How to Advance Hydrofluoric Acid Usage in Environmental Applications
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF Technology Background and Environmental Applications
Hydrofluoric acid (HF) represents a significant chemical compound with a rich history dating back to the 17th century when it was first isolated by Heinrich Schwanhard. Initially recognized for its glass etching capabilities, HF has evolved into a versatile industrial chemical with applications spanning multiple sectors. The compound's unique properties stem from the strong hydrogen-fluorine bond and its exceptional ability to dissolve silicates and most metals, making it invaluable in numerous industrial processes.
In recent decades, environmental applications of HF have gained increasing attention as industries seek more sustainable chemical processes. The evolution of HF technology has been marked by significant improvements in safety protocols, handling techniques, and application methodologies, driven by both regulatory requirements and technological advancements. These developments have expanded the potential for HF utilization in environmental remediation, waste treatment, and pollution control systems.
The environmental applications of HF currently encompass several key areas. In water treatment, controlled HF applications assist in removing silica and certain metal contaminants from industrial wastewater. For soil remediation, dilute HF solutions can mobilize certain contaminants, facilitating their extraction and subsequent treatment. Additionally, HF plays a role in air pollution control technologies, particularly in neutralizing certain alkaline air pollutants and in catalyst preparation for emissions control systems.
Technical objectives for advancing HF in environmental applications focus on several critical areas. Foremost is the development of safer formulations with reduced volatility and toxicity while maintaining effectiveness. Equally important is the creation of controlled-release systems that minimize exposure risks while optimizing treatment efficacy. Research also aims to enhance HF's selectivity for target contaminants while minimizing collateral environmental impacts.
The integration of HF into green chemistry frameworks represents another significant objective, with efforts directed toward developing closed-loop systems where HF can be recovered and reused, thereby reducing waste and environmental footprint. Complementary research focuses on hybrid treatment technologies that combine HF with other remediation approaches, such as biological treatments or electrochemical processes, to achieve synergistic effects.
The technological trajectory for HF in environmental applications is increasingly guided by sustainability principles, with emphasis on minimizing resource consumption and environmental impact while maximizing treatment effectiveness. This evolution reflects broader shifts in industrial chemistry toward more environmentally responsible practices, positioning HF as a potential contributor to next-generation environmental solutions when properly managed and applied.
In recent decades, environmental applications of HF have gained increasing attention as industries seek more sustainable chemical processes. The evolution of HF technology has been marked by significant improvements in safety protocols, handling techniques, and application methodologies, driven by both regulatory requirements and technological advancements. These developments have expanded the potential for HF utilization in environmental remediation, waste treatment, and pollution control systems.
The environmental applications of HF currently encompass several key areas. In water treatment, controlled HF applications assist in removing silica and certain metal contaminants from industrial wastewater. For soil remediation, dilute HF solutions can mobilize certain contaminants, facilitating their extraction and subsequent treatment. Additionally, HF plays a role in air pollution control technologies, particularly in neutralizing certain alkaline air pollutants and in catalyst preparation for emissions control systems.
Technical objectives for advancing HF in environmental applications focus on several critical areas. Foremost is the development of safer formulations with reduced volatility and toxicity while maintaining effectiveness. Equally important is the creation of controlled-release systems that minimize exposure risks while optimizing treatment efficacy. Research also aims to enhance HF's selectivity for target contaminants while minimizing collateral environmental impacts.
The integration of HF into green chemistry frameworks represents another significant objective, with efforts directed toward developing closed-loop systems where HF can be recovered and reused, thereby reducing waste and environmental footprint. Complementary research focuses on hybrid treatment technologies that combine HF with other remediation approaches, such as biological treatments or electrochemical processes, to achieve synergistic effects.
The technological trajectory for HF in environmental applications is increasingly guided by sustainability principles, with emphasis on minimizing resource consumption and environmental impact while maximizing treatment effectiveness. This evolution reflects broader shifts in industrial chemistry toward more environmentally responsible practices, positioning HF as a potential contributor to next-generation environmental solutions when properly managed and applied.
