Hydrofluoric Acid and Hydroxide: Neutralization Reaction Study
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF-Hydroxide Neutralization Background and Objectives
Hydrofluoric acid (HF) represents a unique chemical compound with distinctive properties that set it apart from other mineral acids. Since its discovery in the late 18th century, HF has evolved from a laboratory curiosity to an essential industrial reagent. The acid's ability to dissolve silica and silicates has made it invaluable in numerous applications, from glass etching to semiconductor manufacturing. This technical evolution has been accompanied by growing awareness of its extreme hazards, necessitating specialized handling protocols.
The neutralization of hydrofluoric acid with hydroxide compounds represents a critical area of study with significant implications for industrial safety, environmental protection, and medical treatment protocols. Unlike other acid-base reactions, HF neutralization presents unique challenges due to the acid's weak dissociation properties and the formation of various fluoride species that can exhibit complex behavior in solution.
Recent technological advancements have driven increased industrial utilization of HF across sectors including petrochemical processing, electronics manufacturing, and materials science. This expanded application landscape has correspondingly elevated the importance of effective neutralization methodologies. The global HF market, valued at approximately $2.7 billion in 2022, is projected to grow at a CAGR of 5.2% through 2030, underscoring the economic significance of this chemical and its safe management.
The primary objective of this technical research is to comprehensively evaluate the reaction mechanisms, kinetics, and thermodynamics governing the neutralization of hydrofluoric acid with various hydroxide compounds. We aim to establish optimized protocols for different concentration regimes and application scenarios, with particular emphasis on industrial spill management and medical treatment contexts.
Secondary objectives include characterizing the formation and behavior of intermediate fluoride species during neutralization processes, assessing the efficacy of different hydroxide compounds (including sodium, potassium, calcium, and magnesium hydroxides) for specific applications, and developing predictive models for reaction outcomes under varying conditions of temperature, concentration, and mixing parameters.
The technological trajectory in this field points toward the development of advanced neutralization formulations with enhanced safety profiles, improved reaction kinetics, and reduced environmental impact. Emerging research suggests potential for novel composite neutralizing agents that combine multiple hydroxide species to optimize performance across diverse scenarios, representing a promising direction for future innovation.
This study addresses critical knowledge gaps in current neutralization practices, particularly regarding the behavior of HF in complex matrices and the long-term stability of neutralization products. The findings will inform the development of next-generation safety protocols and remediation technologies for this hazardous but industrially essential compound.
The neutralization of hydrofluoric acid with hydroxide compounds represents a critical area of study with significant implications for industrial safety, environmental protection, and medical treatment protocols. Unlike other acid-base reactions, HF neutralization presents unique challenges due to the acid's weak dissociation properties and the formation of various fluoride species that can exhibit complex behavior in solution.
Recent technological advancements have driven increased industrial utilization of HF across sectors including petrochemical processing, electronics manufacturing, and materials science. This expanded application landscape has correspondingly elevated the importance of effective neutralization methodologies. The global HF market, valued at approximately $2.7 billion in 2022, is projected to grow at a CAGR of 5.2% through 2030, underscoring the economic significance of this chemical and its safe management.
The primary objective of this technical research is to comprehensively evaluate the reaction mechanisms, kinetics, and thermodynamics governing the neutralization of hydrofluoric acid with various hydroxide compounds. We aim to establish optimized protocols for different concentration regimes and application scenarios, with particular emphasis on industrial spill management and medical treatment contexts.
Secondary objectives include characterizing the formation and behavior of intermediate fluoride species during neutralization processes, assessing the efficacy of different hydroxide compounds (including sodium, potassium, calcium, and magnesium hydroxides) for specific applications, and developing predictive models for reaction outcomes under varying conditions of temperature, concentration, and mixing parameters.
The technological trajectory in this field points toward the development of advanced neutralization formulations with enhanced safety profiles, improved reaction kinetics, and reduced environmental impact. Emerging research suggests potential for novel composite neutralizing agents that combine multiple hydroxide species to optimize performance across diverse scenarios, representing a promising direction for future innovation.
