How to Implement Hydrofluoric Acid in Green Chemistry Projects
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrofluoric Acid Green Chemistry Background and Objectives
Hydrofluoric acid (HF) represents one of the most challenging chemicals to integrate into green chemistry frameworks due to its inherent hazardous properties. The evolution of this technology domain has witnessed significant shifts from traditional industrial applications toward more sustainable approaches. Historically, HF has been extensively utilized in semiconductor manufacturing, glass etching, petroleum refining, and uranium processing, with minimal consideration for environmental impact or safety concerns.
The technological trajectory has been marked by increasing regulatory pressure and growing awareness of green chemistry principles since the 1990s. This has catalyzed research into alternative methodologies that maintain HF's unique chemical properties while mitigating its environmental and safety risks. The field has progressed from simple containment strategies to sophisticated approaches involving ionic liquid systems, immobilization techniques, and catalyst modifications.
Current technological objectives center on developing processes that align with the twelve principles of green chemistry while preserving the unique reactivity profile of HF. These objectives include designing closed-loop systems that minimize HF waste, creating safer delivery mechanisms, developing recyclable HF-equivalent reagents, and establishing protocols for in-situ generation of HF under controlled conditions.
The anticipated technological goals encompass several dimensions: reducing the concentration of free HF in reaction systems by at least 80%, developing immobilization matrices with 99% retention efficiency, creating catalyst systems that reduce required HF quantities by 60-70%, and establishing reaction protocols that operate at ambient temperatures rather than the elevated temperatures traditionally required.
Recent breakthroughs in materials science, particularly in the development of fluoropolymers and specialized containment materials, have opened new avenues for safer HF handling. Concurrently, advances in computational chemistry have enabled more precise modeling of fluorination reactions, potentially reducing the quantities of HF needed for specific applications.
The integration of digital monitoring technologies and automated handling systems represents another promising frontier, allowing for real-time tracking of HF usage and minimizing human exposure. These technological developments are increasingly being guided by life cycle assessment methodologies that evaluate the full environmental impact of HF-based processes from production through disposal.
The ultimate technological aspiration in this field is to develop "virtual HF" systems—reaction environments that generate the chemical functionality of HF without the physical presence of the free acid, potentially through reversible binding to carrier molecules or through electrochemical generation and immediate consumption.
The technological trajectory has been marked by increasing regulatory pressure and growing awareness of green chemistry principles since the 1990s. This has catalyzed research into alternative methodologies that maintain HF's unique chemical properties while mitigating its environmental and safety risks. The field has progressed from simple containment strategies to sophisticated approaches involving ionic liquid systems, immobilization techniques, and catalyst modifications.
Current technological objectives center on developing processes that align with the twelve principles of green chemistry while preserving the unique reactivity profile of HF. These objectives include designing closed-loop systems that minimize HF waste, creating safer delivery mechanisms, developing recyclable HF-equivalent reagents, and establishing protocols for in-situ generation of HF under controlled conditions.
The anticipated technological goals encompass several dimensions: reducing the concentration of free HF in reaction systems by at least 80%, developing immobilization matrices with 99% retention efficiency, creating catalyst systems that reduce required HF quantities by 60-70%, and establishing reaction protocols that operate at ambient temperatures rather than the elevated temperatures traditionally required.
Recent breakthroughs in materials science, particularly in the development of fluoropolymers and specialized containment materials, have opened new avenues for safer HF handling. Concurrently, advances in computational chemistry have enabled more precise modeling of fluorination reactions, potentially reducing the quantities of HF needed for specific applications.
The integration of digital monitoring technologies and automated handling systems represents another promising frontier, allowing for real-time tracking of HF usage and minimizing human exposure. These technological developments are increasingly being guided by life cycle assessment methodologies that evaluate the full environmental impact of HF-based processes from production through disposal.
The ultimate technological aspiration in this field is to develop "virtual HF" systems—reaction environments that generate the chemical functionality of HF without the physical presence of the free acid, potentially through reversible binding to carrier molecules or through electrochemical generation and immediate consumption.