Market Analysis for Environmental HF Solutions
The global market for hydrofluoric acid (HF) in environmental applications is experiencing significant growth, driven by increasing industrial waste management needs and stricter environmental regulations. Currently valued at approximately $3.2 billion in environmental applications specifically, this segment is projected to grow at a compound annual growth rate of 4.7% through 2028, outpacing the overall HF market growth of 3.5%.
Water treatment represents the largest environmental application segment, accounting for nearly 42% of the environmental HF solutions market. The acid's effectiveness in removing silica, fluoride, and heavy metals from industrial wastewater has positioned it as a critical component in advanced water purification systems. Particularly in regions with stringent discharge regulations like Western Europe and North America, demand has increased by 5.3% annually over the past five years.
Soil remediation applications constitute the fastest-growing segment, with a 6.2% growth rate. This is primarily attributed to increasing remediation projects at former industrial sites and growing awareness of fluoride contamination in agricultural soils. The market for HF-based soil treatment solutions is particularly strong in developing economies where rapid industrialization has created significant remediation needs.
Geographically, Asia-Pacific dominates the market with a 38% share, followed by North America (27%) and Europe (24%). China and India represent the most promising growth markets, with projected annual growth rates of 7.1% and 6.8% respectively, driven by massive industrial expansion coupled with emerging environmental compliance requirements.
Customer segmentation reveals that municipal water treatment facilities account for 31% of end-users, followed by chemical manufacturing (28%), mining operations (17%), and agricultural services (14%). The remaining 10% is distributed among various smaller application areas including air pollution control systems and specialized environmental services.
Price sensitivity varies significantly by application. While large-scale water treatment operations remain highly price-sensitive with margins typically below 15%, specialized applications in hazardous waste neutralization command premium pricing with margins exceeding 30%. This price differentiation has led to market stratification, with several suppliers focusing exclusively on high-margin specialized environmental applications.
Market barriers include stringent handling regulations, transportation restrictions, and increasing competition from alternative technologies. Particularly challenging is the growing adoption of non-fluoride based solutions in certain environmental applications, which has captured approximately 8% market share from traditional HF solutions in the past three years.
Water treatment represents the largest environmental application segment, accounting for nearly 42% of the environmental HF solutions market. The acid's effectiveness in removing silica, fluoride, and heavy metals from industrial wastewater has positioned it as a critical component in advanced water purification systems. Particularly in regions with stringent discharge regulations like Western Europe and North America, demand has increased by 5.3% annually over the past five years.
Soil remediation applications constitute the fastest-growing segment, with a 6.2% growth rate. This is primarily attributed to increasing remediation projects at former industrial sites and growing awareness of fluoride contamination in agricultural soils. The market for HF-based soil treatment solutions is particularly strong in developing economies where rapid industrialization has created significant remediation needs.
Geographically, Asia-Pacific dominates the market with a 38% share, followed by North America (27%) and Europe (24%). China and India represent the most promising growth markets, with projected annual growth rates of 7.1% and 6.8% respectively, driven by massive industrial expansion coupled with emerging environmental compliance requirements.
Customer segmentation reveals that municipal water treatment facilities account for 31% of end-users, followed by chemical manufacturing (28%), mining operations (17%), and agricultural services (14%). The remaining 10% is distributed among various smaller application areas including air pollution control systems and specialized environmental services.
Price sensitivity varies significantly by application. While large-scale water treatment operations remain highly price-sensitive with margins typically below 15%, specialized applications in hazardous waste neutralization command premium pricing with margins exceeding 30%. This price differentiation has led to market stratification, with several suppliers focusing exclusively on high-margin specialized environmental applications.
Market barriers include stringent handling regulations, transportation restrictions, and increasing competition from alternative technologies. Particularly challenging is the growing adoption of non-fluoride based solutions in certain environmental applications, which has captured approximately 8% market share from traditional HF solutions in the past three years.