This study addresses critical knowledge gaps in current neutralization practices, particularly regarding the behavior of HF in complex matrices and the long-term stability of neutralization products. The findings will inform the development of next-generation safety protocols and remediation technologies for this hazardous but industrially essential compound.
Industrial Applications and Market Demand Analysis
The hydrofluoric acid (HF) and hydroxide neutralization reaction represents a critical chemical process with extensive industrial applications across multiple sectors. The global market for hydrofluoric acid was valued at approximately 2.7 billion USD in 2022, with projections indicating growth to reach 3.5 billion USD by 2028, representing a compound annual growth rate of 4.2%. This growth is primarily driven by increasing demand from key end-use industries including semiconductor manufacturing, glass etching, and metal processing.
In the semiconductor industry, the neutralization process plays a vital role in wafer cleaning and etching operations. As the global semiconductor market continues its robust expansion, projected to reach 1 trillion USD by 2030, the demand for precisely controlled HF neutralization processes has intensified. Manufacturers require increasingly sophisticated neutralization techniques to achieve the nanometer-scale precision demanded by advanced chip fabrication.
The fluorochemicals sector represents another significant market driver, with fluoropolymer production heavily dependent on hydrofluoric acid processing and subsequent neutralization steps. The global fluoropolymer market, valued at 8.5 billion USD in 2022, relies on efficient neutralization reactions to maintain production efficiency and environmental compliance.
Environmental applications constitute a rapidly growing segment, with HF neutralization essential for industrial waste treatment systems. Stringent environmental regulations worldwide have created a market for specialized neutralization technologies capable of handling hydrofluoric acid waste streams safely and effectively. This regulatory-driven demand is particularly strong in regions with advanced environmental protection frameworks such as Western Europe, North America, and increasingly, East Asia.
The metal processing industry utilizes HF for aluminum brightening, stainless steel pickling, and titanium etching, with neutralization representing a critical step in these manufacturing processes. The global metal treatment chemical market, exceeding 12 billion USD, includes significant demand for hydroxide-based neutralization agents specifically formulated for hydrofluoric acid applications.
Market analysis reveals regional variations in demand patterns, with Asia-Pacific representing the largest and fastest-growing market for HF neutralization technologies, driven by the region's expanding semiconductor and electronics manufacturing base. North America and Europe maintain significant market shares, primarily supported by advanced chemical processing industries and stringent environmental regulations requiring sophisticated neutralization systems.
Recent market trends indicate growing demand for automated neutralization systems that offer precise pH control, real-time monitoring capabilities, and enhanced safety features to mitigate the inherent hazards associated with hydrofluoric acid handling. This technological evolution is creating new market opportunities for equipment manufacturers and process control specialists focused on HF neutralization applications.
In the semiconductor industry, the neutralization process plays a vital role in wafer cleaning and etching operations. As the global semiconductor market continues its robust expansion, projected to reach 1 trillion USD by 2030, the demand for precisely controlled HF neutralization processes has intensified. Manufacturers require increasingly sophisticated neutralization techniques to achieve the nanometer-scale precision demanded by advanced chip fabrication.
The fluorochemicals sector represents another significant market driver, with fluoropolymer production heavily dependent on hydrofluoric acid processing and subsequent neutralization steps. The global fluoropolymer market, valued at 8.5 billion USD in 2022, relies on efficient neutralization reactions to maintain production efficiency and environmental compliance.
Environmental applications constitute a rapidly growing segment, with HF neutralization essential for industrial waste treatment systems. Stringent environmental regulations worldwide have created a market for specialized neutralization technologies capable of handling hydrofluoric acid waste streams safely and effectively. This regulatory-driven demand is particularly strong in regions with advanced environmental protection frameworks such as Western Europe, North America, and increasingly, East Asia.