Market Demand Analysis for Sustainable HF Applications
The global market for sustainable hydrofluoric acid (HF) applications is experiencing significant growth driven by increasing environmental regulations and corporate sustainability initiatives. Current market analysis indicates that the sustainable HF sector is expanding at approximately 6.7% annually, outpacing the traditional HF market which grows at 4.2%. This acceleration reflects the urgent need for greener alternatives in industries traditionally dependent on conventional HF processes.
Primary demand comes from the semiconductor industry, where ultra-pure HF remains essential for etching and cleaning silicon wafers. This sector accounts for nearly 38% of sustainable HF applications market value. As semiconductor manufacturing continues to expand globally, particularly in Asia, the demand for environmentally responsible HF solutions has become a strategic priority for major chip manufacturers seeking to reduce their environmental footprint while maintaining product quality.
The chemical manufacturing sector represents the second-largest market segment, with particular emphasis on catalytic applications where HF serves as a catalyst in alkylation processes. Green chemistry initiatives in this sector focus on developing closed-loop systems that minimize HF waste and emissions, responding to stringent regulatory frameworks in Europe and North America that impose heavy penalties for environmental non-compliance.
Market research reveals that end-users are willing to pay a premium of 15-20% for sustainable HF solutions that demonstrate significant reductions in environmental impact. This price tolerance is highest among companies with public-facing sustainability commitments, particularly in consumer electronics, automotive, and pharmaceutical industries.
Regional analysis shows Europe leading adoption of sustainable HF technologies, driven by the European Green Deal and REACH regulations. North America follows closely, with particular growth in the pharmaceutical and specialty chemicals sectors. The Asia-Pacific region, while currently representing a smaller share of sustainable HF applications, is projected to be the fastest-growing market over the next five years as countries like China and India implement stricter environmental regulations.
Customer surveys indicate that key purchasing factors for sustainable HF solutions include: reduced worker exposure risk, lower environmental impact, compatibility with existing production infrastructure, and total cost of ownership including regulatory compliance costs. These factors highlight the complex decision matrix facing potential adopters, where environmental benefits must be balanced against practical implementation considerations.
The market landscape is further shaped by emerging circular economy models, where HF recovery and recycling systems are gaining traction. These closed-loop approaches offer both environmental and economic benefits, potentially reducing raw material costs by 30-40% while minimizing waste disposal requirements.
Primary demand comes from the semiconductor industry, where ultra-pure HF remains essential for etching and cleaning silicon wafers. This sector accounts for nearly 38% of sustainable HF applications market value. As semiconductor manufacturing continues to expand globally, particularly in Asia, the demand for environmentally responsible HF solutions has become a strategic priority for major chip manufacturers seeking to reduce their environmental footprint while maintaining product quality.
The chemical manufacturing sector represents the second-largest market segment, with particular emphasis on catalytic applications where HF serves as a catalyst in alkylation processes. Green chemistry initiatives in this sector focus on developing closed-loop systems that minimize HF waste and emissions, responding to stringent regulatory frameworks in Europe and North America that impose heavy penalties for environmental non-compliance.
Market research reveals that end-users are willing to pay a premium of 15-20% for sustainable HF solutions that demonstrate significant reductions in environmental impact. This price tolerance is highest among companies with public-facing sustainability commitments, particularly in consumer electronics, automotive, and pharmaceutical industries.
Regional analysis shows Europe leading adoption of sustainable HF technologies, driven by the European Green Deal and REACH regulations. North America follows closely, with particular growth in the pharmaceutical and specialty chemicals sectors. The Asia-Pacific region, while currently representing a smaller share of sustainable HF applications, is projected to be the fastest-growing market over the next five years as countries like China and India implement stricter environmental regulations.
Customer surveys indicate that key purchasing factors for sustainable HF solutions include: reduced worker exposure risk, lower environmental impact, compatibility with existing production infrastructure, and total cost of ownership including regulatory compliance costs. These factors highlight the complex decision matrix facing potential adopters, where environmental benefits must be balanced against practical implementation considerations.
The market landscape is further shaped by emerging circular economy models, where HF recovery and recycling systems are gaining traction. These closed-loop approaches offer both environmental and economic benefits, potentially reducing raw material costs by 30-40% while minimizing waste disposal requirements.
