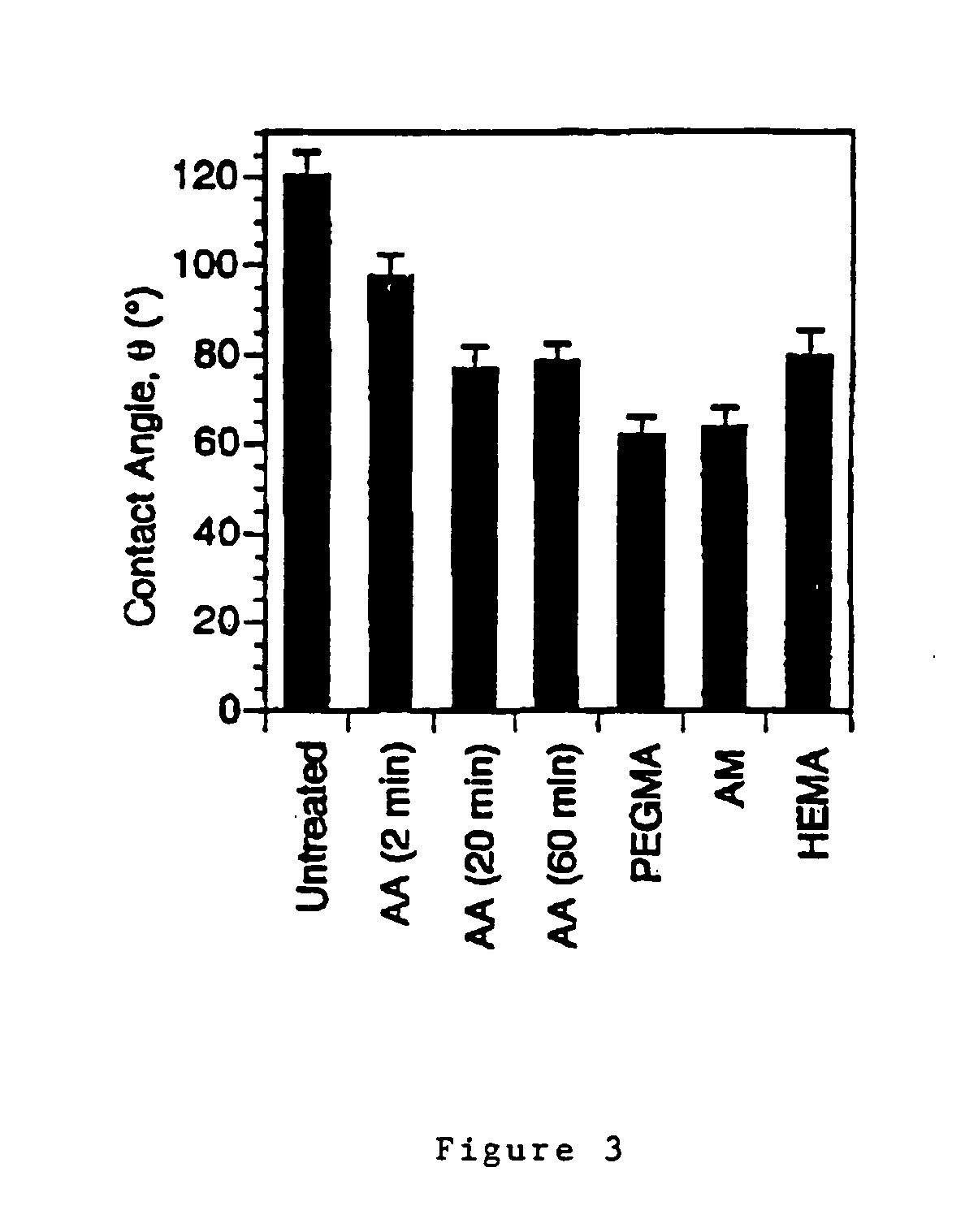
How Surface Modification Enhances Biomedical Polymer Applications
OCT 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Surface Modification Background and Objectives
Biomedical polymers have emerged as critical materials in healthcare applications over the past several decades, evolving from simple structural components to sophisticated functional materials. The trajectory of these materials has been marked by continuous innovation, particularly in surface modification techniques that enhance biocompatibility, functionality, and performance. Initially limited by surface properties that often led to adverse biological responses, polymeric biomaterials have undergone significant transformation through various modification strategies.
