How to Ensure Hydrofluoric Acid Precision in Material Analysis
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrofluoric Acid Analysis Background and Objectives
Hydrofluoric acid (HF) has emerged as a critical reagent in modern material analysis, particularly in semiconductor manufacturing, geological sample preparation, and advanced materials research. The evolution of this technology dates back to the early 20th century when HF was first utilized in glass etching applications. Over subsequent decades, its analytical applications have expanded significantly, driven by increasing demands for precision in microelectronics and nanomaterials characterization.
The technological trajectory of HF in analytical chemistry has been marked by continuous refinement in handling protocols, concentration control methodologies, and safety measures. Initially employed as a bulk etchant, HF applications have progressively shifted toward precision-controlled processes requiring nanometer-scale accuracy. This evolution reflects broader trends in materials science toward miniaturization and atomic-level control of surfaces and interfaces.
Current technological objectives in HF precision analysis center on achieving reproducible sub-nanometer etching control, developing real-time concentration monitoring systems, and establishing standardized protocols that ensure consistent results across different laboratory environments. These objectives are particularly crucial as semiconductor feature sizes continue to shrink below 5nm, demanding unprecedented precision in surface preparation and analysis.
The scientific community has identified several key parameters affecting HF precision, including temperature stability, concentration homogeneity, exposure time control, and contamination prevention. Recent advances in microfluidic delivery systems and in-situ monitoring technologies have significantly improved control over these parameters, yet challenges remain in achieving the ultra-high precision required for next-generation materials characterization.
From a global perspective, research into HF precision techniques has concentrated in regions with strong semiconductor and advanced materials industries, notably East Asia, North America, and Western Europe. Collaborative international efforts have accelerated in recent years, focusing on establishing universal standards for HF analytical methodologies.
The ultimate technological goal in this field is to develop fully automated, closed-loop systems capable of maintaining HF concentration with precision better than ±0.01% while simultaneously monitoring and adjusting for environmental variables that might affect analytical outcomes. Such systems would represent a significant advancement over current manual or semi-automated approaches, enabling more reliable material characterization across diverse industrial and research applications.
The technological trajectory of HF in analytical chemistry has been marked by continuous refinement in handling protocols, concentration control methodologies, and safety measures. Initially employed as a bulk etchant, HF applications have progressively shifted toward precision-controlled processes requiring nanometer-scale accuracy. This evolution reflects broader trends in materials science toward miniaturization and atomic-level control of surfaces and interfaces.
Current technological objectives in HF precision analysis center on achieving reproducible sub-nanometer etching control, developing real-time concentration monitoring systems, and establishing standardized protocols that ensure consistent results across different laboratory environments. These objectives are particularly crucial as semiconductor feature sizes continue to shrink below 5nm, demanding unprecedented precision in surface preparation and analysis.
The scientific community has identified several key parameters affecting HF precision, including temperature stability, concentration homogeneity, exposure time control, and contamination prevention. Recent advances in microfluidic delivery systems and in-situ monitoring technologies have significantly improved control over these parameters, yet challenges remain in achieving the ultra-high precision required for next-generation materials characterization.
From a global perspective, research into HF precision techniques has concentrated in regions with strong semiconductor and advanced materials industries, notably East Asia, North America, and Western Europe. Collaborative international efforts have accelerated in recent years, focusing on establishing universal standards for HF analytical methodologies.
The ultimate technological goal in this field is to develop fully automated, closed-loop systems capable of maintaining HF concentration with precision better than ±0.01% while simultaneously monitoring and adjusting for environmental variables that might affect analytical outcomes. Such systems would represent a significant advancement over current manual or semi-automated approaches, enabling more reliable material characterization across diverse industrial and research applications.
Market Demand for Precision Material Analysis
The global market for precision material analysis has witnessed substantial growth in recent years, driven primarily by advancements in semiconductor manufacturing, pharmaceutical research, and environmental monitoring. The demand for hydrofluoric acid (HF) precision in material analysis specifically has intensified as industries require increasingly accurate compositional data for quality control and research applications.
