Hydrofluoric Acid Role in Metal Surface Treatment Safety
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF Acid Evolution and Treatment Objectives
Hydrofluoric acid (HF) has been a cornerstone in metal surface treatment since the early 20th century, evolving from rudimentary applications to sophisticated industrial processes. Initially utilized primarily for glass etching, HF's unique ability to dissolve silicon dioxide and various metal oxides quickly expanded its application into metal manufacturing sectors. The 1950s marked a significant turning point when aerospace and electronics industries began adopting HF-based treatments for specialized metal surface preparation, catalyzing research into more controlled application methodologies.
The evolution of HF acid technology has been driven by dual imperatives: enhancing treatment effectiveness while simultaneously addressing serious safety concerns. Unlike other acids, HF's distinctive hazard profile stems from its ability to penetrate skin and tissues, causing deep-tissue damage and potential systemic toxicity through calcium sequestration. This has necessitated parallel development tracks focusing on both technical efficacy and comprehensive safety protocols.
Modern HF treatment objectives center around achieving precise surface modifications while implementing rigorous safety standards. Primary technical goals include controlled etching depth, uniform surface preparation, removal of specific contaminants and oxides, and enhancement of subsequent coating adhesion properties. These objectives must be balanced with increasingly stringent worker safety requirements and environmental regulations that have become more prominent since the 1990s.
Recent technological advancements have focused on developing buffered HF formulations that maintain effectiveness while reducing hazards. These innovations include incorporating stabilizing agents, implementing precise concentration control systems, and designing closed-loop application equipment that minimizes worker exposure. The industry has established treatment objectives that quantify success metrics such as surface roughness parameters, contamination reduction percentages, and adhesion strength improvements.
The current trajectory of HF acid evolution emphasizes finding alternative technologies that can match or exceed HF's performance while eliminating its inherent dangers. Research into plasma treatments, supercritical CO2 processes, and ionic liquid alternatives represents promising directions. However, complete replacement remains challenging due to HF's unique chemical properties and cost-effectiveness in certain applications.
Treatment objectives now incorporate comprehensive risk assessment frameworks that evaluate not only technical performance but also safety margins, environmental impact, and regulatory compliance. The industry has established standardized protocols that define acceptable risk thresholds and mandatory safety controls, creating a more holistic approach to technology implementation that considers the entire lifecycle of HF-based processes.
The evolution of HF acid technology has been driven by dual imperatives: enhancing treatment effectiveness while simultaneously addressing serious safety concerns. Unlike other acids, HF's distinctive hazard profile stems from its ability to penetrate skin and tissues, causing deep-tissue damage and potential systemic toxicity through calcium sequestration. This has necessitated parallel development tracks focusing on both technical efficacy and comprehensive safety protocols.
Modern HF treatment objectives center around achieving precise surface modifications while implementing rigorous safety standards. Primary technical goals include controlled etching depth, uniform surface preparation, removal of specific contaminants and oxides, and enhancement of subsequent coating adhesion properties. These objectives must be balanced with increasingly stringent worker safety requirements and environmental regulations that have become more prominent since the 1990s.
Recent technological advancements have focused on developing buffered HF formulations that maintain effectiveness while reducing hazards. These innovations include incorporating stabilizing agents, implementing precise concentration control systems, and designing closed-loop application equipment that minimizes worker exposure. The industry has established treatment objectives that quantify success metrics such as surface roughness parameters, contamination reduction percentages, and adhesion strength improvements.
The current trajectory of HF acid evolution emphasizes finding alternative technologies that can match or exceed HF's performance while eliminating its inherent dangers. Research into plasma treatments, supercritical CO2 processes, and ionic liquid alternatives represents promising directions. However, complete replacement remains challenging due to HF's unique chemical properties and cost-effectiveness in certain applications.
Treatment objectives now incorporate comprehensive risk assessment frameworks that evaluate not only technical performance but also safety margins, environmental impact, and regulatory compliance. The industry has established standardized protocols that define acceptable risk thresholds and mandatory safety controls, creating a more holistic approach to technology implementation that considers the entire lifecycle of HF-based processes.
Market Analysis for HF-Based Surface Treatment
The global market for hydrofluoric acid (HF) in metal surface treatment applications has demonstrated consistent growth over the past decade, driven primarily by expanding industrial manufacturing sectors and increasing demand for high-performance metal components. The market size for HF-based surface treatment solutions reached approximately $2.3 billion in 2022, with projections indicating a compound annual growth rate of 4.7% through 2028.