The evolution of surface modification techniques for biomedical polymers can be traced back to the 1960s with rudimentary physical treatments. By the 1980s, chemical modification methods gained prominence, followed by the development of plasma treatments in the 1990s. The early 2000s witnessed the rise of biomolecule immobilization techniques, while recent years have seen remarkable advancements in nanoscale modifications and stimuli-responsive surfaces.
Current technological trends indicate a shift toward precision engineering of polymer surfaces at the molecular level, enabling unprecedented control over cell-material interactions. The integration of computational modeling with experimental approaches has accelerated innovation in this field, allowing for predictive design of surface properties tailored to specific biomedical applications.
The primary objective of biomedical polymer surface modification is to overcome the inherent limitations of bulk materials while preserving their beneficial mechanical and physical properties. Specific goals include enhancing biocompatibility to minimize foreign body responses, improving hemocompatibility for blood-contacting devices, promoting selective cell adhesion for tissue engineering applications, and incorporating antimicrobial properties to prevent device-associated infections.
Additionally, surface modification aims to enable controlled drug release profiles from polymeric delivery systems, improve the durability and longevity of implantable devices, and create responsive interfaces that can adapt to changing physiological conditions. These objectives align with broader healthcare trends toward personalized medicine, minimally invasive procedures, and long-term implantable solutions.
The technological landscape is increasingly focused on sustainable and scalable modification methods that can transition effectively from laboratory research to commercial manufacturing. This includes the development of environmentally friendly processes, reduction of toxic reagents, and implementation of quality control measures suitable for regulated medical device production.
As biomedical technologies continue to advance, the goals for polymer surface modification are expanding to include integration with digital health platforms, compatibility with imaging modalities, and adaptability to 3D printing and other advanced manufacturing techniques. These developments represent the convergence of materials science, biology, and information technology in addressing complex healthcare challenges.
The evolution of surface modification techniques for biomedical polymers can be traced back to the 1960s with rudimentary physical treatments. By the 1980s, chemical modification methods gained prominence, followed by the development of plasma treatments in the 1990s. The early 2000s witnessed the rise of biomolecule immobilization techniques, while recent years have seen remarkable advancements in nanoscale modifications and stimuli-responsive surfaces.
Current technological trends indicate a shift toward precision engineering of polymer surfaces at the molecular level, enabling unprecedented control over cell-material interactions. The integration of computational modeling with experimental approaches has accelerated innovation in this field, allowing for predictive design of surface properties tailored to specific biomedical applications.
The primary objective of biomedical polymer surface modification is to overcome the inherent limitations of bulk materials while preserving their beneficial mechanical and physical properties. Specific goals include enhancing biocompatibility to minimize foreign body responses, improving hemocompatibility for blood-contacting devices, promoting selective cell adhesion for tissue engineering applications, and incorporating antimicrobial properties to prevent device-associated infections.
Additionally, surface modification aims to enable controlled drug release profiles from polymeric delivery systems, improve the durability and longevity of implantable devices, and create responsive interfaces that can adapt to changing physiological conditions. These objectives align with broader healthcare trends toward personalized medicine, minimally invasive procedures, and long-term implantable solutions.
The technological landscape is increasingly focused on sustainable and scalable modification methods that can transition effectively from laboratory research to commercial manufacturing. This includes the development of environmentally friendly processes, reduction of toxic reagents, and implementation of quality control measures suitable for regulated medical device production.
As biomedical technologies continue to advance, the goals for polymer surface modification are expanding to include integration with digital health platforms, compatibility with imaging modalities, and adaptability to 3D printing and other advanced manufacturing techniques. These developments represent the convergence of materials science, biology, and information technology in addressing complex healthcare challenges.