In the semiconductor industry, which currently represents approximately 40% of the precision material analysis market, the need for ultra-pure materials and precise etching processes has created significant demand for accurate HF concentration control. As chip manufacturers continue to reduce node sizes below 5nm, even minor variations in HF concentration can lead to critical defects, translating to substantial financial losses in production.
The pharmaceutical and life sciences sector has emerged as another major driver, with stringent regulatory requirements necessitating precise material characterization. This sector's demand for HF precision has grown at a compound annual rate exceeding 8% over the past five years, particularly in applications involving glass etching for specialized laboratory equipment and in certain drug development processes.
Environmental monitoring represents a rapidly expanding market segment, with governmental regulations worldwide mandating increasingly sensitive detection of fluoride compounds in water, soil, and air samples. This regulatory pressure has created substantial market opportunities for precision HF analysis technologies that can detect concentrations at parts-per-billion levels.
Geographically, Asia-Pacific dominates the market demand, accounting for over 45% of global consumption, primarily due to the concentration of semiconductor manufacturing facilities in countries like Taiwan, South Korea, and China. North America and Europe follow with significant market shares, driven by pharmaceutical research and environmental applications respectively.
Market analysts project the global precision material analysis market to reach $12.7 billion by 2027, with HF precision technologies representing a specialized but growing segment within this broader market. This growth trajectory is supported by increasing industrial automation, rising quality control standards, and the expanding application of advanced materials in emerging technologies.
The market also shows clear segmentation between high-end precision instruments for research applications and more cost-effective solutions for routine industrial quality control. This bifurcation has created distinct market opportunities for technology providers focusing on different price-performance ratios, with premium solutions commanding margins up to 60% higher than standard offerings.
In the semiconductor industry, which currently represents approximately 40% of the precision material analysis market, the need for ultra-pure materials and precise etching processes has created significant demand for accurate HF concentration control. As chip manufacturers continue to reduce node sizes below 5nm, even minor variations in HF concentration can lead to critical defects, translating to substantial financial losses in production.
The pharmaceutical and life sciences sector has emerged as another major driver, with stringent regulatory requirements necessitating precise material characterization. This sector's demand for HF precision has grown at a compound annual rate exceeding 8% over the past five years, particularly in applications involving glass etching for specialized laboratory equipment and in certain drug development processes.
Environmental monitoring represents a rapidly expanding market segment, with governmental regulations worldwide mandating increasingly sensitive detection of fluoride compounds in water, soil, and air samples. This regulatory pressure has created substantial market opportunities for precision HF analysis technologies that can detect concentrations at parts-per-billion levels.
Geographically, Asia-Pacific dominates the market demand, accounting for over 45% of global consumption, primarily due to the concentration of semiconductor manufacturing facilities in countries like Taiwan, South Korea, and China. North America and Europe follow with significant market shares, driven by pharmaceutical research and environmental applications respectively.
Market analysts project the global precision material analysis market to reach $12.7 billion by 2027, with HF precision technologies representing a specialized but growing segment within this broader market. This growth trajectory is supported by increasing industrial automation, rising quality control standards, and the expanding application of advanced materials in emerging technologies.
The market also shows clear segmentation between high-end precision instruments for research applications and more cost-effective solutions for routine industrial quality control. This bifurcation has created distinct market opportunities for technology providers focusing on different price-performance ratios, with premium solutions commanding margins up to 60% higher than standard offerings.
Current Challenges in Hydrofluoric Acid Handling
Hydrofluoric acid (HF) presents significant handling challenges in material analysis applications due to its unique chemical properties. Unlike other strong acids, HF is weakly ionized but highly penetrative, capable of passing through tissues and causing severe damage to deep tissue layers and bone. This characteristic makes it exceptionally dangerous compared to other laboratory acids, requiring specialized handling protocols beyond standard acid safety measures.
The precision requirements for HF in material analysis create a fundamental tension between analytical accuracy and safety considerations. Concentration control represents a primary challenge, as HF's volatility leads to concentration fluctuations during storage and use. Even minor variations can significantly impact analytical results, particularly in semiconductor manufacturing where trace impurity detection requires concentration consistency at parts-per-billion levels.
