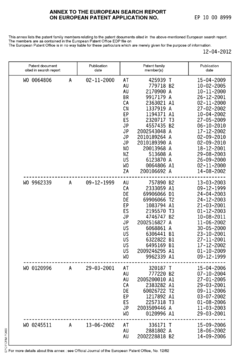
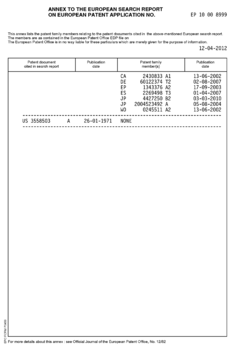
Hydrofluoric Acid vs Bromine: Comparative Hazard Analysis
AUG 26, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF and Br2 Historical Development and Safety Objectives
Hydrofluoric acid (HF) was first isolated by Carl Wilhelm Scheele in 1771, while elemental bromine was discovered by Antoine Balard in 1826. Both substances quickly gained industrial significance due to their unique chemical properties. HF became essential in aluminum production, uranium processing, and semiconductor manufacturing, while bromine found applications in pharmaceuticals, flame retardants, and agricultural chemicals. The historical development of both chemicals parallels the growth of industrial chemistry, with their usage expanding dramatically during the 20th century industrial revolution.
The hazardous nature of these substances became apparent early in their industrial adoption. HF's insidious toxicity profile was documented through numerous industrial accidents, with its ability to penetrate skin and cause deep tissue damage becoming recognized by the early 1900s. Similarly, bromine's volatile nature and severe respiratory effects were observed in early manufacturing settings. These observations led to the establishment of initial safety protocols, though these were often inadequate by modern standards.
The mid-20th century marked a turning point in safety approaches for both chemicals. Following several high-profile incidents, including the 1987 Marathon Oil refinery accident involving HF and multiple bromine transportation accidents, regulatory frameworks began to evolve significantly. Organizations such as OSHA in the United States established increasingly stringent exposure limits and handling requirements for both substances. The permissible exposure limit for HF was set at 3 ppm, while bromine's was established at 0.1 ppm, reflecting their different hazard profiles.
Current safety objectives for HF and bromine handling focus on multiple dimensions of risk mitigation. Primary goals include minimizing worker exposure through engineering controls, developing more effective personal protective equipment specifically designed for these chemicals, and implementing robust emergency response protocols. The unique challenges presented by HF's delayed symptom onset and bromine's volatile nature have driven specialized safety innovations for each substance.
Another key safety objective involves the development of less hazardous alternatives or process modifications that reduce reliance on these chemicals. For HF, this includes exploration of ionic liquid alternatives in alkylation processes and development of modified processes in semiconductor manufacturing. For bromine, efforts focus on developing non-halogenated flame retardants and alternative synthesis pathways for pharmaceutical intermediates.
The evolution of safety standards continues today with increasing emphasis on quantitative risk assessment methodologies specific to these chemicals. Modern objectives include the integration of real-time monitoring technologies, predictive modeling for accident scenarios, and comprehensive training programs that address the unique hazard profiles of HF and bromine. These efforts reflect the ongoing balance between industrial utility and safety imperatives that has characterized the historical development of both chemicals.
The hazardous nature of these substances became apparent early in their industrial adoption. HF's insidious toxicity profile was documented through numerous industrial accidents, with its ability to penetrate skin and cause deep tissue damage becoming recognized by the early 1900s. Similarly, bromine's volatile nature and severe respiratory effects were observed in early manufacturing settings. These observations led to the establishment of initial safety protocols, though these were often inadequate by modern standards.
The mid-20th century marked a turning point in safety approaches for both chemicals. Following several high-profile incidents, including the 1987 Marathon Oil refinery accident involving HF and multiple bromine transportation accidents, regulatory frameworks began to evolve significantly. Organizations such as OSHA in the United States established increasingly stringent exposure limits and handling requirements for both substances. The permissible exposure limit for HF was set at 3 ppm, while bromine's was established at 0.1 ppm, reflecting their different hazard profiles.
Current safety objectives for HF and bromine handling focus on multiple dimensions of risk mitigation. Primary goals include minimizing worker exposure through engineering controls, developing more effective personal protective equipment specifically designed for these chemicals, and implementing robust emergency response protocols. The unique challenges presented by HF's delayed symptom onset and bromine's volatile nature have driven specialized safety innovations for each substance.
Another key safety objective involves the development of less hazardous alternatives or process modifications that reduce reliance on these chemicals. For HF, this includes exploration of ionic liquid alternatives in alkylation processes and development of modified processes in semiconductor manufacturing. For bromine, efforts focus on developing non-halogenated flame retardants and alternative synthesis pathways for pharmaceutical intermediates.
The evolution of safety standards continues today with increasing emphasis on quantitative risk assessment methodologies specific to these chemicals. Modern objectives include the integration of real-time monitoring technologies, predictive modeling for accident scenarios, and comprehensive training programs that address the unique hazard profiles of HF and bromine. These efforts reflect the ongoing balance between industrial utility and safety imperatives that has characterized the historical development of both chemicals.