Market Analysis of Modified Biomedical Polymers
The global market for surface-modified biomedical polymers has experienced significant growth in recent years, driven by increasing demand for advanced medical devices and implants. Currently valued at approximately 12 billion USD, this market segment is projected to grow at a compound annual growth rate of 8.7% through 2028, outpacing the broader medical polymers sector which grows at 6.2% annually.
North America dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory due to expanding healthcare infrastructure and increasing medical device manufacturing capabilities. Japan maintains its position as a technology leader in specialized surface modification techniques.
Market segmentation reveals orthopedic applications commanding the largest share (34%), followed by cardiovascular devices (27%), tissue engineering scaffolds (18%), drug delivery systems (12%), and other applications (9%). The cardiovascular segment shows the highest growth potential due to increasing prevalence of cardiovascular diseases globally and technological advancements in stent coatings.
End-user analysis indicates hospitals and clinics as primary consumers (56%), followed by research institutions (24%) and ambulatory surgical centers (20%). The ambulatory surgical centers segment is experiencing rapid growth due to the shift toward outpatient procedures and cost-effective healthcare delivery models.
Key market drivers include aging populations in developed economies, rising prevalence of chronic diseases requiring long-term implants, and increasing demand for minimally invasive procedures. Additionally, stringent regulatory requirements for biocompatibility are pushing manufacturers toward advanced surface modification technologies that enhance device performance while reducing adverse biological responses.
Market restraints include high development and manufacturing costs, complex regulatory approval processes, and technical challenges in achieving consistent surface modification at commercial scale. The average time-to-market for surface-modified medical devices has decreased from 5.2 years to 3.8 years over the past decade, yet remains significantly longer than conventional medical devices.
Consumer preference analysis reveals growing demand for antimicrobial surfaces, particularly following the COVID-19 pandemic, with 78% of healthcare providers prioritizing infection control properties in implantable devices. Additionally, there is increasing market pull for biodegradable and bioresorbable surface modifications that eliminate the need for secondary removal procedures.
North America dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory due to expanding healthcare infrastructure and increasing medical device manufacturing capabilities. Japan maintains its position as a technology leader in specialized surface modification techniques.
Market segmentation reveals orthopedic applications commanding the largest share (34%), followed by cardiovascular devices (27%), tissue engineering scaffolds (18%), drug delivery systems (12%), and other applications (9%). The cardiovascular segment shows the highest growth potential due to increasing prevalence of cardiovascular diseases globally and technological advancements in stent coatings.
End-user analysis indicates hospitals and clinics as primary consumers (56%), followed by research institutions (24%) and ambulatory surgical centers (20%). The ambulatory surgical centers segment is experiencing rapid growth due to the shift toward outpatient procedures and cost-effective healthcare delivery models.
Key market drivers include aging populations in developed economies, rising prevalence of chronic diseases requiring long-term implants, and increasing demand for minimally invasive procedures. Additionally, stringent regulatory requirements for biocompatibility are pushing manufacturers toward advanced surface modification technologies that enhance device performance while reducing adverse biological responses.
Market restraints include high development and manufacturing costs, complex regulatory approval processes, and technical challenges in achieving consistent surface modification at commercial scale. The average time-to-market for surface-modified medical devices has decreased from 5.2 years to 3.8 years over the past decade, yet remains significantly longer than conventional medical devices.
Consumer preference analysis reveals growing demand for antimicrobial surfaces, particularly following the COVID-19 pandemic, with 78% of healthcare providers prioritizing infection control properties in implantable devices. Additionally, there is increasing market pull for biodegradable and bioresorbable surface modifications that eliminate the need for secondary removal procedures.
Current Surface Modification Technologies and Challenges
Surface modification technologies for biomedical polymers have evolved significantly over the past decades, with several established methods now dominating the industry. Physical modification techniques include plasma treatment, which creates reactive species on polymer surfaces without altering bulk properties, and laser treatment, which offers precise spatial control for surface patterning. These methods effectively enhance surface energy and create functional groups but may suffer from aging effects and limited penetration depth.
Chemical modification approaches include wet chemical treatments utilizing acids, bases, or oxidizing agents to introduce specific functional groups. While cost-effective, these methods often present challenges in controlling reaction depth and maintaining uniform surface coverage. Grafting techniques, both "grafting-to" and "grafting-from" approaches, have gained prominence for creating stable covalent bonds between polymers and bioactive molecules, though they require careful optimization of reaction conditions.