Temperature management during HF handling presents another substantial challenge. The acid's reactivity changes dramatically with temperature variations, affecting both safety parameters and analytical precision. Maintaining consistent temperature conditions throughout analytical procedures requires sophisticated environmental controls that many laboratories struggle to implement effectively.
Contamination control poses a persistent difficulty, as HF's aggressive nature means it readily reacts with container materials, sampling equipment, and environmental contaminants. These interactions can introduce artifacts into analytical results, compromising data integrity. The challenge is magnified when working with ultra-high purity requirements in advanced materials research.
Storage stability represents an ongoing concern, with HF solutions demonstrating concentration drift over time due to evaporation, container interactions, and potential contamination. This necessitates frequent standardization and verification procedures that introduce additional complexity to analytical workflows.
The equipment compatibility issue cannot be overstated, as HF attacks glass, many metals, and certain polymers. This severely limits material choices for analytical apparatus, often forcing compromises between optimal analytical design and HF resistance. Specialized equipment constructed from HF-resistant materials frequently lacks the precision engineering found in standard analytical instruments.
Personnel safety during precise analytical work creates perhaps the most challenging operational constraint. The protective equipment necessary for HF handling (heavy-duty gloves, face shields, etc.) reduces tactile sensitivity and visual clarity, directly impacting an analyst's ability to perform precise manipulations required for accurate measurements. This fundamental conflict between safety requirements and precision handling represents one of the most difficult aspects of HF utilization in advanced material analysis.
The precision requirements for HF in material analysis create a fundamental tension between analytical accuracy and safety considerations. Concentration control represents a primary challenge, as HF's volatility leads to concentration fluctuations during storage and use. Even minor variations can significantly impact analytical results, particularly in semiconductor manufacturing where trace impurity detection requires concentration consistency at parts-per-billion levels.
Temperature management during HF handling presents another substantial challenge. The acid's reactivity changes dramatically with temperature variations, affecting both safety parameters and analytical precision. Maintaining consistent temperature conditions throughout analytical procedures requires sophisticated environmental controls that many laboratories struggle to implement effectively.
Contamination control poses a persistent difficulty, as HF's aggressive nature means it readily reacts with container materials, sampling equipment, and environmental contaminants. These interactions can introduce artifacts into analytical results, compromising data integrity. The challenge is magnified when working with ultra-high purity requirements in advanced materials research.
Storage stability represents an ongoing concern, with HF solutions demonstrating concentration drift over time due to evaporation, container interactions, and potential contamination. This necessitates frequent standardization and verification procedures that introduce additional complexity to analytical workflows.
The equipment compatibility issue cannot be overstated, as HF attacks glass, many metals, and certain polymers. This severely limits material choices for analytical apparatus, often forcing compromises between optimal analytical design and HF resistance. Specialized equipment constructed from HF-resistant materials frequently lacks the precision engineering found in standard analytical instruments.
Personnel safety during precise analytical work creates perhaps the most challenging operational constraint. The protective equipment necessary for HF handling (heavy-duty gloves, face shields, etc.) reduces tactile sensitivity and visual clarity, directly impacting an analyst's ability to perform precise manipulations required for accurate measurements. This fundamental conflict between safety requirements and precision handling represents one of the most difficult aspects of HF utilization in advanced material analysis.
Current Precision Control Solutions
01 Precision etching applications in semiconductor manufacturing
Hydrofluoric acid is widely used in semiconductor manufacturing for precision etching of silicon dioxide and other materials. The controlled application of hydrofluoric acid solutions allows for precise removal of thin films and surface layers, which is critical for creating microelectronic components with high precision. Various formulations and concentrations are employed to achieve specific etch rates and selectivity for different materials in the fabrication process.- Precision etching applications in semiconductor manufacturing: Hydrofluoric acid is widely used in semiconductor manufacturing for precision etching of silicon dioxide and other materials. The controlled application of hydrofluoric acid solutions allows for precise removal of thin films and surface layers, which is critical in microelectronic fabrication processes. Various concentrations and formulations are employed to achieve specific etch rates and selectivity for different substrate materials, enabling the creation of intricate circuit patterns and structures at nanometer scales.