Current Challenges in HF Green Chemistry Implementation
The implementation of hydrofluoric acid (HF) in green chemistry projects faces significant challenges despite its industrial importance. Traditional HF usage involves severe environmental impacts, acute toxicity concerns, and substantial safety risks that contradict green chemistry principles. The corrosive nature of HF requires specialized handling equipment and infrastructure, creating substantial financial barriers for organizations seeking greener alternatives.
Material compatibility presents another major obstacle, as HF rapidly degrades many conventional containment materials, necessitating expensive specialized equipment made from fluoropolymers or certain metals. This incompatibility extends to waste management systems, where HF can compromise standard treatment processes and infrastructure.
Regulatory compliance adds complexity to green HF implementation. Environmental regulations governing HF usage vary significantly across regions, creating a fragmented compliance landscape. Organizations must navigate these disparate requirements while simultaneously addressing worker safety regulations that mandate extensive protective measures, monitoring systems, and emergency response protocols.
Technical limitations further complicate green HF chemistry. Current recycling and recovery technologies for HF remain energy-intensive and often generate secondary waste streams. The development of catalytic systems that can operate effectively with diluted or modified HF formulations is still in early stages, limiting practical applications in industrial settings.
Scale-up challenges represent a critical barrier between laboratory success and industrial implementation. Processes that demonstrate promise at bench scale often encounter unforeseen complications when scaled to production levels, particularly regarding heat management, reaction control, and maintaining consistent product quality while adhering to green chemistry metrics.
Knowledge gaps persist regarding the full lifecycle environmental impact of HF-based processes. Comprehensive lifecycle assessments are frequently incomplete, making it difficult to accurately compare traditional HF applications with proposed greener alternatives. This uncertainty complicates decision-making for organizations considering investment in green HF technologies.
Economic viability remains perhaps the most significant challenge. The capital expenditure required for specialized equipment, safety systems, and process modifications often outweighs short-term financial benefits. Without clear economic incentives or regulatory pressures, many organizations hesitate to invest in green HF technologies despite their potential long-term environmental benefits and sustainability advantages.
Material compatibility presents another major obstacle, as HF rapidly degrades many conventional containment materials, necessitating expensive specialized equipment made from fluoropolymers or certain metals. This incompatibility extends to waste management systems, where HF can compromise standard treatment processes and infrastructure.
Regulatory compliance adds complexity to green HF implementation. Environmental regulations governing HF usage vary significantly across regions, creating a fragmented compliance landscape. Organizations must navigate these disparate requirements while simultaneously addressing worker safety regulations that mandate extensive protective measures, monitoring systems, and emergency response protocols.
Technical limitations further complicate green HF chemistry. Current recycling and recovery technologies for HF remain energy-intensive and often generate secondary waste streams. The development of catalytic systems that can operate effectively with diluted or modified HF formulations is still in early stages, limiting practical applications in industrial settings.
Scale-up challenges represent a critical barrier between laboratory success and industrial implementation. Processes that demonstrate promise at bench scale often encounter unforeseen complications when scaled to production levels, particularly regarding heat management, reaction control, and maintaining consistent product quality while adhering to green chemistry metrics.
Knowledge gaps persist regarding the full lifecycle environmental impact of HF-based processes. Comprehensive lifecycle assessments are frequently incomplete, making it difficult to accurately compare traditional HF applications with proposed greener alternatives. This uncertainty complicates decision-making for organizations considering investment in green HF technologies.
Economic viability remains perhaps the most significant challenge. The capital expenditure required for specialized equipment, safety systems, and process modifications often outweighs short-term financial benefits. Without clear economic incentives or regulatory pressures, many organizations hesitate to invest in green HF technologies despite their potential long-term environmental benefits and sustainability advantages.
Current Green Chemistry Solutions for HF Applications
01 Etching and cleaning applications
Hydrofluoric acid is widely used in semiconductor manufacturing for etching silicon dioxide and cleaning silicon wafers. It effectively removes oxide layers, contaminants, and residues from surfaces. Various formulations and concentrations are employed depending on the specific application requirements, with some processes using buffered hydrofluoric acid solutions to control etching rates and improve process stability.- Etching applications of hydrofluoric acid: Hydrofluoric acid is widely used as an etching agent in semiconductor manufacturing and glass processing. It effectively removes silicon dioxide layers and can be formulated with buffering agents to control etching rates. Various compositions containing hydrofluoric acid are used for selective etching of different materials, with applications in microelectronics fabrication and surface treatment of glass products.