The metal processing industry utilizes HF for aluminum brightening, stainless steel pickling, and titanium etching, with neutralization representing a critical step in these manufacturing processes. The global metal treatment chemical market, exceeding 12 billion USD, includes significant demand for hydroxide-based neutralization agents specifically formulated for hydrofluoric acid applications.
Market analysis reveals regional variations in demand patterns, with Asia-Pacific representing the largest and fastest-growing market for HF neutralization technologies, driven by the region's expanding semiconductor and electronics manufacturing base. North America and Europe maintain significant market shares, primarily supported by advanced chemical processing industries and stringent environmental regulations requiring sophisticated neutralization systems.
Recent market trends indicate growing demand for automated neutralization systems that offer precise pH control, real-time monitoring capabilities, and enhanced safety features to mitigate the inherent hazards associated with hydrofluoric acid handling. This technological evolution is creating new market opportunities for equipment manufacturers and process control specialists focused on HF neutralization applications.
Current Challenges in HF Neutralization Processes
The neutralization of hydrofluoric acid (HF) presents significant challenges due to its unique chemical properties and hazardous nature. Current industrial processes face several critical obstacles that limit efficiency, safety, and environmental sustainability. One primary challenge is the incomplete neutralization phenomenon, where residual HF remains even after treatment with standard neutralizing agents. This occurs because HF forms complex equilibria in aqueous solutions, with dissociation behavior that differs markedly from other strong acids.
Temperature control represents another major hurdle in HF neutralization processes. The reaction between HF and hydroxides is highly exothermic, requiring sophisticated cooling systems to prevent dangerous temperature spikes. Many industrial facilities struggle with heat management during large-scale neutralization operations, particularly when dealing with concentrated HF solutions where reaction rates can accelerate unpredictably.
Material compatibility issues further complicate neutralization procedures. HF's corrosive nature attacks many common materials used in containment and processing equipment. Even specialized materials like certain grades of stainless steel can experience stress corrosion cracking when exposed to partially neutralized HF solutions. This necessitates frequent equipment replacement and maintenance, increasing operational costs and downtime.
The formation of insoluble fluoride salts during neutralization creates additional processing difficulties. When calcium hydroxide is used as a neutralizing agent, the resulting calcium fluoride precipitates can clog filtration systems and reduce process efficiency. These precipitates also create waste disposal challenges, as they may contain unreacted HF or other hazardous components requiring specialized handling.
Worker safety concerns remain paramount in HF neutralization operations. Despite engineering controls and personal protective equipment, the risk of exposure persists due to HF's ability to penetrate skin rapidly and cause deep tissue damage without immediate symptoms. Current detection systems for HF vapor have limitations in sensitivity and response time, potentially allowing dangerous concentrations to go undetected.
Environmental regulations present growing compliance challenges for facilities performing HF neutralization. Wastewater discharge limits for fluoride compounds have become increasingly stringent, requiring more sophisticated treatment processes. Many existing neutralization systems struggle to consistently achieve these lower concentration thresholds without significant process modifications or additional treatment steps.
Analytical monitoring of neutralization processes represents another technical challenge. Real-time measurement of fluoride ion concentration in complex industrial mixtures remains difficult, with current methods often requiring sample preparation steps that introduce delays in process control decisions. This analytical gap hampers the development of more efficient, automated neutralization systems.
Temperature control represents another major hurdle in HF neutralization processes. The reaction between HF and hydroxides is highly exothermic, requiring sophisticated cooling systems to prevent dangerous temperature spikes. Many industrial facilities struggle with heat management during large-scale neutralization operations, particularly when dealing with concentrated HF solutions where reaction rates can accelerate unpredictably.
Material compatibility issues further complicate neutralization procedures. HF's corrosive nature attacks many common materials used in containment and processing equipment. Even specialized materials like certain grades of stainless steel can experience stress corrosion cracking when exposed to partially neutralized HF solutions. This necessitates frequent equipment replacement and maintenance, increasing operational costs and downtime.