Aerospace and automotive industries represent the largest end-user segments, collectively accounting for over 53% of market consumption. This dominance stems from the critical need for precision surface preparation in these sectors, where component reliability directly impacts safety and performance. The electronics industry follows as the third-largest consumer, utilizing HF-based treatments for semiconductor manufacturing and circuit board production.
Regionally, Asia-Pacific leads the market with a 42% share, bolstered by rapid industrialization in China and India, alongside established manufacturing hubs in Japan and South Korea. North America and Europe follow with 27% and 23% market shares respectively, where demand is primarily driven by high-tech manufacturing and stringent quality requirements in aerospace applications.
A significant market trend is the growing demand for safer HF alternatives and enhanced safety systems. This shift is evidenced by the 18% annual growth in sales of buffered HF formulations and enclosed treatment systems with advanced ventilation. Companies investing in these safer technologies report reduced workplace incidents and lower insurance premiums, creating a compelling economic case beyond regulatory compliance.
The competitive landscape features both chemical manufacturing giants and specialized surface treatment solution providers. Key market players include Honeywell, Solvay, Stella Chemifa, and Morita Chemical Industries, who collectively control approximately 65% of global supply. Regional players with specialized expertise in safety systems are gaining market share, particularly in developed economies with stringent workplace safety regulations.
Customer segmentation reveals distinct purchasing patterns: large-scale manufacturers prioritize consistent supply chains and bulk pricing, while specialized fabricators emphasize technical support and customized formulations. This dichotomy has led to the emergence of service-oriented business models, where suppliers offer comprehensive packages including chemicals, equipment, training, and waste management solutions.
Price sensitivity varies significantly by region and application, with aerospace and medical device manufacturers demonstrating willingness to pay premium prices for higher purity grades and enhanced safety features. Conversely, general industrial applications remain highly price-competitive, driving innovation in cost-effective delivery and recovery systems.
Aerospace and automotive industries represent the largest end-user segments, collectively accounting for over 53% of market consumption. This dominance stems from the critical need for precision surface preparation in these sectors, where component reliability directly impacts safety and performance. The electronics industry follows as the third-largest consumer, utilizing HF-based treatments for semiconductor manufacturing and circuit board production.
Regionally, Asia-Pacific leads the market with a 42% share, bolstered by rapid industrialization in China and India, alongside established manufacturing hubs in Japan and South Korea. North America and Europe follow with 27% and 23% market shares respectively, where demand is primarily driven by high-tech manufacturing and stringent quality requirements in aerospace applications.
A significant market trend is the growing demand for safer HF alternatives and enhanced safety systems. This shift is evidenced by the 18% annual growth in sales of buffered HF formulations and enclosed treatment systems with advanced ventilation. Companies investing in these safer technologies report reduced workplace incidents and lower insurance premiums, creating a compelling economic case beyond regulatory compliance.
The competitive landscape features both chemical manufacturing giants and specialized surface treatment solution providers. Key market players include Honeywell, Solvay, Stella Chemifa, and Morita Chemical Industries, who collectively control approximately 65% of global supply. Regional players with specialized expertise in safety systems are gaining market share, particularly in developed economies with stringent workplace safety regulations.
Customer segmentation reveals distinct purchasing patterns: large-scale manufacturers prioritize consistent supply chains and bulk pricing, while specialized fabricators emphasize technical support and customized formulations. This dichotomy has led to the emergence of service-oriented business models, where suppliers offer comprehensive packages including chemicals, equipment, training, and waste management solutions.
Price sensitivity varies significantly by region and application, with aerospace and medical device manufacturers demonstrating willingness to pay premium prices for higher purity grades and enhanced safety features. Conversely, general industrial applications remain highly price-competitive, driving innovation in cost-effective delivery and recovery systems.
Current Challenges in HF Surface Treatment Safety
Despite significant advancements in metal surface treatment technologies, hydrofluoric acid (HF) continues to present substantial safety challenges across industrial applications. The inherent dual hazard profile of HF—combining both corrosive and toxic properties—creates a complex risk landscape that current safety protocols struggle to fully mitigate. When absorbed through skin, HF penetrates deep into tissues, causing severe damage to bones and internal organs that may not be immediately apparent, complicating emergency response procedures.