Current Challenges in Environmental HF Usage
Despite the potential benefits of hydrofluoric acid (HF) in environmental applications, several significant challenges currently impede its widespread adoption and advancement. The primary concern remains its extreme toxicity and corrosivity, which pose substantial risks to human health and safety. Even at low concentrations, HF can cause severe tissue damage, and inhalation of its vapors can lead to pulmonary edema. These hazards necessitate rigorous safety protocols, specialized handling equipment, and extensive personnel training, all of which increase operational costs and complexity.
Containment and transportation of HF present additional challenges. The acid's ability to penetrate glass and many common materials requires specialized storage solutions using fluoropolymer-lined vessels or high-grade alloys. This requirement significantly increases infrastructure costs and creates logistical complications for field applications, particularly in remote environmental remediation sites.
Regulatory compliance represents another substantial hurdle. Environmental applications of HF face increasingly stringent regulations across jurisdictions, with some regions implementing outright bans on certain uses. Navigating this complex regulatory landscape requires considerable resources and expertise, creating barriers to entry for smaller organizations and limiting innovation potential.
The environmental impact of HF usage itself presents a paradoxical challenge. While the acid may serve beneficial environmental purposes, accidental releases can cause severe ecological damage, including soil acidification, groundwater contamination, and harm to aquatic ecosystems. This contradiction complicates the justification for HF use in environmental contexts and necessitates comprehensive risk assessment frameworks.
Technical limitations further constrain HF applications. Current methods for controlling reaction rates and neutralizing the acid after use remain imperfect, leading to efficiency losses and potential secondary contamination issues. Additionally, monitoring technologies for detecting HF leaks or measuring concentrations in environmental matrices lack the sensitivity and reliability required for certain applications.
Economic factors also present significant barriers. The high costs associated with safety measures, specialized equipment, and regulatory compliance often render HF-based solutions economically unviable compared to alternatives, despite potential technical advantages. This cost-benefit imbalance has limited research investment and commercial development of novel HF applications.
Public perception and stakeholder acceptance represent final but crucial challenges. The negative public image of HF, reinforced by high-profile industrial accidents, creates resistance to its use even in beneficial environmental applications. Overcoming this perception barrier requires transparent communication, community engagement, and demonstrable safety records.
Containment and transportation of HF present additional challenges. The acid's ability to penetrate glass and many common materials requires specialized storage solutions using fluoropolymer-lined vessels or high-grade alloys. This requirement significantly increases infrastructure costs and creates logistical complications for field applications, particularly in remote environmental remediation sites.
Regulatory compliance represents another substantial hurdle. Environmental applications of HF face increasingly stringent regulations across jurisdictions, with some regions implementing outright bans on certain uses. Navigating this complex regulatory landscape requires considerable resources and expertise, creating barriers to entry for smaller organizations and limiting innovation potential.
The environmental impact of HF usage itself presents a paradoxical challenge. While the acid may serve beneficial environmental purposes, accidental releases can cause severe ecological damage, including soil acidification, groundwater contamination, and harm to aquatic ecosystems. This contradiction complicates the justification for HF use in environmental contexts and necessitates comprehensive risk assessment frameworks.
Technical limitations further constrain HF applications. Current methods for controlling reaction rates and neutralizing the acid after use remain imperfect, leading to efficiency losses and potential secondary contamination issues. Additionally, monitoring technologies for detecting HF leaks or measuring concentrations in environmental matrices lack the sensitivity and reliability required for certain applications.
Economic factors also present significant barriers. The high costs associated with safety measures, specialized equipment, and regulatory compliance often render HF-based solutions economically unviable compared to alternatives, despite potential technical advantages. This cost-benefit imbalance has limited research investment and commercial development of novel HF applications.
Public perception and stakeholder acceptance represent final but crucial challenges. The negative public image of HF, reinforced by high-profile industrial accidents, creates resistance to its use even in beneficial environmental applications. Overcoming this perception barrier requires transparent communication, community engagement, and demonstrable safety records.