The formation of insoluble fluoride salts during neutralization creates additional processing difficulties. When calcium hydroxide is used as a neutralizing agent, the resulting calcium fluoride precipitates can clog filtration systems and reduce process efficiency. These precipitates also create waste disposal challenges, as they may contain unreacted HF or other hazardous components requiring specialized handling.
Worker safety concerns remain paramount in HF neutralization operations. Despite engineering controls and personal protective equipment, the risk of exposure persists due to HF's ability to penetrate skin rapidly and cause deep tissue damage without immediate symptoms. Current detection systems for HF vapor have limitations in sensitivity and response time, potentially allowing dangerous concentrations to go undetected.
Environmental regulations present growing compliance challenges for facilities performing HF neutralization. Wastewater discharge limits for fluoride compounds have become increasingly stringent, requiring more sophisticated treatment processes. Many existing neutralization systems struggle to consistently achieve these lower concentration thresholds without significant process modifications or additional treatment steps.
Analytical monitoring of neutralization processes represents another technical challenge. Real-time measurement of fluoride ion concentration in complex industrial mixtures remains difficult, with current methods often requiring sample preparation steps that introduce delays in process control decisions. This analytical gap hampers the development of more efficient, automated neutralization systems.
Established Neutralization Protocols and Techniques
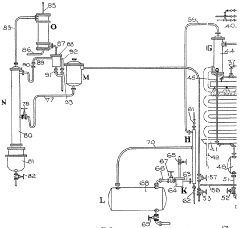
01 Neutralization of hydrofluoric acid with hydroxide compounds
The neutralization reaction between hydrofluoric acid (HF) and hydroxide compounds involves the reaction of H+ ions from the acid with OH- ions from the base to form water. This process is commonly used in industrial settings to neutralize hydrofluoric acid waste or spills. The reaction typically produces fluoride salts as byproducts, which are less hazardous than the original acid. Various hydroxide compounds such as sodium hydroxide, potassium hydroxide, or calcium hydroxide can be used as neutralizing agents.- Neutralization of hydrofluoric acid with hydroxide compounds: Hydrofluoric acid can be neutralized using various hydroxide compounds such as sodium hydroxide, potassium hydroxide, or calcium hydroxide. The neutralization reaction produces fluoride salts and water. This process is commonly used in industrial settings to treat hydrofluoric acid waste before disposal, reducing its corrosive and hazardous properties. The reaction typically requires careful monitoring of pH levels to ensure complete neutralization.
- Wastewater treatment systems for hydrofluoric acid neutralization: Specialized wastewater treatment systems have been developed for neutralizing hydrofluoric acid in industrial effluents. These systems often incorporate multiple stages of neutralization with hydroxide compounds, followed by precipitation and filtration to remove fluoride salts. The treatment process may include pH adjustment tanks, mixing chambers, and monitoring equipment to ensure effective neutralization. Such systems are particularly important in semiconductor manufacturing, glass etching, and metal processing industries where hydrofluoric acid is commonly used.
- Safety equipment and procedures for hydrofluoric acid neutralization: Due to the highly corrosive and toxic nature of hydrofluoric acid, specialized safety equipment and procedures are essential during neutralization processes. This includes automated neutralization systems that minimize human exposure, emergency neutralization kits containing calcium hydroxide or other neutralizing agents, and specialized containment vessels resistant to both acid and the heat generated during neutralization reactions. Personal protective equipment and monitoring systems are also critical components of safe neutralization procedures.
- Neutralization monitoring and control systems: Advanced monitoring and control systems have been developed to ensure precise and complete neutralization of hydrofluoric acid with hydroxide compounds. These systems typically include real-time pH sensors, automated reagent dosing equipment, temperature monitors, and computerized control interfaces. Some systems incorporate predictive algorithms to optimize the neutralization process, reducing chemical usage while ensuring complete neutralization. These technologies are particularly important in high-volume industrial applications where consistent neutralization is critical for safety and environmental compliance.