Coating technologies represent another major category, with dip-coating and spin-coating being widely employed for their simplicity. However, these methods face adhesion issues and coating stability concerns in physiological environments. Layer-by-layer assembly offers nanoscale control over surface properties but is time-consuming for thick coatings.
Despite these advances, significant challenges persist in the field. Achieving long-term stability of modified surfaces remains problematic, as many modifications deteriorate under physiological conditions or sterilization processes. The trade-off between surface functionality and bulk material properties presents another obstacle, as aggressive modification techniques may compromise mechanical integrity or introduce unwanted changes to the polymer backbone.
Scalability and reproducibility issues hinder industrial implementation, with many laboratory-scale techniques proving difficult to translate to mass production. Regulatory hurdles further complicate commercialization, as novel surface modifications require extensive safety validation and standardization.
Biocompatibility assessment presents unique challenges, with in vitro tests often failing to predict in vivo performance accurately. The complexity of biological responses to modified surfaces necessitates comprehensive testing protocols that consider both immediate and long-term host reactions.
Emerging concerns include the environmental impact of modification processes, with many techniques utilizing hazardous chemicals or generating significant waste. The industry is increasingly focused on developing greener alternatives that maintain performance while reducing ecological footprint.
Cost-effectiveness remains a critical consideration, as complex modification technologies may significantly increase product costs, limiting widespread adoption in healthcare settings where budget constraints are prevalent.
Chemical modification approaches include wet chemical treatments utilizing acids, bases, or oxidizing agents to introduce specific functional groups. While cost-effective, these methods often present challenges in controlling reaction depth and maintaining uniform surface coverage. Grafting techniques, both "grafting-to" and "grafting-from" approaches, have gained prominence for creating stable covalent bonds between polymers and bioactive molecules, though they require careful optimization of reaction conditions.
Coating technologies represent another major category, with dip-coating and spin-coating being widely employed for their simplicity. However, these methods face adhesion issues and coating stability concerns in physiological environments. Layer-by-layer assembly offers nanoscale control over surface properties but is time-consuming for thick coatings.
Despite these advances, significant challenges persist in the field. Achieving long-term stability of modified surfaces remains problematic, as many modifications deteriorate under physiological conditions or sterilization processes. The trade-off between surface functionality and bulk material properties presents another obstacle, as aggressive modification techniques may compromise mechanical integrity or introduce unwanted changes to the polymer backbone.
Scalability and reproducibility issues hinder industrial implementation, with many laboratory-scale techniques proving difficult to translate to mass production. Regulatory hurdles further complicate commercialization, as novel surface modifications require extensive safety validation and standardization.
Biocompatibility assessment presents unique challenges, with in vitro tests often failing to predict in vivo performance accurately. The complexity of biological responses to modified surfaces necessitates comprehensive testing protocols that consider both immediate and long-term host reactions.
Emerging concerns include the environmental impact of modification processes, with many techniques utilizing hazardous chemicals or generating significant waste. The industry is increasingly focused on developing greener alternatives that maintain performance while reducing ecological footprint.
Cost-effectiveness remains a critical consideration, as complex modification technologies may significantly increase product costs, limiting widespread adoption in healthcare settings where budget constraints are prevalent.
Established Surface Modification Techniques for Biomedical Applications
01 Plasma treatment for surface modification
Plasma treatment is used to modify the surface properties of biomedical polymers, enhancing their biocompatibility and functionality. This technique involves exposing the polymer surface to ionized gas, which creates reactive species that can alter the surface chemistry without affecting the bulk properties. Plasma treatment can introduce functional groups, increase surface energy, and improve wettability, making the polymers more suitable for biomedical applications such as implants and tissue engineering scaffolds.- Plasma treatment for surface modification: Plasma treatment is used to modify the surface of biomedical polymers to enhance their biocompatibility and functionality. This technique involves exposing the polymer surface to ionized gas, which creates reactive species that can alter the surface properties without affecting the bulk material. Plasma treatment can introduce functional groups, increase surface energy, and improve wettability, which enhances cell adhesion and protein binding on biomedical devices.