Current HF Management Solutions
01 Etching applications of hydrofluoric acid
Hydrofluoric acid is widely used as an etching agent in semiconductor manufacturing and glass processing. It effectively removes silicon dioxide layers and can be formulated with various additives to control etching rates and surface quality. These formulations are crucial for microelectronics fabrication processes where precise etching is required for circuit patterns and surface preparation.- Etching and cleaning applications: Hydrofluoric acid is widely used in semiconductor manufacturing for etching silicon dioxide and cleaning silicon wafers. It effectively removes oxide layers, contaminants, and residues from surfaces. Various formulations and methods have been developed to optimize the etching rate, selectivity, and safety of hydrofluoric acid solutions for specific applications in microelectronics fabrication.
- Production and purification methods: Various methods for producing and purifying hydrofluoric acid have been developed to meet industrial requirements. These include processes for manufacturing high-purity hydrofluoric acid from fluoride-containing raw materials, recovery techniques from waste streams, and purification methods to remove contaminants. Advanced distillation, filtration, and chemical treatment processes are employed to achieve the desired purity levels for different applications.
- Safety measures and handling techniques: Due to its highly corrosive and toxic nature, specialized safety measures and handling techniques have been developed for hydrofluoric acid. These include containment systems, neutralization methods, personal protective equipment, and emergency response protocols. Various formulations incorporate buffering agents, indicators, or thickeners to improve safety while maintaining effectiveness for specific applications.
- Waste treatment and environmental protection: Methods for treating hydrofluoric acid waste to minimize environmental impact have been developed. These include neutralization processes, recovery systems, and conversion to less hazardous compounds. Techniques for capturing fluoride from waste streams, monitoring emissions, and ensuring compliance with environmental regulations are important aspects of hydrofluoric acid usage in industrial settings.
- Industrial applications beyond semiconductors: Hydrofluoric acid is utilized in various industrial applications beyond semiconductor manufacturing. These include glass etching and frosting, metal surface treatment, oil refining catalysts, and fluorochemical production. Specialized formulations have been developed for specific industries, optimizing concentration, additives, and application methods to achieve desired results while minimizing hazards.
02 Purification and recovery methods for hydrofluoric acid
Various techniques have been developed for purifying and recovering hydrofluoric acid from industrial waste streams. These methods include distillation, adsorption, membrane separation, and chemical precipitation processes. Purification is essential to remove contaminants such as metal ions and particulates, allowing for the recycling of hydrofluoric acid in manufacturing processes and reducing environmental impact.Expand Specific Solutions03 Safety measures and neutralization techniques
Due to its highly corrosive and toxic nature, specialized safety protocols and neutralization methods have been developed for handling hydrofluoric acid. These include calcium-based neutralizing agents, specialized containment systems, and emergency treatment formulations. Innovations in this area focus on rapid neutralization to minimize damage to tissues and materials, as well as environmental protection during storage and transport.Expand Specific Solutions04 Production methods of hydrofluoric acid
Various processes have been developed for manufacturing hydrofluoric acid, primarily from fluorite (calcium fluoride) and sulfuric acid. Innovations include continuous production systems, catalytic methods, and techniques for improving yield and purity. These methods focus on energy efficiency, reducing byproducts, and minimizing environmental impact during production.Expand Specific Solutions05 Specialized formulations for specific industries
Hydrofluoric acid is formulated with various additives for specific industrial applications. These include mixtures with other acids for metal surface treatment, buffered solutions for controlled reactivity, and stabilized formulations for extended shelf life. Specialized compositions have been developed for applications in the petroleum industry, electronics manufacturing, and glass processing, each tailored to provide optimal performance for the specific application requirements.Expand Specific Solutions
Key Industry Players and Stakeholders
The hydrofluoric acid environmental applications market is currently in a growth phase, with increasing demand for sustainable solutions driving innovation. The global market size is expanding as industries seek environmentally responsible alternatives for traditional chemical processes. Technologically, the sector shows varying maturity levels across applications. Leading players like Honeywell International, The Chemours Co., and DuPont de Nemours are advancing high-purity HF technologies for electronics and semiconductor applications. Daikin Industries and 3M Innovative Properties focus on developing fluoropolymers with reduced environmental impact. Meanwhile, companies such as Anhui Chaoyue Environmental Technology and Sinochem Lantian are pioneering HF recovery and recycling systems. Emerging players like Do-Fluoride New Materials and Resonac Holdings are investing in green chemistry approaches to minimize HF's environmental footprint.