- Safety and handling improvements for hydrofluoric acid: Due to the highly corrosive and toxic nature of hydrofluoric acid, various safety and handling improvements have been developed. These include specialized containment systems, neutralizing agents, and personal protective equipment designed specifically for hydrofluoric acid applications. Advanced monitoring systems can detect minute leaks or vapor concentrations to prevent exposure. Additionally, buffered formulations and dilution techniques have been created to reduce risks while maintaining the acid's effectiveness for precision applications.
- Purification and quality control of hydrofluoric acid: High-precision applications require ultra-pure hydrofluoric acid with minimal contaminants. Various purification methods have been developed, including distillation, filtration, and ion exchange techniques to remove metal ions, particles, and organic impurities. Advanced analytical methods enable precise measurement of trace contaminants at parts-per-billion levels. Quality control protocols ensure consistent acid concentration and purity, which is critical for reproducible results in precision manufacturing processes.
- Hydrofluoric acid mixtures and formulations for enhanced performance: Specialized hydrofluoric acid formulations combine the acid with other chemicals to enhance performance for specific applications. These include buffered oxide etch (BOE) solutions containing ammonium fluoride, mixtures with nitric acid for silicon etching, and formulations with surfactants for improved wetting and uniformity. Some compositions incorporate organic solvents or additives to modify etch selectivity, reduce surface tension, or improve material compatibility. These tailored formulations enable precise control over etching profiles and rates.
- Recovery and recycling systems for hydrofluoric acid: Environmental and economic considerations have driven the development of recovery and recycling systems for hydrofluoric acid used in precision applications. These systems capture spent acid from manufacturing processes, purify it through various separation techniques, and reconcentrate it for reuse. Advanced distillation, membrane separation, and chemical treatment methods enable the removal of contaminants accumulated during use. Closed-loop systems minimize waste generation and reduce the environmental impact of hydrofluoric acid applications while maintaining the high purity required for precision processes.
02 Purification and concentration control methods
Methods for purifying hydrofluoric acid and controlling its concentration with high precision are essential for advanced industrial applications. These techniques involve distillation processes, filtration systems, and analytical methods to remove impurities and maintain consistent acid strength. Precise concentration control is achieved through specialized measurement techniques and dilution protocols that ensure the acid meets stringent purity requirements for high-precision applications.Expand Specific Solutions03 Safety and handling innovations for precision work
Innovations in the safe handling of hydrofluoric acid for precision applications include specialized containment systems, neutralization methods, and protective equipment. These developments focus on minimizing risks while maintaining the precision required for technical applications. Advanced monitoring systems detect potential leaks or exposure, while automated handling systems reduce direct human contact with the acid during precision operations.Expand Specific Solutions04 Surface treatment and cleaning applications
Hydrofluoric acid formulations are used for precision surface treatment and cleaning in various industries. These applications include the removal of oxides, contaminants, and residues from metal surfaces, glass, and electronic components. Specialized techniques have been developed to achieve uniform surface characteristics while maintaining dimensional accuracy. The acid's ability to selectively dissolve certain materials makes it valuable for precision cleaning where other chemicals may be ineffective.Expand Specific Solutions05 Precision measurement and quality control systems
Systems for the precise measurement of hydrofluoric acid properties and quality control in manufacturing processes ensure consistent performance in high-precision applications. These include advanced analytical techniques for monitoring acid concentration, purity, and reactivity in real-time. Automated feedback systems adjust process parameters based on these measurements to maintain precision throughout extended operations. Quality control protocols verify that the acid meets specifications before use in critical applications.Expand Specific Solutions
Key Industry Players in Material Analysis
The hydrofluoric acid precision material analysis market is currently in a growth phase, with increasing demand driven by semiconductor, electronics, and automotive industries. The global market size is estimated to exceed $1.5 billion, expanding at approximately 5-7% CAGR. Technologically, the field shows varying maturity levels across applications, with companies like Do-Fluoride New Materials and Stella Chemifa leading in high-purity HF production, while Siemens, Fujitsu, and Renesas Electronics focus on advanced analytical instrumentation. HORIBA Advanced Techno and Hitachi High-Tech America have developed sophisticated measurement technologies, while research institutions like AIST and CEA are pioneering next-generation techniques. Semiconductor manufacturers including Macronix and JFE Steel represent key end-users driving precision requirements in material analysis applications.