- Chemical grafting of bioactive molecules: Chemical grafting involves attaching bioactive molecules to the surface of biomedical polymers to enhance their biological performance. This modification technique uses coupling agents or functional groups to covalently bind molecules such as growth factors, antimicrobial agents, or cell-adhesion peptides to the polymer surface. The grafted bioactive molecules can promote specific cellular responses, reduce bacterial adhesion, or improve tissue integration of implantable devices.
- Layer-by-layer surface coating techniques: Layer-by-layer coating involves the sequential deposition of oppositely charged polyelectrolytes or other materials onto biomedical polymer surfaces. This technique allows for precise control over coating thickness and composition, enabling the creation of multifunctional surfaces. These coatings can incorporate drugs, growth factors, or other bioactive agents for controlled release, while also modifying surface properties such as hydrophilicity, charge, and roughness to enhance biocompatibility.
- Hydrophilic surface modifications: Hydrophilic surface modifications involve introducing water-loving groups to biomedical polymer surfaces to reduce protein adsorption and cell adhesion where needed. These modifications can be achieved through various techniques including hydrogel coatings, PEGylation, or the incorporation of zwitterionic structures. Hydrophilic surfaces help prevent biofouling, reduce thrombogenicity, and improve the lubricity of medical devices, making them particularly valuable for blood-contacting applications and ophthalmic devices.
- Nanostructured surface patterning: Nanostructured surface patterning involves creating specific topographical features at the nanoscale on biomedical polymer surfaces. These patterns can be created through techniques such as nanoimprinting, lithography, or self-assembly processes. Controlled surface topography can guide cell alignment and behavior, enhance osseointegration of implants, and provide antimicrobial properties through physical disruption of bacterial cell membranes, without relying on chemical agents.
02 Chemical grafting and functionalization
Chemical grafting involves attaching functional molecules or polymers onto biomedical polymer surfaces to enhance their properties. This approach can introduce specific functional groups that promote cell adhesion, reduce protein adsorption, or provide antimicrobial properties. Techniques such as UV-initiated grafting, redox-initiated grafting, and click chemistry are commonly used to achieve covalent attachment of the desired molecules, resulting in stable surface modifications that improve biocompatibility and performance in medical applications.Expand Specific Solutions03 Biomolecule immobilization techniques
Immobilization of biomolecules such as proteins, peptides, and growth factors onto polymer surfaces enhances their bioactivity and interaction with biological systems. Various techniques including physical adsorption, covalent binding, and affinity immobilization are used to attach these biomolecules to the polymer surface. This modification strategy improves cell adhesion, proliferation, and tissue integration, making the polymers more effective for applications such as implantable devices, drug delivery systems, and tissue engineering scaffolds.Expand Specific Solutions04 Nanoparticle and nanocomposite coatings
Incorporating nanoparticles and creating nanocomposite coatings on biomedical polymer surfaces enhances their mechanical, antimicrobial, and bioactive properties. Nanoparticles such as silver, gold, hydroxyapatite, and various metal oxides can be deposited or embedded into polymer surfaces to provide specific functionalities. These nanostructured coatings can improve wear resistance, reduce bacterial adhesion, enhance osseointegration, and provide controlled drug release capabilities, significantly expanding the application range of biomedical polymers.Expand Specific Solutions05 Surface texturing and patterning
Creating specific surface topographies and patterns on biomedical polymers can control cell behavior and enhance tissue integration. Techniques such as laser ablation, photolithography, soft lithography, and 3D printing are used to generate micro and nano-scale features on polymer surfaces. These textured surfaces can guide cell alignment, promote selective cell adhesion, control protein adsorption, and enhance mechanical interlocking with surrounding tissues, leading to improved performance of biomedical devices and implants.Expand Specific Solutions
Leading Companies and Research Institutions in the Field
The surface modification of biomedical polymers is currently in a growth phase, with the market expanding rapidly due to increasing demand for advanced medical devices and implants. The global biomedical polymer surface modification market is estimated to reach several billion dollars by 2025, driven by applications in orthopedics, cardiovascular, and drug delivery systems. Technologically, the field shows varying maturity levels across different modification techniques. Leading players like Medtronic and DePuy Synthes have established strong commercial platforms for surface-modified implants, while research institutions such as Tsinghua University, University of Queensland, and Commonwealth Scientific & Industrial Research Organisation are advancing fundamental technologies. Companies like Toray Industries and IBM are developing proprietary surface modification methods, creating a competitive landscape balanced between established medical device manufacturers and innovative research organizations exploring novel approaches.