- Specialized neutralization methods for semiconductor manufacturing: The semiconductor industry has developed specialized methods for neutralizing hydrofluoric acid used in etching and cleaning processes. These methods often involve precise neutralization with specific hydroxide compounds that minimize contamination of sensitive electronic components. The neutralization processes may include multiple dilution steps, controlled reaction environments, and specialized waste handling procedures. Some methods incorporate recovery systems that allow for recycling of fluoride compounds, reducing waste and environmental impact while maintaining the high purity standards required in semiconductor manufacturing.
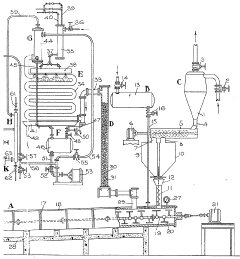
02 Neutralization systems for hydrofluoric acid in semiconductor manufacturing
Specialized neutralization systems are designed for handling hydrofluoric acid in semiconductor manufacturing processes. These systems often incorporate automated pH monitoring and control mechanisms to ensure complete neutralization of the acid with hydroxide compounds. The neutralization process is critical in semiconductor fabrication where hydrofluoric acid is used for etching silicon dioxide and cleaning silicon wafers. Proper neutralization prevents equipment damage and ensures worker safety while meeting environmental discharge regulations.Expand Specific Solutions03 Waste treatment apparatus for hydrofluoric acid neutralization
Various apparatus designs are employed for the treatment of hydrofluoric acid waste through neutralization with hydroxide solutions. These systems typically include reaction chambers, mixing mechanisms, pH sensors, and control systems to ensure complete neutralization. Some designs incorporate multiple stages of neutralization to handle high concentrations of hydrofluoric acid effectively. Advanced systems may also include heat exchangers to manage the exothermic nature of the neutralization reaction and filtration components to remove precipitated fluoride salts.Expand Specific Solutions04 Neutralization methods for environmental protection
Environmental protection methods focus on the neutralization of hydrofluoric acid to prevent ecological damage. These methods often employ specific hydroxide compounds and controlled neutralization processes to ensure that the resulting effluent meets environmental discharge standards. The neutralization process may include monitoring systems to verify complete reaction and prevent the release of harmful fluoride compounds. Some methods incorporate additional treatment steps such as precipitation of fluorides as insoluble calcium fluoride to further reduce environmental impact.Expand Specific Solutions05 Safety systems for hydrofluoric acid neutralization in industrial settings
Safety systems are designed specifically for neutralizing hydrofluoric acid spills or leaks in industrial environments using hydroxide solutions. These systems often include emergency shower stations, spill containment areas, and rapid-response neutralization kits containing appropriate hydroxide compounds. Advanced safety systems may incorporate automated detection of hydrofluoric acid vapors that trigger neutralization processes. Training protocols and specialized personal protective equipment are also essential components of these safety systems to protect workers during neutralization procedures.Expand Specific Solutions
Key Industry Players and Research Institutions
The hydrofluoric acid and hydroxide neutralization reaction market is currently in a growth phase, with increasing applications across pharmaceutical, chemical manufacturing, and water treatment sectors. The global market size for these neutralization technologies is estimated at $3.5-4 billion annually, expanding at approximately 5-7% CAGR. Leading players include diversified chemical giants like DuPont, BASF, and Honeywell International, who leverage their extensive R&D capabilities to develop advanced neutralization solutions. Specialized companies such as Stella Chemifa Corp. and Arkema France SA focus on high-purity chemical applications, while Kurita Water Industries and Condias GmbH are pioneering water treatment applications. Academic institutions including Nanyang Technological University and California Institute of Technology contribute significantly to technological advancement, particularly in developing safer handling protocols and environmentally sustainable neutralization methods.