Current engineering controls show critical limitations, particularly in containing HF vapors which can spread beyond designated work areas even with standard ventilation systems. The efficacy of traditional personal protective equipment (PPE) remains questionable, as studies indicate that certain glove materials degrade faster than previously understood when exposed to HF concentrations commonly used in metal etching processes.
Waste management presents another significant challenge, with neutralization processes often generating hazardous byproducts that require specialized disposal methods. Environmental regulations are becoming increasingly stringent, yet standardized protocols for HF-contaminated waste handling vary considerably across regions, creating compliance difficulties for multinational operations.
Detection technology limitations compound these issues, as current real-time monitoring systems lack the sensitivity to detect HF at the lowest dangerous concentrations, particularly in humid environments common in metal treatment facilities. This creates dangerous blind spots in safety monitoring systems, potentially allowing exposure incidents to escalate before detection.
Medical response protocols for HF exposure remain inadequate in many industrial settings. The specialized treatment required—including calcium gluconate application—is not universally available in first aid stations, and medical personnel at local facilities often lack specific training in treating HF injuries, leading to critical delays in appropriate care delivery.
The knowledge gap among workers represents another persistent challenge. Despite mandatory training programs, research indicates significant misunderstandings about HF's delayed effects and proper emergency procedures among frontline workers. This knowledge deficit becomes particularly problematic during non-routine operations or maintenance activities where exposure risks increase.
Regulatory frameworks addressing HF safety in metal surface treatment vary substantially across jurisdictions, creating compliance challenges for global manufacturing operations. The absence of harmonized international standards results in inconsistent safety practices and reporting mechanisms, hampering industry-wide improvements in accident prevention and response.
Current engineering controls show critical limitations, particularly in containing HF vapors which can spread beyond designated work areas even with standard ventilation systems. The efficacy of traditional personal protective equipment (PPE) remains questionable, as studies indicate that certain glove materials degrade faster than previously understood when exposed to HF concentrations commonly used in metal etching processes.
Waste management presents another significant challenge, with neutralization processes often generating hazardous byproducts that require specialized disposal methods. Environmental regulations are becoming increasingly stringent, yet standardized protocols for HF-contaminated waste handling vary considerably across regions, creating compliance difficulties for multinational operations.
Detection technology limitations compound these issues, as current real-time monitoring systems lack the sensitivity to detect HF at the lowest dangerous concentrations, particularly in humid environments common in metal treatment facilities. This creates dangerous blind spots in safety monitoring systems, potentially allowing exposure incidents to escalate before detection.
Medical response protocols for HF exposure remain inadequate in many industrial settings. The specialized treatment required—including calcium gluconate application—is not universally available in first aid stations, and medical personnel at local facilities often lack specific training in treating HF injuries, leading to critical delays in appropriate care delivery.
The knowledge gap among workers represents another persistent challenge. Despite mandatory training programs, research indicates significant misunderstandings about HF's delayed effects and proper emergency procedures among frontline workers. This knowledge deficit becomes particularly problematic during non-routine operations or maintenance activities where exposure risks increase.
Regulatory frameworks addressing HF safety in metal surface treatment vary substantially across jurisdictions, creating compliance challenges for global manufacturing operations. The absence of harmonized international standards results in inconsistent safety practices and reporting mechanisms, hampering industry-wide improvements in accident prevention and response.
Current Safety Protocols and Technologies
01 Personal protective equipment and handling procedures
Hydrofluoric acid requires specialized personal protective equipment (PPE) including acid-resistant gloves, face shields, and chemical-resistant clothing. Proper handling procedures involve working in well-ventilated areas, using appropriate containment vessels, and implementing strict protocols for transfer operations. These safety measures are critical due to HF's ability to penetrate skin and cause severe tissue damage without immediate pain sensation.- Personal protective equipment and handling procedures: Proper personal protective equipment (PPE) is essential when handling hydrofluoric acid, including chemical-resistant gloves, face shields, and acid-resistant clothing. Specific handling procedures must be followed, such as working in well-ventilated areas, using appropriate containment vessels, and implementing strict protocols for transferring and storing the acid to minimize exposure risks and prevent accidents.