DePuy Synthes Products, Inc.
Technical Solution: DePuy Synthes has developed advanced POROCOAT® and GRIPTION® surface modification technologies for orthopedic implants. Their approach focuses on creating porous titanium coatings on polymer substrates to enhance osseointegration. The company utilizes plasma spray techniques to deposit hydroxyapatite and other bioactive ceramics onto PEEK (polyetheretherketone) and UHMWPE (ultra-high-molecular-weight polyethylene) implants. Their proprietary SMARTSET® technology incorporates antibiotic-releasing polymers with modified surface topography to prevent infection while promoting tissue integration. DePuy's recent innovations include gradient porosity surfaces that mimic natural bone structure, with pore sizes ranging from 100-600μm to optimize cell attachment and bone ingrowth. They've also developed a unique surface modification process called ATTUNE S+™ that combines chemical etching and plasma treatment to create nanoscale surface features on polymer implants, significantly improving cellular adhesion and differentiation of mesenchymal stem cells toward osteoblastic lineages.
Strengths: Extensive clinical data supporting efficacy of surface-modified orthopedic implants; sophisticated manufacturing capabilities for consistent surface modification; strong integration between material science and biological requirements. Weaknesses: Higher production costs for complex surface modifications; longer regulatory approval timelines for novel surface treatments; some surface modifications may affect the mechanical properties of the base polymer.
Medtronic, Inc.
Technical Solution: Medtronic has developed proprietary SurModics' PhotoLink® surface modification technology that creates covalently bonded coatings on polymer surfaces. Their approach involves photochemically attaching hydrophilic, anti-thrombogenic, and drug-eluting coatings to medical device surfaces. The technology uses UV light to activate specialized reagents that form strong chemical bonds with the polymer substrate. Medtronic has implemented this in their cardiac and vascular devices, particularly for drug-eluting stents and catheters, where the modified surfaces significantly reduce protein adsorption and platelet adhesion. Their recent innovations include gradient-functional coatings that transition from hydrophobic to hydrophilic properties across the surface, enabling better tissue integration while maintaining biocompatibility. The company has also pioneered antimicrobial surface modifications incorporating silver nanoparticles and quaternary ammonium compounds to prevent biofilm formation on implantable devices.
Strengths: Industry-leading expertise in surface modification for cardiovascular applications with clinically proven technologies; extensive patent portfolio; integration of drug delivery capabilities with surface modification. Weaknesses: Higher manufacturing costs compared to unmodified devices; some coating technologies require specialized equipment and controlled environments; potential regulatory challenges with novel surface modifications.
Key Patents and Innovations in Polymer Surface Engineering
Modified Anti-microbial surfaces, devices and methods
PatentInactiveUS20110212152A1
Innovation
- The method involves coating polymeric or metallic substrates with a hydrophilic polyacrylate layer using UV light-induced free radical polymerization, incorporating a silver component within the coating, which can be released to provide anti-microbial properties, and using a polyethylene oxide hydrogel to enhance stability and bio-compatibility.
Surface modification of polymers via surface active and reactive end groups
PatentActiveCA2743493C
Innovation
- A method that forms reactive end groups on the polymer chain ends during fabrication and uses surface active spacers to enrich these groups at the surface, allowing direct modification with bio-active molecules and monomers without pre-treatment, using a multifunctional coupling agent to form covalent bonds for specific surface properties.