Arkema France SA
Technical Solution: Arkema has developed advanced neutralization processes for hydrofluoric acid (HF) using specialized hydroxide compounds. Their patented technology employs calcium hydroxide slurries with controlled particle size distribution to optimize neutralization efficiency while minimizing dangerous heat generation during the exothermic reaction. The process incorporates continuous monitoring systems that adjust reagent addition rates based on real-time pH measurements, maintaining optimal neutralization conditions. Arkema's industrial-scale implementation includes specialized corrosion-resistant equipment designed specifically for HF handling, with double-containment systems and specialized agitation mechanisms to ensure complete reaction. Their approach also addresses the challenge of calcium fluoride precipitation management through proprietary filtration systems that enable efficient solid waste separation and potential recovery of fluoride compounds for secondary applications in industries such as aluminum production and ceramics manufacturing.
Strengths: Superior safety protocols with automated monitoring systems; high neutralization efficiency (>99.5%); ability to handle industrial-scale HF waste streams; recovery potential for fluoride byproducts. Weaknesses: Higher capital equipment costs compared to basic neutralization methods; requires specialized operator training; process generates significant solid waste requiring disposal.
DuPont de Nemours, Inc.
Technical Solution: DuPont has pioneered a comprehensive HF neutralization technology utilizing a multi-stage hydroxide treatment approach. Their system employs a primary neutralization with sodium hydroxide followed by secondary treatment with magnesium hydroxide slurries to achieve complete neutralization while controlling reaction kinetics. The process incorporates proprietary mixing technology that creates micro-turbulence zones for enhanced mass transfer without excessive heat buildup, critical for managing the highly exothermic HF-hydroxide reaction. DuPont's approach includes integrated heat exchangers that capture and dissipate reaction heat, maintaining optimal temperature profiles throughout the neutralization process. Their technology also features advanced fluoride precipitation control mechanisms that produce more manageable solid waste forms with reduced leaching potential. The system is complemented by real-time monitoring of multiple parameters including pH, temperature, and fluoride concentration, with automated feedback controls that adjust reagent addition rates to maintain optimal neutralization conditions across varying HF concentrations and flow rates.
Strengths: Exceptional reaction control with minimal risk of runaway exothermic reactions; high neutralization efficiency across wide concentration ranges; integrated heat management systems; produces more stable and environmentally benign waste forms. Weaknesses: Complex multi-stage process requires sophisticated control systems; higher operational costs; requires significant technical expertise to operate and maintain.
Critical Patents and Research on HF-Hydroxide Reactions
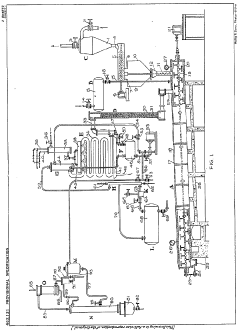
Improvements in or relating to the manufacture of hydrofluoric acid
PatentInactiveGB462131A
Innovation
- A continuous process under superatmospheric pressure reacts a fluoride with an acid, followed by preliminary cooling and condensation to separate dilute and concentrated hydrofluoric acid, using a reactor and condensers to achieve high yields and purity.
Improvements in and relating to the recovery of fluorine as hydrofluoric acid
PatentInactiveGB166228A
Innovation
- The process involves adding ferric oxide to the solution to precipitate out silica and form iron fluoride, followed by heat treatment in the presence of water vapor to release hydrogen fluoride, which is then absorbed to produce hydrofluoric acid.
Safety Protocols and Hazard Mitigation Strategies
The handling of hydrofluoric acid (HF) and hydroxide compounds during neutralization reactions requires rigorous safety protocols due to their highly hazardous nature. HF presents unique dangers compared to other acids, as it can penetrate skin and cause deep tissue damage without immediate pain sensation. Hydroxides, particularly concentrated solutions, pose severe corrosion risks to skin, eyes, and respiratory systems. Comprehensive safety management must be implemented at all stages of experimentation and industrial applications.