- Emergency response and first aid measures: Immediate and appropriate emergency response is critical for hydrofluoric acid exposure. This includes specific first aid protocols such as calcium gluconate application for skin exposure, specialized eye washing procedures for eye contact, and emergency medical treatment guidelines. Rapid response systems and training for personnel working with hydrofluoric acid are essential to mitigate the severe health effects of exposure.
- Neutralization and disposal methods: Safe neutralization and disposal of hydrofluoric acid requires specialized techniques and materials. Methods include using specific neutralizing agents like calcium compounds or specialized absorbents, followed by proper containment and disposal according to hazardous waste regulations. These processes must be carefully controlled to prevent secondary reactions and ensure complete neutralization before disposal.
- Facility design and engineering controls: Facilities handling hydrofluoric acid require specialized design features and engineering controls to ensure safety. These include dedicated ventilation systems with acid-resistant components, specialized storage areas with secondary containment, emergency shower and eyewash stations, and monitoring systems to detect leaks or releases. Proper facility design minimizes the risk of exposure and facilitates rapid response to incidents.
- Detection and monitoring systems: Advanced detection and monitoring systems are crucial for hydrofluoric acid safety. These include specialized sensors and alarms that can detect acid vapors at low concentrations, continuous monitoring equipment for work areas, and personal monitoring devices for workers. Early detection systems allow for prompt evacuation and response measures before dangerous exposure levels are reached.
02 Neutralization and emergency response systems
Effective emergency response for hydrofluoric acid exposure includes immediate access to calcium gluconate gel for skin contact and specialized neutralization agents. Safety systems should include emergency showers, eyewash stations, and spill containment equipment. Rapid response protocols must be established with clear procedures for different exposure scenarios, as HF burns can continue to cause damage hours after initial contact if not properly treated.Expand Specific Solutions03 Storage and containment solutions
Hydrofluoric acid must be stored in specialized containers made of compatible materials such as polyethylene or Teflon, as it can corrode glass and many metals. Storage areas require secondary containment systems, proper ventilation, temperature control, and should be physically separated from incompatible chemicals. Specialized labeling and regular inspection of storage facilities are essential to prevent leaks and accidental exposure.Expand Specific Solutions04 Detection and monitoring systems
Advanced detection systems for hydrofluoric acid vapors are crucial for workplace safety. These include electronic sensors, colorimetric detection tubes, and automated alarm systems that can detect HF at concentrations below dangerous levels. Continuous monitoring in areas where HF is used or stored provides early warning of leaks or releases, allowing for prompt evacuation and response before harmful exposure occurs.Expand Specific Solutions05 Waste treatment and disposal methods
Safe disposal of hydrofluoric acid waste requires specialized neutralization processes before discharge. Treatment methods include controlled neutralization with calcium or magnesium compounds to form insoluble fluoride salts. Waste handling systems must incorporate secondary containment, specialized piping materials, and proper ventilation. Environmental regulations typically require verification of complete neutralization and proper documentation of disposal procedures.Expand Specific Solutions
Critical Patents in HF Handling Safety
Acid inhibitor composition and process in hydrofluoric acid chemical cleaning
PatentInactiveUS4104303A
Innovation
- A Mannich base and thiourea inhibitor composition is used in hydrofluoric acid cleaning solutions to significantly reduce acid attack on ferrous metal surfaces, with the Mannich base being a condensation product of a primary or secondary amine, an alpha ketone, and formaldehyde, and thiourea enhancing the inhibiting ability, along with nonionic surfactants for improved solubility and cleaning action.
Hydrofluoric acid generating composition and method of treating surfaces
PatentInactiveUS6821351B2
Innovation
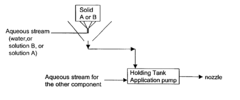
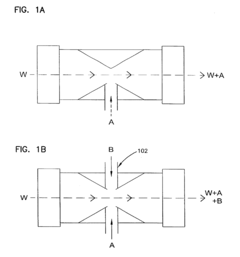
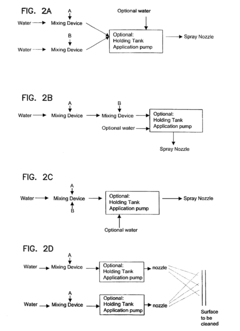
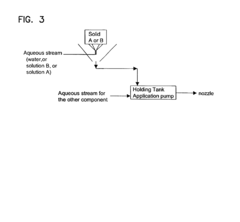
- A two-part composition system where a fluoride source and an acid are mixed on-site or immediately before application to generate hydrofluoric acid, reducing exposure risks and enhancing surface brightness through controlled mixing techniques, such as using an eductor or spray nozzle, allowing for efficient and safe application on metal surfaces.