Siemens AG
Technical Solution: Siemens has engineered the ULTRAMAT HF-6000, an advanced process analytical system for precise hydrofluoric acid monitoring in material analysis applications. The technology combines tunable diode laser absorption spectroscopy (TDLAS) with proprietary multivariate calibration algorithms to achieve measurement accuracy of ±0.05% across the full concentration range. Their system features a non-contact flow-through cell design constructed from specialized fluoropolymers that eliminate sample contamination while providing exceptional chemical resistance. The ULTRAMAT platform incorporates automatic validation routines using sealed reference cells containing certified HF concentrations, ensuring measurement traceability and long-term stability. Siemens' technology includes advanced temperature and pressure compensation algorithms that maintain accuracy across varying environmental conditions. The system integrates with Siemens' broader industrial automation ecosystem, enabling seamless data transfer and process control integration for material analysis applications requiring precise acid concentration management.
Strengths: Robust industrial design suitable for continuous operation in demanding environments; comprehensive diagnostic capabilities ensure measurement integrity; extensive global support network; proven reliability in critical applications. Weaknesses: Significant footprint compared to laboratory-scale analyzers; higher power requirements; complex installation requiring specialized utilities; substantial initial investment compared to manual analytical methods.
Stella Chemifa Corp.
Technical Solution: Stella Chemifa has developed advanced ultra-high purity hydrofluoric acid (UPSS) technology specifically designed for semiconductor manufacturing and materials analysis. Their proprietary purification process achieves 99.9999% (6N) purity levels through multi-stage distillation and specialized filtration systems. The company employs real-time monitoring with ion chromatography and ICP-MS (Inductively Coupled Plasma Mass Spectrometry) to ensure consistent parts-per-trillion (ppt) level impurity control. Their analytical grade hydrofluoric acid incorporates stabilizing agents that maintain concentration accuracy over extended storage periods while minimizing container interaction. Stella Chemifa's precision dispensing systems feature temperature-controlled delivery mechanisms with flow rate accuracy of ±0.1%, critical for maintaining analytical precision in material testing applications.
Strengths: Industry-leading purity levels suitable for the most demanding semiconductor applications; proprietary stabilization technology extends shelf life while maintaining precision; advanced analytical verification methods ensure batch-to-batch consistency. Weaknesses: Higher cost compared to standard industrial grades; requires specialized handling equipment; limited global distribution network compared to larger chemical conglomerates.
Critical Technologies in HF Concentration Measurement
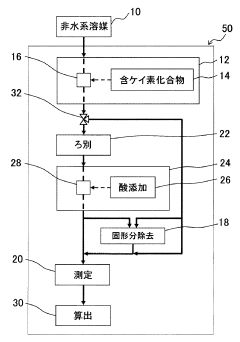
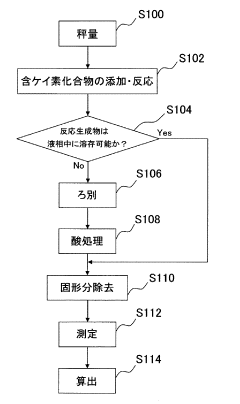
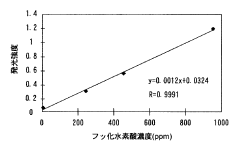
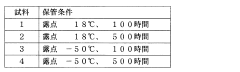
Measuring method and measuring device of nonaqueous solution
PatentInactiveJP2009054353A
Innovation
- A method involving the addition of a silicon-containing compound, such as silicon dioxide or silica gel, to react with hydrofluoric acid to form a reaction product, followed by inductively coupled plasma atomic emission spectrometry to measure the amount of hydrofluoric acid based on the reaction product.
Method for the detection of fluoride or hydrogen fluoride and detection kit
PatentInactiveUS20050227368A1
Innovation
- A method involving a silylated organic compound that undergoes desilylation in the presence of HF, allowing for separate detection of the silylated and desilylated forms, using reagents like BTSFA and MTBSTFA, and employing immunological tests for enhanced sensitivity and specificity.