Personal protective equipment (PPE) forms the first line of defense when working with these chemicals. This includes chemical-resistant gloves (specifically rated for HF), face shields, goggles, lab coats, and in some cases, full-body protection suits. Respiratory protection with appropriate filters is essential when there is potential for vapor formation. All PPE must be regularly inspected for integrity and replaced according to manufacturer guidelines.
Engineering controls represent critical infrastructure for hazard mitigation. Properly functioning fume hoods with adequate face velocity (typically 80-120 fpm) are mandatory for all neutralization procedures. Secondary containment systems must be in place to capture potential spills, with specialized HF-resistant materials used for all storage and reaction vessels. Emergency eyewash stations and safety showers must be located within 10 seconds of travel time from work areas.
Emergency response protocols must be clearly established and regularly practiced. This includes immediate access to calcium gluconate gel (2.5%) for HF exposure, which counteracts fluoride ion penetration by forming insoluble calcium fluoride. Detailed written procedures for spill containment and neutralization must be prominently displayed, with specialized HF spill kits readily available. Regular emergency drills ensure all personnel can execute these protocols under stress.
Monitoring and detection systems provide crucial early warning capabilities. HF-specific gas detectors should be installed in work areas, with regular calibration schedules maintained. pH monitoring during neutralization reactions helps prevent unexpected concentration spikes. Environmental monitoring ensures that waste streams comply with regulatory requirements before disposal.
Training requirements must be stringent and regularly reinforced. All personnel working with these chemicals require documented training on hazard recognition, proper handling techniques, neutralization procedures, and emergency response. Refresher training should be conducted at least annually, with competency verification through practical demonstrations and written assessments.
Waste management protocols must address the specific challenges of HF and hydroxide neutralization byproducts. Neutralized solutions require verification of pH (typically between 6-8) before disposal. Precipitation of fluoride salts may be necessary, with subsequent solid waste handled according to local regulations. Documentation of all waste treatment procedures must be maintained for regulatory compliance.
Personal protective equipment (PPE) forms the first line of defense when working with these chemicals. This includes chemical-resistant gloves (specifically rated for HF), face shields, goggles, lab coats, and in some cases, full-body protection suits. Respiratory protection with appropriate filters is essential when there is potential for vapor formation. All PPE must be regularly inspected for integrity and replaced according to manufacturer guidelines.
Engineering controls represent critical infrastructure for hazard mitigation. Properly functioning fume hoods with adequate face velocity (typically 80-120 fpm) are mandatory for all neutralization procedures. Secondary containment systems must be in place to capture potential spills, with specialized HF-resistant materials used for all storage and reaction vessels. Emergency eyewash stations and safety showers must be located within 10 seconds of travel time from work areas.
Emergency response protocols must be clearly established and regularly practiced. This includes immediate access to calcium gluconate gel (2.5%) for HF exposure, which counteracts fluoride ion penetration by forming insoluble calcium fluoride. Detailed written procedures for spill containment and neutralization must be prominently displayed, with specialized HF spill kits readily available. Regular emergency drills ensure all personnel can execute these protocols under stress.
Monitoring and detection systems provide crucial early warning capabilities. HF-specific gas detectors should be installed in work areas, with regular calibration schedules maintained. pH monitoring during neutralization reactions helps prevent unexpected concentration spikes. Environmental monitoring ensures that waste streams comply with regulatory requirements before disposal.
Training requirements must be stringent and regularly reinforced. All personnel working with these chemicals require documented training on hazard recognition, proper handling techniques, neutralization procedures, and emergency response. Refresher training should be conducted at least annually, with competency verification through practical demonstrations and written assessments.
Waste management protocols must address the specific challenges of HF and hydroxide neutralization byproducts. Neutralized solutions require verification of pH (typically between 6-8) before disposal. Precipitation of fluoride salts may be necessary, with subsequent solid waste handled according to local regulations. Documentation of all waste treatment procedures must be maintained for regulatory compliance.