Regulatory Compliance Framework
The regulatory landscape governing hydrofluoric acid (HF) usage in metal surface treatment operations is complex and multifaceted, spanning international, national, and local jurisdictions. At the international level, the United Nations' Globally Harmonized System of Classification and Labeling of Chemicals (GHS) provides standardized hazard communication protocols that manufacturers and users must follow when handling HF. These standards have been widely adopted across industrial nations, creating a baseline for safety compliance.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific permissible exposure limits (PELs) for HF at 3 parts per million (ppm) as an 8-hour time-weighted average. Additionally, OSHA's Hazard Communication Standard (29 CFR 1910.1200) mandates comprehensive hazard information disclosure, employee training, and proper labeling of HF-containing products. The Environmental Protection Agency (EPA) regulates HF under the Toxic Substances Control Act (TSCA) and requires reporting of releases exceeding reportable quantities under CERCLA.
The European Union's regulatory framework is particularly stringent, with HF falling under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation and the Classification, Labeling and Packaging (CLP) regulation. These frameworks impose strict documentation requirements, safety assessments, and usage limitations. The EU has also established workplace exposure limits through Directive 2017/164/EU, setting both short-term and long-term exposure values.
Industry-specific standards complement these governmental regulations. The National Association of Corrosion Engineers (NACE) and the American Society for Testing and Materials (ASTM) have developed technical standards for HF applications in metal treatment processes that emphasize both efficacy and safety. These standards often become de facto requirements for companies seeking to demonstrate due diligence and best practices.
Compliance verification mechanisms include mandatory workplace monitoring programs, regular third-party audits, and documentation of employee training. Many jurisdictions require facilities to maintain emergency response plans specifically addressing HF exposure scenarios and to conduct regular drills testing these protocols. Increasingly, regulatory frameworks are incorporating requirements for technological controls such as automated handling systems, closed-loop processing equipment, and real-time monitoring technologies to minimize human exposure.
The regulatory landscape continues to evolve, with a clear trend toward more stringent controls, lower exposure limits, and greater emphasis on substitution with less hazardous alternatives where technically feasible. Companies operating globally must navigate this complex regulatory environment while maintaining operational efficiency and competitive advantage.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific permissible exposure limits (PELs) for HF at 3 parts per million (ppm) as an 8-hour time-weighted average. Additionally, OSHA's Hazard Communication Standard (29 CFR 1910.1200) mandates comprehensive hazard information disclosure, employee training, and proper labeling of HF-containing products. The Environmental Protection Agency (EPA) regulates HF under the Toxic Substances Control Act (TSCA) and requires reporting of releases exceeding reportable quantities under CERCLA.
The European Union's regulatory framework is particularly stringent, with HF falling under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation and the Classification, Labeling and Packaging (CLP) regulation. These frameworks impose strict documentation requirements, safety assessments, and usage limitations. The EU has also established workplace exposure limits through Directive 2017/164/EU, setting both short-term and long-term exposure values.
Industry-specific standards complement these governmental regulations. The National Association of Corrosion Engineers (NACE) and the American Society for Testing and Materials (ASTM) have developed technical standards for HF applications in metal treatment processes that emphasize both efficacy and safety. These standards often become de facto requirements for companies seeking to demonstrate due diligence and best practices.
Compliance verification mechanisms include mandatory workplace monitoring programs, regular third-party audits, and documentation of employee training. Many jurisdictions require facilities to maintain emergency response plans specifically addressing HF exposure scenarios and to conduct regular drills testing these protocols. Increasingly, regulatory frameworks are incorporating requirements for technological controls such as automated handling systems, closed-loop processing equipment, and real-time monitoring technologies to minimize human exposure.
The regulatory landscape continues to evolve, with a clear trend toward more stringent controls, lower exposure limits, and greater emphasis on substitution with less hazardous alternatives where technically feasible. Companies operating globally must navigate this complex regulatory environment while maintaining operational efficiency and competitive advantage.