Safety Protocols and Risk Management
Working with hydrofluoric acid (HF) in material analysis laboratories presents significant safety challenges due to its highly corrosive nature and ability to penetrate skin and cause deep tissue damage. Comprehensive safety protocols must be established and rigorously enforced to protect personnel and ensure accurate analytical results. These protocols should begin with mandatory specialized training for all staff handling HF, covering proper handling techniques, emergency response procedures, and the unique hazards associated with this acid.
Personal protective equipment (PPE) requirements for HF handling must exceed standard laboratory protection measures. This includes chemical-resistant clothing, face shields, specialized gloves (typically neoprene or heavy nitrile), and appropriate respiratory protection when working with concentrated solutions or when there is a risk of vapor formation. Engineering controls such as properly functioning fume hoods with specific flow rates and specialized containment systems are essential for preventing exposure to HF vapors.
Risk management for HF operations necessitates detailed standard operating procedures (SOPs) that outline step-by-step processes for each analytical method utilizing the acid. These SOPs should include specific concentration preparation guidelines, handling procedures, waste disposal protocols, and clearly defined responsibilities. Regular audits of these procedures ensure compliance and identify areas for improvement in safety practices.
Emergency response planning represents a critical component of HF safety management. Laboratories must maintain calcium gluconate gel antidotes in multiple accessible locations and train all personnel in their proper application. Emergency eyewash stations and safety showers must be readily available, regularly tested, and specifically designed for chemical emergencies. Clear evacuation routes and communication protocols should be established for major spill scenarios.
Implementing a comprehensive monitoring system for tracking HF usage, storage conditions, and disposal is essential for risk management. This includes regular inventory checks, proper labeling of all containers, and secure storage in acid-resistant cabinets with appropriate ventilation. Waste management protocols must address the specific challenges of HF disposal, including neutralization procedures and compliance with environmental regulations.
Regular safety drills and simulations of HF exposure scenarios help maintain preparedness and identify potential weaknesses in emergency response systems. Documentation of all safety incidents, near-misses, and procedural violations provides valuable data for continuous improvement of safety protocols. This information should be reviewed regularly by safety committees to update procedures and address emerging risks in HF handling for material analysis applications.
Personal protective equipment (PPE) requirements for HF handling must exceed standard laboratory protection measures. This includes chemical-resistant clothing, face shields, specialized gloves (typically neoprene or heavy nitrile), and appropriate respiratory protection when working with concentrated solutions or when there is a risk of vapor formation. Engineering controls such as properly functioning fume hoods with specific flow rates and specialized containment systems are essential for preventing exposure to HF vapors.
Risk management for HF operations necessitates detailed standard operating procedures (SOPs) that outline step-by-step processes for each analytical method utilizing the acid. These SOPs should include specific concentration preparation guidelines, handling procedures, waste disposal protocols, and clearly defined responsibilities. Regular audits of these procedures ensure compliance and identify areas for improvement in safety practices.
Emergency response planning represents a critical component of HF safety management. Laboratories must maintain calcium gluconate gel antidotes in multiple accessible locations and train all personnel in their proper application. Emergency eyewash stations and safety showers must be readily available, regularly tested, and specifically designed for chemical emergencies. Clear evacuation routes and communication protocols should be established for major spill scenarios.
Implementing a comprehensive monitoring system for tracking HF usage, storage conditions, and disposal is essential for risk management. This includes regular inventory checks, proper labeling of all containers, and secure storage in acid-resistant cabinets with appropriate ventilation. Waste management protocols must address the specific challenges of HF disposal, including neutralization procedures and compliance with environmental regulations.
Regular safety drills and simulations of HF exposure scenarios help maintain preparedness and identify potential weaknesses in emergency response systems. Documentation of all safety incidents, near-misses, and procedural violations provides valuable data for continuous improvement of safety protocols. This information should be reviewed regularly by safety committees to update procedures and address emerging risks in HF handling for material analysis applications.