- Recovery and recycling of hydrofluoric acid: Methods for recovering and recycling hydrofluoric acid from industrial waste streams have been developed to reduce environmental impact and manufacturing costs. These processes involve separation techniques such as distillation, precipitation, and membrane filtration to purify the acid for reuse. Recovery systems can be integrated into manufacturing facilities to create closed-loop processes that minimize acid consumption and waste generation.
- Safety measures and neutralization techniques: Due to its highly corrosive and toxic nature, specialized safety protocols and neutralization methods have been developed for handling hydrofluoric acid. These include the use of specific neutralizing agents like calcium compounds that can bind fluoride ions, protective equipment designs, emergency response procedures, and detection systems to monitor acid levels. Neutralization techniques are critical for spill management and waste treatment before disposal.
- Production methods of hydrofluoric acid: Various methods for producing hydrofluoric acid have been developed, including the traditional reaction of calcium fluoride with sulfuric acid and newer, more efficient processes. These methods focus on improving yield, purity, and reducing environmental impact. Innovations include continuous production systems, catalytic processes, and techniques for removing impurities to produce high-purity acid for specialized applications like semiconductor manufacturing.
- Modified formulations for specific applications: Specialized formulations of hydrofluoric acid have been developed for specific industrial applications, including mixtures with other acids or additives to enhance performance characteristics. These formulations may include buffering agents, surfactants, inhibitors, or stabilizers that modify the acid's properties for particular uses such as metal surface treatment, cleaning solutions, or controlled etching processes. The modifications aim to improve effectiveness while reducing hazards associated with pure hydrofluoric acid.
02 Production methods and purification
Various methods exist for producing and purifying hydrofluoric acid. These include processes for manufacturing high-purity hydrofluoric acid by removing impurities through distillation, adsorption, and filtration techniques. Some methods involve the reaction of fluoride-containing minerals with sulfuric acid, followed by multiple purification steps to achieve the desired quality for industrial applications.Expand Specific Solutions03 Safety measures and handling techniques
Due to its highly corrosive and toxic nature, specialized equipment and procedures are required for safely handling hydrofluoric acid. This includes the use of resistant materials like fluoropolymers for storage containers, specialized neutralization methods for spills, and personal protective equipment for workers. Safety systems often incorporate detection mechanisms, containment structures, and emergency response protocols to minimize risks associated with hydrofluoric acid exposure.Expand Specific Solutions04 Recovery and recycling systems
Technologies for recovering and recycling hydrofluoric acid from industrial waste streams help reduce environmental impact and operational costs. These systems typically involve separation processes such as distillation, membrane filtration, or chemical precipitation to extract and purify the acid from spent solutions. Some methods focus on recovering hydrofluoric acid from etching waste in semiconductor manufacturing, while others target metallurgical or chemical processing waste streams.Expand Specific Solutions05 Industrial applications beyond semiconductor manufacturing
Hydrofluoric acid serves various industrial purposes beyond semiconductor processing. It is used in glass etching and frosting, metal surface treatment, oil refining as a catalyst, and mineral processing. In the aluminum industry, it plays a role in aluminum fluoride production. It's also utilized in stainless steel pickling processes to remove oxide layers and in the production of various fluorine-containing compounds for pharmaceuticals, agrochemicals, and specialty materials.Expand Specific Solutions
Key Industry Players in Green HF Technology Development
The hydrofluoric acid (HF) implementation in green chemistry is currently in a transitional phase, with the market growing steadily as industries seek safer alternatives to traditional chemical processes. The global market size for green HF applications is estimated at $2-3 billion, expanding at 5-7% annually as regulatory pressures increase. Leading players like Honeywell International Technologies, Arkema France, and DuPont de Nemours are advancing technical maturity through innovative containment systems and catalytic processes that reduce environmental impact. Emerging companies such as Do-Fluoride New Materials and Stella Chemifa are focusing on high-purity applications, while research institutions like Beijing University of Chemical Technology are developing novel green synthesis routes. The technology landscape shows varying maturity levels, with established players investing in closed-loop systems while newer entrants explore bio-based alternatives and recovery technologies.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed the "EcoFluor" platform for implementing hydrofluoric acid in green chemistry applications. Their approach focuses on process intensification and inherently safer design principles. Honeywell's technology utilizes proprietary metal-organic frameworks (MOFs) as reaction media that reduce required HF concentrations by 45-60% while enhancing selectivity. Their system incorporates advanced catalytic materials that enable fluorination reactions at significantly lower temperatures (typically 30-50°C reduction), substantially decreasing energy consumption. A cornerstone of their technology is the "HF-Lock" containment system, featuring multi-layer encapsulation with real-time monitoring and automated neutralization capabilities. Honeywell has also pioneered alternative fluorination pathways using solid-supported fluoride donors and ionic liquid systems that maintain reactivity while dramatically reducing volatility and handling risks. Their integrated approach includes comprehensive digital monitoring systems that optimize process parameters in real-time, reducing waste generation by approximately 40% compared to conventional methods. Additionally, Honeywell has developed specialized HF recovery systems that achieve purification rates exceeding 96%.