Environmental Impact Assessment
The environmental impact of hydrofluoric acid (HF) in metal surface treatment processes represents a significant concern for both industry stakeholders and regulatory bodies. HF releases can occur at various stages of metal treatment operations, including storage, application, rinsing, and waste disposal. When improperly managed, these releases contaminate soil, groundwater, and surface water systems, creating persistent environmental hazards due to fluoride's high mobility and stability in aqueous environments.
Air emissions containing HF vapors pose substantial risks to surrounding ecosystems, causing vegetation damage through direct contact and contributing to acid rain formation. Studies have documented reduced biodiversity in areas adjacent to facilities with inadequate HF emission controls, with particularly severe impacts on sensitive aquatic organisms and plant species.
Water pollution from HF-containing effluent disrupts aquatic ecosystems by altering pH levels and introducing toxic fluoride compounds. Bioaccumulation of fluoride in aquatic organisms has been observed, potentially affecting entire food chains. Treatment facilities generating HF-contaminated wastewater must implement specialized neutralization and precipitation processes to remove fluoride ions before discharge, significantly increasing operational costs and resource requirements.
The energy-intensive nature of HF production and transportation contributes substantially to the carbon footprint of metal treatment operations. Life cycle assessments indicate that HF-based processes generate approximately 2.5 times more greenhouse gas emissions compared to alternative surface treatment technologies, when accounting for production, transportation, use, and disposal phases.
Regulatory frameworks governing HF use have evolved significantly, with the EU's REACH regulation and the US EPA's Toxic Substances Control Act imposing increasingly stringent requirements for handling and disposal. Many regions now mandate comprehensive environmental impact assessments before permitting new HF-utilizing facilities, focusing on potential groundwater contamination pathways and atmospheric dispersion modeling.
Industry response has accelerated the development of closed-loop systems that capture and recycle HF, reducing environmental releases by up to 95% compared to traditional open systems. Advanced monitoring technologies, including real-time fluoride sensors and atmospheric HF detectors, enable more effective environmental management and rapid response to potential releases, though implementation costs remain prohibitive for smaller operations.
The transition toward less environmentally harmful alternatives represents the most promising approach to mitigating HF's environmental impact. Emerging technologies utilizing supercritical CO2, plasma treatments, and biodegradable chelating agents demonstrate comparable surface treatment effectiveness with dramatically reduced environmental footprints, though commercial-scale implementation remains limited by technical and economic barriers.
Air emissions containing HF vapors pose substantial risks to surrounding ecosystems, causing vegetation damage through direct contact and contributing to acid rain formation. Studies have documented reduced biodiversity in areas adjacent to facilities with inadequate HF emission controls, with particularly severe impacts on sensitive aquatic organisms and plant species.
Water pollution from HF-containing effluent disrupts aquatic ecosystems by altering pH levels and introducing toxic fluoride compounds. Bioaccumulation of fluoride in aquatic organisms has been observed, potentially affecting entire food chains. Treatment facilities generating HF-contaminated wastewater must implement specialized neutralization and precipitation processes to remove fluoride ions before discharge, significantly increasing operational costs and resource requirements.
The energy-intensive nature of HF production and transportation contributes substantially to the carbon footprint of metal treatment operations. Life cycle assessments indicate that HF-based processes generate approximately 2.5 times more greenhouse gas emissions compared to alternative surface treatment technologies, when accounting for production, transportation, use, and disposal phases.
Regulatory frameworks governing HF use have evolved significantly, with the EU's REACH regulation and the US EPA's Toxic Substances Control Act imposing increasingly stringent requirements for handling and disposal. Many regions now mandate comprehensive environmental impact assessments before permitting new HF-utilizing facilities, focusing on potential groundwater contamination pathways and atmospheric dispersion modeling.
Industry response has accelerated the development of closed-loop systems that capture and recycle HF, reducing environmental releases by up to 95% compared to traditional open systems. Advanced monitoring technologies, including real-time fluoride sensors and atmospheric HF detectors, enable more effective environmental management and rapid response to potential releases, though implementation costs remain prohibitive for smaller operations.
The transition toward less environmentally harmful alternatives represents the most promising approach to mitigating HF's environmental impact. Emerging technologies utilizing supercritical CO2, plasma treatments, and biodegradable chelating agents demonstrate comparable surface treatment effectiveness with dramatically reduced environmental footprints, though commercial-scale implementation remains limited by technical and economic barriers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!