Environmental Impact and Sustainable Practices
The use of hydrofluoric acid (HF) in material analysis presents significant environmental challenges that require careful consideration and sustainable management practices. HF is classified as a highly hazardous substance with potential for severe environmental contamination if improperly handled or disposed of. When released into aquatic ecosystems, even at low concentrations, it can dramatically alter pH levels and introduce fluoride ions that are toxic to aquatic organisms, disrupting entire ecological communities.
Atmospheric emissions from HF-based analytical processes contribute to air pollution and can lead to acid rain formation when combined with atmospheric moisture. This precipitation can damage vegetation, acidify soil and water bodies, and accelerate the weathering of building materials and cultural artifacts. The environmental persistence of fluoride compounds further compounds these concerns, as they can accumulate in soil and sediments, creating long-term environmental liabilities.
Current regulatory frameworks worldwide have established increasingly stringent standards for HF handling and disposal. The European Union's REACH regulations, the United States EPA guidelines, and similar frameworks in Asia-Pacific regions mandate comprehensive environmental risk assessments and mitigation strategies for facilities utilizing HF in analytical applications. These regulations have catalyzed the development of more sustainable practices within the industry.
Leading laboratories and research institutions have pioneered several sustainable approaches to minimize the environmental footprint of HF-based analysis. Micro-analytical techniques have significantly reduced required acid volumes, sometimes by factors of 10-100 compared to traditional methods. Closed-loop recycling systems now enable the recovery and purification of spent HF solutions, substantially decreasing waste generation while conserving resources.
Substitution strategies represent another promising direction, with research focused on developing less hazardous alternatives that maintain analytical precision. Ammonium bifluoride, organic acid mixtures, and certain chelating agents have shown potential as partial replacements in specific analytical applications, though complete substitution remains challenging for many high-precision requirements.
Advanced treatment technologies for HF waste have also emerged, including neutralization with calcium compounds to form insoluble calcium fluoride, ion exchange systems for fluoride removal, and specialized membrane filtration processes. These technologies can reduce environmental discharge concentrations to parts-per-billion levels, minimizing ecological impact while meeting regulatory requirements.
The industry is increasingly adopting life cycle assessment methodologies to evaluate the comprehensive environmental impact of analytical processes involving HF, from raw material extraction through disposal. This holistic approach enables more informed decision-making regarding analytical method selection and optimization, balancing precision requirements against environmental considerations.
Atmospheric emissions from HF-based analytical processes contribute to air pollution and can lead to acid rain formation when combined with atmospheric moisture. This precipitation can damage vegetation, acidify soil and water bodies, and accelerate the weathering of building materials and cultural artifacts. The environmental persistence of fluoride compounds further compounds these concerns, as they can accumulate in soil and sediments, creating long-term environmental liabilities.
Current regulatory frameworks worldwide have established increasingly stringent standards for HF handling and disposal. The European Union's REACH regulations, the United States EPA guidelines, and similar frameworks in Asia-Pacific regions mandate comprehensive environmental risk assessments and mitigation strategies for facilities utilizing HF in analytical applications. These regulations have catalyzed the development of more sustainable practices within the industry.
Leading laboratories and research institutions have pioneered several sustainable approaches to minimize the environmental footprint of HF-based analysis. Micro-analytical techniques have significantly reduced required acid volumes, sometimes by factors of 10-100 compared to traditional methods. Closed-loop recycling systems now enable the recovery and purification of spent HF solutions, substantially decreasing waste generation while conserving resources.
Substitution strategies represent another promising direction, with research focused on developing less hazardous alternatives that maintain analytical precision. Ammonium bifluoride, organic acid mixtures, and certain chelating agents have shown potential as partial replacements in specific analytical applications, though complete substitution remains challenging for many high-precision requirements.
Advanced treatment technologies for HF waste have also emerged, including neutralization with calcium compounds to form insoluble calcium fluoride, ion exchange systems for fluoride removal, and specialized membrane filtration processes. These technologies can reduce environmental discharge concentrations to parts-per-billion levels, minimizing ecological impact while meeting regulatory requirements.
The industry is increasingly adopting life cycle assessment methodologies to evaluate the comprehensive environmental impact of analytical processes involving HF, from raw material extraction through disposal. This holistic approach enables more informed decision-making regarding analytical method selection and optimization, balancing precision requirements against environmental considerations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!