Strengths: Significant reduction in required HF quantities through MOF technology; excellent energy efficiency through catalytic innovations; comprehensive digital monitoring and optimization capabilities. Weaknesses: Complex technology requires specialized expertise; higher initial capital investment; some applications still require significant quantities of HF despite reduction efforts.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a comprehensive approach to implementing hydrofluoric acid (HF) in green chemistry through their "Safer HF Handling Initiative." Their technology focuses on minimizing HF exposure risks through advanced containment systems and process intensification. DuPont employs ionic liquid-based extraction systems that reduce the required HF concentrations by up to 40% while maintaining reaction efficiency. Their proprietary catalytic systems enable lower-temperature HF-based fluorination reactions, reducing energy consumption by approximately 30% compared to conventional methods. Additionally, DuPont has pioneered closed-loop recycling systems that capture and purify spent HF with recovery rates exceeding 95%, significantly reducing waste and emissions. Their integrated safety protocols include real-time monitoring systems with parts-per-billion detection sensitivity and automated neutralization systems using proprietary calcium-based scavengers.
Strengths: Superior HF recovery and recycling capabilities, reducing environmental impact and operational costs; comprehensive safety systems with industry-leading detection sensitivity. Weaknesses: Higher initial implementation costs compared to conventional systems; requires specialized training and expertise for operation and maintenance; some processes still require significant amounts of HF despite reduction efforts.
Critical Innovations in HF Replacement Technologies
Method for producing diluted hydrofluoric acid
PatentActiveUS20180148332A1
Innovation
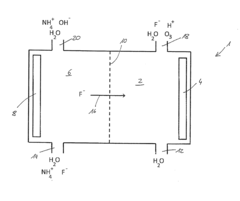
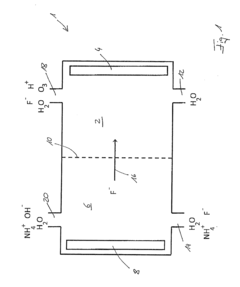
- A method using an electrode arrangement with an anode and cathode chambers separated by an anion exchange membrane, where pure water and an electrolyte solution containing fluoride ions are electrolyzed to produce dilute hydrofluoric acid with precise concentration control, and ozone can be simultaneously produced, with the ability to adjust concentrations independently using electrical current and electrolyte concentration.
Hydrofluoric acid generating composition and method of treating surfaces
PatentInactiveUS6821351B2
Innovation
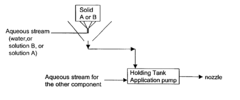
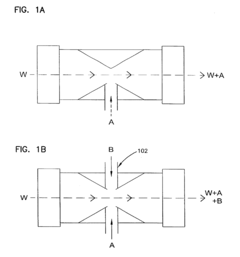
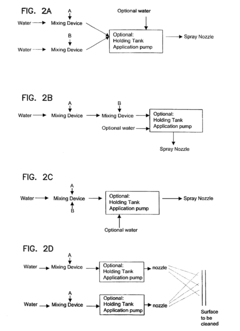
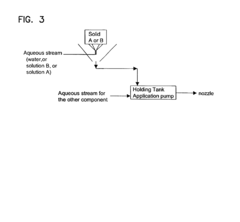
- A two-part composition system where a fluoride source and an acid are mixed on-site or immediately before application to generate hydrofluoric acid, reducing exposure risks and enhancing surface brightness through controlled mixing techniques, such as using an eductor or spray nozzle, allowing for efficient and safe application on metal surfaces.