Environmental Impact and Waste Management Considerations
The neutralization reaction between hydrofluoric acid and hydroxide compounds generates waste streams that require careful environmental management. These reactions produce fluoride salts which, while less hazardous than HF itself, still present significant environmental concerns if improperly discharged. Fluoride ions can persist in aquatic environments, potentially affecting freshwater ecosystems through bioaccumulation in aquatic organisms and disruption of calcium metabolism in various species.
Wastewater from neutralization processes typically contains residual fluoride concentrations that exceed permissible discharge limits established by environmental protection agencies worldwide. The EPA, for instance, has set a maximum contaminant level for fluoride at 4.0 mg/L in drinking water, necessitating additional treatment steps before effluent release.
Current best practices for managing neutralization waste streams include precipitation methods using calcium compounds to form insoluble calcium fluoride (CaF₂), which can be separated and disposed of as solid waste. Advanced treatment technologies such as ion exchange resins and reverse osmosis systems are increasingly employed to further reduce fluoride concentrations to environmentally acceptable levels.
The solid waste generated from these treatment processes presents its own management challenges. Calcium fluoride precipitates are typically classified as industrial waste requiring specialized disposal in designated landfills with appropriate liners and leachate collection systems to prevent groundwater contamination. Some facilities have implemented recovery processes to reclaim fluoride compounds for industrial reuse, creating potential circular economy opportunities.
Atmospheric emissions during neutralization reactions must also be considered, particularly when volatile fluoride compounds may form. Modern facilities incorporate scrubber systems designed specifically to capture fluoride-containing vapors, preventing their release into the atmosphere where they could contribute to air pollution or acid rain formation.
Life cycle assessment studies of HF neutralization processes indicate that environmental impacts can be significantly reduced through process optimization. Techniques such as precise reagent dosing, continuous monitoring systems, and closed-loop processing have demonstrated reduction in waste generation by up to 30% in industrial applications.
Regulatory frameworks governing HF neutralization waste continue to evolve, with increasing emphasis on producer responsibility and cradle-to-grave management approaches. Organizations conducting these neutralization reactions must maintain comprehensive waste tracking systems and regularly report disposal methods and quantities to regulatory authorities.
Wastewater from neutralization processes typically contains residual fluoride concentrations that exceed permissible discharge limits established by environmental protection agencies worldwide. The EPA, for instance, has set a maximum contaminant level for fluoride at 4.0 mg/L in drinking water, necessitating additional treatment steps before effluent release.
Current best practices for managing neutralization waste streams include precipitation methods using calcium compounds to form insoluble calcium fluoride (CaF₂), which can be separated and disposed of as solid waste. Advanced treatment technologies such as ion exchange resins and reverse osmosis systems are increasingly employed to further reduce fluoride concentrations to environmentally acceptable levels.
The solid waste generated from these treatment processes presents its own management challenges. Calcium fluoride precipitates are typically classified as industrial waste requiring specialized disposal in designated landfills with appropriate liners and leachate collection systems to prevent groundwater contamination. Some facilities have implemented recovery processes to reclaim fluoride compounds for industrial reuse, creating potential circular economy opportunities.
Atmospheric emissions during neutralization reactions must also be considered, particularly when volatile fluoride compounds may form. Modern facilities incorporate scrubber systems designed specifically to capture fluoride-containing vapors, preventing their release into the atmosphere where they could contribute to air pollution or acid rain formation.
Life cycle assessment studies of HF neutralization processes indicate that environmental impacts can be significantly reduced through process optimization. Techniques such as precise reagent dosing, continuous monitoring systems, and closed-loop processing have demonstrated reduction in waste generation by up to 30% in industrial applications.
Regulatory frameworks governing HF neutralization waste continue to evolve, with increasing emphasis on producer responsibility and cradle-to-grave management approaches. Organizations conducting these neutralization reactions must maintain comprehensive waste tracking systems and regularly report disposal methods and quantities to regulatory authorities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!