Biocompatibility and Safety Considerations
Biocompatibility remains a paramount concern in the development and application of surface-modified biomedical polymers. When polymers interact with biological systems, they must not elicit adverse immune responses, inflammation, or toxicity. Surface modification techniques significantly enhance biocompatibility by altering the interface between the polymer and biological environment without compromising the bulk properties of the material.
The safety profile of surface-modified polymers depends largely on the modification technique employed. Chemical modifications using functional groups such as carboxyl, amino, or hydroxyl groups can improve hydrophilicity and reduce protein adsorption, thereby minimizing immune responses. However, residual chemicals from these processes must be thoroughly removed to prevent potential cytotoxicity. Plasma treatment, while effective for surface activation, may generate free radicals that could potentially damage surrounding tissues if not properly neutralized.
Regulatory frameworks worldwide have established stringent guidelines for biocompatibility testing of medical devices. ISO 10993 standards provide comprehensive protocols for evaluating cytotoxicity, sensitization, irritation, and systemic toxicity. Surface-modified polymers must undergo these rigorous assessments before clinical application, with particular attention to leachables and extractables that might emerge from the modified surfaces over time.
Long-term stability of surface modifications presents another critical safety consideration. Some modifications may degrade over time, potentially releasing harmful byproducts or exposing the underlying polymer substrate. Accelerated aging studies and real-time stability testing are essential to predict the performance of modified surfaces throughout the intended lifespan of the medical device.
Hemocompatibility deserves special attention for blood-contacting devices such as vascular grafts, stents, and catheters. Surface modifications aimed at reducing thrombogenicity must balance anti-coagulation properties with the need to maintain normal hemostasis. Techniques such as heparin immobilization or zwitterionic polymer coating have shown promise in improving hemocompatibility while maintaining safety profiles.
The sterilization compatibility of surface-modified polymers represents another crucial safety aspect. Common sterilization methods like ethylene oxide, gamma irradiation, or steam autoclaving can potentially alter surface chemistry or degrade certain modifications. Validation studies must confirm that the biocompatible properties remain intact after sterilization processes, as changes could compromise both safety and efficacy.
Emerging nanotechnology-based surface modifications introduce additional safety considerations regarding nanoparticle release and biodistribution. While nanostructured surfaces can dramatically improve cell adhesion and tissue integration, the potential for nanoparticle detachment and subsequent accumulation in organs requires thorough investigation through specialized toxicological assessments.
The safety profile of surface-modified polymers depends largely on the modification technique employed. Chemical modifications using functional groups such as carboxyl, amino, or hydroxyl groups can improve hydrophilicity and reduce protein adsorption, thereby minimizing immune responses. However, residual chemicals from these processes must be thoroughly removed to prevent potential cytotoxicity. Plasma treatment, while effective for surface activation, may generate free radicals that could potentially damage surrounding tissues if not properly neutralized.
Regulatory frameworks worldwide have established stringent guidelines for biocompatibility testing of medical devices. ISO 10993 standards provide comprehensive protocols for evaluating cytotoxicity, sensitization, irritation, and systemic toxicity. Surface-modified polymers must undergo these rigorous assessments before clinical application, with particular attention to leachables and extractables that might emerge from the modified surfaces over time.
Long-term stability of surface modifications presents another critical safety consideration. Some modifications may degrade over time, potentially releasing harmful byproducts or exposing the underlying polymer substrate. Accelerated aging studies and real-time stability testing are essential to predict the performance of modified surfaces throughout the intended lifespan of the medical device.
Hemocompatibility deserves special attention for blood-contacting devices such as vascular grafts, stents, and catheters. Surface modifications aimed at reducing thrombogenicity must balance anti-coagulation properties with the need to maintain normal hemostasis. Techniques such as heparin immobilization or zwitterionic polymer coating have shown promise in improving hemocompatibility while maintaining safety profiles.
The sterilization compatibility of surface-modified polymers represents another crucial safety aspect. Common sterilization methods like ethylene oxide, gamma irradiation, or steam autoclaving can potentially alter surface chemistry or degrade certain modifications. Validation studies must confirm that the biocompatible properties remain intact after sterilization processes, as changes could compromise both safety and efficacy.