The Chemours Co.
Technical Solution: Chemours has developed advanced hydrofluoric acid (HF) treatment technologies focusing on sustainable applications. Their OpteonTM line includes HF derivatives that replace traditional high-global warming potential (GWP) refrigerants and foam expansion agents. For environmental remediation, they've engineered specialized HF-based formulations that can neutralize contaminated soils while minimizing secondary environmental impacts. Their closed-loop recovery systems capture and recycle HF from industrial processes, significantly reducing waste discharge. Chemours has also pioneered membrane filtration technology that selectively removes fluoride ions from wastewater streams, allowing for the recovery and reuse of HF in manufacturing processes. Their environmental applications extend to air pollution control, where HF-derived compounds are used in catalytic systems to reduce harmful emissions from industrial facilities.
Strengths: Industry-leading expertise in fluorochemicals with established infrastructure for safe handling; proprietary recovery technologies that minimize environmental footprint. Weaknesses: Higher implementation costs compared to conventional systems; requires specialized training and equipment for safe deployment.
Dow Global Technologies LLC
Technical Solution: Dow has developed an innovative approach to HF management in environmental applications through their SAFR (Sustainable Acid Fluoride Recovery) technology. This system enables the capture and conversion of HF emissions from industrial processes into usable fluoride compounds. Their technology incorporates specialized scrubber systems with proprietary absorption media that can achieve over 99% HF removal efficiency from waste streams. Dow's environmental applications extend to water treatment, where they've developed selective ion exchange resins specifically designed for fluoride removal from drinking water and industrial effluents. Their HF recovery systems implement advanced monitoring technologies with real-time sensors that optimize chemical usage while ensuring regulatory compliance. Additionally, Dow has pioneered HF-based catalysts for breaking down persistent organic pollutants in contaminated sites, offering a more efficient alternative to traditional remediation methods.
Strengths: Comprehensive approach integrating multiple technologies (absorption, ion exchange, catalysis) for different environmental applications; strong safety protocols and engineering controls. Weaknesses: Systems typically require significant capital investment; technology may be overengineered for smaller-scale applications.
Critical Patents in Environmental HF Applications
Separation of close boiling compounds by addition of a third compound
PatentActiveUS20100187088A1
Innovation
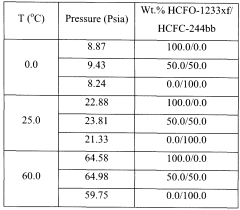
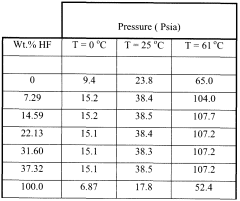
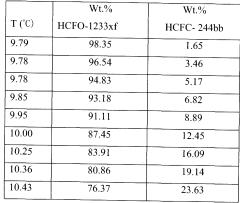
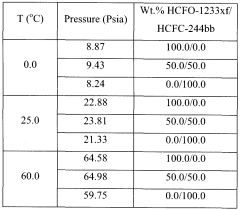
- Adding hydrogen fluoride to the binary azeotropic mixture of HCFC-244bb and HCFO-1233xf forms a ternary azeotrope with a stronger boiling point, allowing for the separation of HCFC-244bb from HCFO-1233xf through fractional distillation, with approximately 60-97% recovery of HCFC-244bb.
Separation of close boiling compounds by addition of a third compound
PatentWO2011126620A2
Innovation
- Adding hydrogen fluoride to the binary azeotropic mixture forms a ternary azeotrope with HCFC-244bb and HCFO-1233xf, allowing for the separation of HCFC-244bb by distillation, with the ternary azeotrope having a concentration of 10.0 - 18.0 wt% HCFC-244bb, 51.0 - 64.0 wt% HCFO-1233xf, and 23 - 35 wt% hydrogen fluoride, enabling efficient separation using conventional fractional distillation equipment.