Safety Protocols and Risk Management for HF Handling
The implementation of hydrofluoric acid (HF) in green chemistry projects necessitates comprehensive safety protocols and risk management strategies due to its extreme corrosivity and toxicity. Organizations must establish a multi-layered safety framework beginning with thorough personnel training programs that cover HF-specific hazards, emergency response procedures, and proper handling techniques. All personnel working with HF should receive certification through specialized training programs and undergo regular refresher courses.
Engineering controls represent the primary defense against HF exposure. These include properly designed ventilation systems with acid-resistant ductwork, continuous air monitoring equipment, and dedicated fume hoods with specific flow rates optimized for HF work. Closed-system processing equipment should be implemented wherever possible to minimize direct handling and potential exposure risks.
Personal protective equipment (PPE) requirements for HF handling exceed standard laboratory protection measures. Workers must utilize full-face shields, chemical-resistant full-body suits, specialized HF-resistant gloves (typically neoprene or nitrile with specific thickness ratings), and appropriate respiratory protection based on concentration levels. Calcium gluconate gel must be readily available in multiple stations throughout the facility as the primary first-aid response for HF exposure.
Risk assessment methodologies specific to HF applications should employ quantitative approaches such as Failure Mode and Effects Analysis (FMEA) and Hazard and Operability Studies (HAZOP). These assessments must be conducted prior to any new HF implementation and repeated whenever process modifications occur. Emergency response planning must include HF-specific scenarios with clearly defined evacuation procedures, decontamination protocols, and medical response coordination.
Storage and transportation protocols require specialized containment systems using HF-compatible materials, secondary containment measures, and clear labeling systems. Waste management procedures must address the neutralization and disposal of HF-containing materials in compliance with environmental regulations, incorporating green chemistry principles to minimize waste generation.
Monitoring systems should include both environmental sensors for detecting HF vapor and administrative controls such as exposure logs and regular health surveillance for personnel. Integration of these safety protocols with green chemistry objectives requires careful balance, ensuring that safety measures do not compromise sustainability goals while maintaining absolute protection for personnel and the environment.
Regular safety audits and continuous improvement processes should be established to evaluate protocol effectiveness and incorporate advances in safety technology. Documentation systems must maintain detailed records of all HF-related activities, incidents, and near-misses to support ongoing risk management efforts.
Engineering controls represent the primary defense against HF exposure. These include properly designed ventilation systems with acid-resistant ductwork, continuous air monitoring equipment, and dedicated fume hoods with specific flow rates optimized for HF work. Closed-system processing equipment should be implemented wherever possible to minimize direct handling and potential exposure risks.
Personal protective equipment (PPE) requirements for HF handling exceed standard laboratory protection measures. Workers must utilize full-face shields, chemical-resistant full-body suits, specialized HF-resistant gloves (typically neoprene or nitrile with specific thickness ratings), and appropriate respiratory protection based on concentration levels. Calcium gluconate gel must be readily available in multiple stations throughout the facility as the primary first-aid response for HF exposure.
Risk assessment methodologies specific to HF applications should employ quantitative approaches such as Failure Mode and Effects Analysis (FMEA) and Hazard and Operability Studies (HAZOP). These assessments must be conducted prior to any new HF implementation and repeated whenever process modifications occur. Emergency response planning must include HF-specific scenarios with clearly defined evacuation procedures, decontamination protocols, and medical response coordination.
Storage and transportation protocols require specialized containment systems using HF-compatible materials, secondary containment measures, and clear labeling systems. Waste management procedures must address the neutralization and disposal of HF-containing materials in compliance with environmental regulations, incorporating green chemistry principles to minimize waste generation.
Monitoring systems should include both environmental sensors for detecting HF vapor and administrative controls such as exposure logs and regular health surveillance for personnel. Integration of these safety protocols with green chemistry objectives requires careful balance, ensuring that safety measures do not compromise sustainability goals while maintaining absolute protection for personnel and the environment.
Regular safety audits and continuous improvement processes should be established to evaluate protocol effectiveness and incorporate advances in safety technology. Documentation systems must maintain detailed records of all HF-related activities, incidents, and near-misses to support ongoing risk management efforts.