Emerging nanotechnology-based surface modifications introduce additional safety considerations regarding nanoparticle release and biodistribution. While nanostructured surfaces can dramatically improve cell adhesion and tissue integration, the potential for nanoparticle detachment and subsequent accumulation in organs requires thorough investigation through specialized toxicological assessments.
Regulatory Pathway for Modified Biomedical Polymers
The regulatory landscape for surface-modified biomedical polymers presents a complex pathway that manufacturers must navigate to bring products to market. In the United States, the FDA oversees these materials through various centers depending on their intended use, with the Center for Devices and Radiological Health (CDRH) handling most polymer-based medical devices. Surface-modified polymers typically require submission through the 510(k) premarket notification process if they are substantially equivalent to predefined predicate devices, or through the more rigorous Premarket Approval (PMA) pathway for novel technologies.
European regulatory frameworks operate under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which replaced the previous Medical Device Directive in 2021. These regulations impose stricter requirements for clinical evidence and post-market surveillance, particularly for Class III devices where many surface-modified polymers are categorized due to their long-term implantable nature or contact with critical body systems.
Risk classification plays a pivotal role in determining the regulatory pathway. Surface modifications that alter biocompatibility, degradation profiles, or introduce novel biological interactions often elevate the risk classification, necessitating more comprehensive testing protocols. Manufacturers must demonstrate that modifications do not compromise the safety profile established for the base polymer material.
Testing requirements for modified biomedical polymers include comprehensive biocompatibility assessments following ISO 10993 standards, with particular emphasis on cytotoxicity, sensitization, and hemocompatibility for blood-contacting devices. Surface-specific characterization techniques such as X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), and contact angle measurements are essential to document the modification's physical and chemical properties.
Documentation requirements include detailed manufacturing process validation, with special attention to the consistency and stability of the surface modification technique. Quality control parameters must be established to ensure batch-to-batch reproducibility of surface properties, which presents unique challenges compared to bulk material manufacturing.
Post-market surveillance has become increasingly important under both FDA and MDR frameworks, requiring manufacturers to implement robust systems for tracking device performance and adverse events specifically related to surface interactions. This includes monitoring for unexpected biological responses that may not have been evident during preclinical testing.
Harmonization efforts between international regulatory bodies have improved in recent years, with initiatives like the Medical Device Single Audit Program (MDSAP) allowing for more streamlined compliance across multiple markets. However, significant regional differences remain in how surface-modified materials are evaluated, particularly regarding acceptable testing methodologies and risk assessment approaches.
European regulatory frameworks operate under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which replaced the previous Medical Device Directive in 2021. These regulations impose stricter requirements for clinical evidence and post-market surveillance, particularly for Class III devices where many surface-modified polymers are categorized due to their long-term implantable nature or contact with critical body systems.
Risk classification plays a pivotal role in determining the regulatory pathway. Surface modifications that alter biocompatibility, degradation profiles, or introduce novel biological interactions often elevate the risk classification, necessitating more comprehensive testing protocols. Manufacturers must demonstrate that modifications do not compromise the safety profile established for the base polymer material.
Testing requirements for modified biomedical polymers include comprehensive biocompatibility assessments following ISO 10993 standards, with particular emphasis on cytotoxicity, sensitization, and hemocompatibility for blood-contacting devices. Surface-specific characterization techniques such as X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), and contact angle measurements are essential to document the modification's physical and chemical properties.
Documentation requirements include detailed manufacturing process validation, with special attention to the consistency and stability of the surface modification technique. Quality control parameters must be established to ensure batch-to-batch reproducibility of surface properties, which presents unique challenges compared to bulk material manufacturing.
Post-market surveillance has become increasingly important under both FDA and MDR frameworks, requiring manufacturers to implement robust systems for tracking device performance and adverse events specifically related to surface interactions. This includes monitoring for unexpected biological responses that may not have been evident during preclinical testing.
Harmonization efforts between international regulatory bodies have improved in recent years, with initiatives like the Medical Device Single Audit Program (MDSAP) allowing for more streamlined compliance across multiple markets. However, significant regional differences remain in how surface-modified materials are evaluated, particularly regarding acceptable testing methodologies and risk assessment approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!