Safety Protocols and Risk Mitigation
The implementation of robust safety protocols and risk mitigation strategies is paramount when advancing hydrofluoric acid (HF) usage in environmental applications. Due to HF's highly corrosive nature and severe health hazards, comprehensive safety frameworks must be established before deploying this chemical in environmental remediation or treatment processes.
Primary engineering controls represent the first line of defense against HF exposure. These include closed-system designs that minimize direct contact opportunities, automated handling systems that reduce manual interaction requirements, and specialized ventilation systems equipped with scrubbers to capture and neutralize any vapor emissions. Material selection for containment systems must prioritize HF-resistant components, typically utilizing fluoropolymer linings or specialized alloys that withstand prolonged exposure.
Personal protective equipment (PPE) protocols specific to HF operations must be rigorously enforced. This includes impermeable suits made from materials like Viton or butyl rubber, full-face respiratory protection with appropriate cartridges, double-glove systems with HF-resistant materials, and face shields combined with chemical splash goggles. Regular inspection and replacement schedules for all PPE components must be documented and strictly followed.
Emergency response planning requires specialized considerations for HF incidents. Calcium gluconate gel must be readily available at all operational sites for immediate application to exposed skin. Decontamination stations with specific neutralizing agents should be strategically positioned throughout facilities. Additionally, medical protocols must be established with local healthcare providers who understand the unique pathophysiology of HF injuries and appropriate treatment methodologies.
Environmental monitoring systems must be deployed to detect potential releases before they become hazardous. These include continuous air monitoring with HF-specific sensors, regular analysis of surrounding soil and water systems, and boundary monitoring to ensure containment within operational areas. Threshold action levels must be established with clear response protocols for various detection scenarios.
Training programs represent a critical component of risk mitigation. All personnel working with or near HF operations require specialized training covering chemical properties, exposure symptoms, emergency procedures, and proper handling techniques. Certification processes should include practical demonstrations and regular refresher courses to maintain competency.
Regulatory compliance frameworks must be integrated into all safety protocols, addressing requirements from multiple agencies including OSHA, EPA, and local environmental authorities. Documentation systems should facilitate transparent reporting and enable continuous improvement of safety measures based on operational experience and emerging best practices.
Primary engineering controls represent the first line of defense against HF exposure. These include closed-system designs that minimize direct contact opportunities, automated handling systems that reduce manual interaction requirements, and specialized ventilation systems equipped with scrubbers to capture and neutralize any vapor emissions. Material selection for containment systems must prioritize HF-resistant components, typically utilizing fluoropolymer linings or specialized alloys that withstand prolonged exposure.
Personal protective equipment (PPE) protocols specific to HF operations must be rigorously enforced. This includes impermeable suits made from materials like Viton or butyl rubber, full-face respiratory protection with appropriate cartridges, double-glove systems with HF-resistant materials, and face shields combined with chemical splash goggles. Regular inspection and replacement schedules for all PPE components must be documented and strictly followed.
Emergency response planning requires specialized considerations for HF incidents. Calcium gluconate gel must be readily available at all operational sites for immediate application to exposed skin. Decontamination stations with specific neutralizing agents should be strategically positioned throughout facilities. Additionally, medical protocols must be established with local healthcare providers who understand the unique pathophysiology of HF injuries and appropriate treatment methodologies.
Environmental monitoring systems must be deployed to detect potential releases before they become hazardous. These include continuous air monitoring with HF-specific sensors, regular analysis of surrounding soil and water systems, and boundary monitoring to ensure containment within operational areas. Threshold action levels must be established with clear response protocols for various detection scenarios.
Training programs represent a critical component of risk mitigation. All personnel working with or near HF operations require specialized training covering chemical properties, exposure symptoms, emergency procedures, and proper handling techniques. Certification processes should include practical demonstrations and regular refresher courses to maintain competency.
Regulatory compliance frameworks must be integrated into all safety protocols, addressing requirements from multiple agencies including OSHA, EPA, and local environmental authorities. Documentation systems should facilitate transparent reporting and enable continuous improvement of safety measures based on operational experience and emerging best practices.