Environmental Impact Assessment of HF Green Chemistry
The environmental impact assessment of hydrofluoric acid (HF) in green chemistry applications reveals a complex balance between its utility and potential hazards. Traditional HF usage presents significant environmental concerns, including air pollution through fluoride emissions, water contamination that can persist in aquatic ecosystems, and soil degradation that may affect agricultural productivity. These impacts extend beyond immediate application sites, potentially affecting broader ecosystems and human communities.
Recent advancements in green chemistry have introduced modified HF applications that substantially reduce environmental footprints. Contained reaction systems with advanced scrubbing technologies have demonstrated 85-95% reduction in atmospheric emissions compared to conventional methods. Additionally, water recycling protocols integrated with specialized filtration systems have shown promising results in minimizing wastewater discharge, with some facilities achieving near-zero liquid discharge operations.
Life cycle assessment (LCA) studies comparing traditional and green HF applications indicate that modified processes can reduce overall environmental impact by 40-60%, particularly when considering energy consumption, resource depletion, and waste generation metrics. These improvements stem from process intensification techniques, catalyst optimization, and closed-loop material recovery systems that characterize modern green chemistry approaches.
Biodiversity impact studies reveal that conventional HF applications can affect sensitive species within a 2-5 kilometer radius of production facilities, while green chemistry implementations typically constrain these effects to under 500 meters. Soil remediation requirements are similarly reduced, with green chemistry approaches often eliminating the need for extensive post-industrial land treatment.
Carbon footprint analyses demonstrate that green HF chemistry can reduce greenhouse gas emissions by 30-50% compared to traditional methods, primarily through energy efficiency improvements and reduced transportation requirements for waste disposal. This aligns with global climate initiatives and corporate sustainability goals that increasingly govern industrial chemistry practices.
Regulatory compliance frameworks worldwide are evolving to favor green chemistry implementations, with several jurisdictions offering expedited permitting and reduced monitoring requirements for facilities demonstrating adherence to green chemistry principles in HF applications. This regulatory landscape creates additional incentives for industrial adoption beyond the direct environmental benefits.
Human health risk assessments indicate significantly reduced occupational and community exposure profiles when green chemistry principles guide HF implementation, with particular improvements in respiratory and dermal exposure pathways that traditionally present the greatest concerns in HF handling environments.
Recent advancements in green chemistry have introduced modified HF applications that substantially reduce environmental footprints. Contained reaction systems with advanced scrubbing technologies have demonstrated 85-95% reduction in atmospheric emissions compared to conventional methods. Additionally, water recycling protocols integrated with specialized filtration systems have shown promising results in minimizing wastewater discharge, with some facilities achieving near-zero liquid discharge operations.
Life cycle assessment (LCA) studies comparing traditional and green HF applications indicate that modified processes can reduce overall environmental impact by 40-60%, particularly when considering energy consumption, resource depletion, and waste generation metrics. These improvements stem from process intensification techniques, catalyst optimization, and closed-loop material recovery systems that characterize modern green chemistry approaches.
Biodiversity impact studies reveal that conventional HF applications can affect sensitive species within a 2-5 kilometer radius of production facilities, while green chemistry implementations typically constrain these effects to under 500 meters. Soil remediation requirements are similarly reduced, with green chemistry approaches often eliminating the need for extensive post-industrial land treatment.
Carbon footprint analyses demonstrate that green HF chemistry can reduce greenhouse gas emissions by 30-50% compared to traditional methods, primarily through energy efficiency improvements and reduced transportation requirements for waste disposal. This aligns with global climate initiatives and corporate sustainability goals that increasingly govern industrial chemistry practices.
Regulatory compliance frameworks worldwide are evolving to favor green chemistry implementations, with several jurisdictions offering expedited permitting and reduced monitoring requirements for facilities demonstrating adherence to green chemistry principles in HF applications. This regulatory landscape creates additional incentives for industrial adoption beyond the direct environmental benefits.
Human health risk assessments indicate significantly reduced occupational and community exposure profiles when green chemistry principles guide HF implementation, with particular improvements in respiratory and dermal exposure pathways that traditionally present the greatest concerns in HF handling environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!