Regulatory Compliance Framework
The regulatory landscape governing hydrofluoric acid (HF) usage in environmental applications is complex and multifaceted, requiring comprehensive understanding for successful implementation. At the international level, the Stockholm Convention on Persistent Organic Pollutants and the Rotterdam Convention establish baseline requirements for hazardous chemical management, while the Basel Convention specifically addresses transboundary movements of hazardous wastes including HF-containing materials.
In the United States, the Environmental Protection Agency (EPA) regulates HF under multiple frameworks including the Toxic Substances Control Act (TSCA), the Resource Conservation and Recovery Act (RCRA), and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). The Occupational Safety and Health Administration (OSHA) further imposes strict workplace safety standards through the Hazard Communication Standard (29 CFR 1910.1200) and specific HF handling protocols.
European regulations are particularly stringent, with HF falling under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework and the Classification, Labelling and Packaging (CLP) Regulation. The Industrial Emissions Directive (IED) establishes additional requirements for facilities utilizing HF in environmental remediation processes.
Compliance documentation requirements represent a significant operational consideration, including detailed record-keeping of usage volumes, exposure monitoring data, waste disposal manifests, and incident reports. Organizations must maintain Safety Data Sheets (SDS) that adhere to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) standards, ensuring consistent hazard communication across jurisdictions.
Permitting processes vary by application and jurisdiction but typically involve environmental impact assessments, public consultation periods, and demonstration of best available techniques (BAT). For environmental remediation applications, additional site-specific permits may be required, particularly for groundwater or soil treatment projects involving HF.
Emerging regulatory trends indicate increasing scrutiny of HF applications, with several jurisdictions implementing more stringent exposure limits and environmental release thresholds. The development of green chemistry alternatives is being incentivized through regulatory frameworks that reward reduced dependence on highly hazardous substances.
Compliance strategies for organizations advancing HF environmental applications should include comprehensive staff training programs, regular compliance audits, engagement with regulatory stakeholders during early development phases, and participation in industry consortia to stay abreast of evolving requirements. Implementation of management systems aligned with ISO 14001 (Environmental Management) and ISO 45001 (Occupational Health and Safety) standards can provide structured frameworks for regulatory compliance.
In the United States, the Environmental Protection Agency (EPA) regulates HF under multiple frameworks including the Toxic Substances Control Act (TSCA), the Resource Conservation and Recovery Act (RCRA), and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). The Occupational Safety and Health Administration (OSHA) further imposes strict workplace safety standards through the Hazard Communication Standard (29 CFR 1910.1200) and specific HF handling protocols.
European regulations are particularly stringent, with HF falling under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework and the Classification, Labelling and Packaging (CLP) Regulation. The Industrial Emissions Directive (IED) establishes additional requirements for facilities utilizing HF in environmental remediation processes.
Compliance documentation requirements represent a significant operational consideration, including detailed record-keeping of usage volumes, exposure monitoring data, waste disposal manifests, and incident reports. Organizations must maintain Safety Data Sheets (SDS) that adhere to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) standards, ensuring consistent hazard communication across jurisdictions.
Permitting processes vary by application and jurisdiction but typically involve environmental impact assessments, public consultation periods, and demonstration of best available techniques (BAT). For environmental remediation applications, additional site-specific permits may be required, particularly for groundwater or soil treatment projects involving HF.
Emerging regulatory trends indicate increasing scrutiny of HF applications, with several jurisdictions implementing more stringent exposure limits and environmental release thresholds. The development of green chemistry alternatives is being incentivized through regulatory frameworks that reward reduced dependence on highly hazardous substances.
Compliance strategies for organizations advancing HF environmental applications should include comprehensive staff training programs, regular compliance audits, engagement with regulatory stakeholders during early development phases, and participation in industry consortia to stay abreast of evolving requirements. Implementation of management systems aligned with ISO 14001 (Environmental Management) and ISO 45001 (Occupational Health and Safety) standards can provide structured frameworks for regulatory compliance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!