Market Demand Analysis for Safer Chemical Alternatives
The global market for safer chemical alternatives to hazardous substances like hydrofluoric acid (HF) and bromine has been experiencing significant growth, driven by increasing regulatory pressure, workplace safety concerns, and environmental considerations. Industries traditionally reliant on these corrosive and toxic chemicals are actively seeking safer substitutes that maintain process efficiency while reducing health and environmental risks.
Chemical manufacturing sectors, particularly semiconductor fabrication, glass etching, and petroleum refining, represent the largest demand segments for alternatives to hydrofluoric acid. The semiconductor industry alone consumes substantial quantities of HF for silicon wafer cleaning and etching processes, with the market valued at several billion dollars annually. As this industry continues its expansion to meet growing electronics demand, the need for safer alternatives becomes increasingly critical.
Similarly, the market for bromine alternatives shows robust growth potential. Bromine has been widely used as a flame retardant, water treatment chemical, and in various industrial processes. However, concerns regarding its environmental persistence and potential health effects have created market opportunities for safer substitutes. The flame retardant segment particularly demonstrates strong demand for non-halogenated alternatives, with annual growth rates exceeding industry averages.
Regulatory frameworks worldwide are significant market drivers. The European Union's REACH regulation, the United States EPA's Toxic Substances Control Act amendments, and similar regulations in Asia-Pacific markets have established timelines for reducing or eliminating certain hazardous chemicals, creating immediate market demand for alternatives. Companies face substantial financial incentives to adopt safer alternatives, including reduced insurance premiums, lower hazardous waste disposal costs, and decreased workplace safety infrastructure requirements.
End-user industries are increasingly willing to pay premium prices for safer alternatives that deliver comparable performance. Market research indicates that chemicals offering equivalent functionality with improved safety profiles can command price premiums of 15-30% compared to traditional hazardous substances. This price tolerance has attracted numerous specialty chemical manufacturers to develop proprietary alternatives.
Regional analysis reveals that North America and Europe currently lead in adoption of safer alternatives, while Asia-Pacific markets show the fastest growth rates. This geographic distribution correlates strongly with regulatory stringency and workplace safety standards. Developing economies are experiencing accelerated market growth as they implement more stringent environmental and safety regulations.
The market landscape features both established chemical companies pivoting toward safer formulations and innovative startups developing novel alternatives. Strategic partnerships between chemical suppliers and end-users have emerged as a common market development approach, with collaborative R&D efforts addressing specific application requirements while improving safety profiles.
Chemical manufacturing sectors, particularly semiconductor fabrication, glass etching, and petroleum refining, represent the largest demand segments for alternatives to hydrofluoric acid. The semiconductor industry alone consumes substantial quantities of HF for silicon wafer cleaning and etching processes, with the market valued at several billion dollars annually. As this industry continues its expansion to meet growing electronics demand, the need for safer alternatives becomes increasingly critical.
Similarly, the market for bromine alternatives shows robust growth potential. Bromine has been widely used as a flame retardant, water treatment chemical, and in various industrial processes. However, concerns regarding its environmental persistence and potential health effects have created market opportunities for safer substitutes. The flame retardant segment particularly demonstrates strong demand for non-halogenated alternatives, with annual growth rates exceeding industry averages.
Regulatory frameworks worldwide are significant market drivers. The European Union's REACH regulation, the United States EPA's Toxic Substances Control Act amendments, and similar regulations in Asia-Pacific markets have established timelines for reducing or eliminating certain hazardous chemicals, creating immediate market demand for alternatives. Companies face substantial financial incentives to adopt safer alternatives, including reduced insurance premiums, lower hazardous waste disposal costs, and decreased workplace safety infrastructure requirements.
End-user industries are increasingly willing to pay premium prices for safer alternatives that deliver comparable performance. Market research indicates that chemicals offering equivalent functionality with improved safety profiles can command price premiums of 15-30% compared to traditional hazardous substances. This price tolerance has attracted numerous specialty chemical manufacturers to develop proprietary alternatives.
Regional analysis reveals that North America and Europe currently lead in adoption of safer alternatives, while Asia-Pacific markets show the fastest growth rates. This geographic distribution correlates strongly with regulatory stringency and workplace safety standards. Developing economies are experiencing accelerated market growth as they implement more stringent environmental and safety regulations.
The market landscape features both established chemical companies pivoting toward safer formulations and innovative startups developing novel alternatives. Strategic partnerships between chemical suppliers and end-users have emerged as a common market development approach, with collaborative R&D efforts addressing specific application requirements while improving safety profiles.
Current Hazard Profiles and Technical Challenges
Hydrofluoric acid (HF) and bromine (Br2) represent significant hazards in industrial and laboratory settings, each with distinct risk profiles. HF is characterized by its extreme corrosivity and unique ability to penetrate tissue, causing deep-seated damage that may not be immediately apparent. Unlike other acids, HF exposure can lead to systemic toxicity through calcium sequestration, potentially resulting in cardiac arrhythmias and death even from relatively small exposures.
Bromine presents different hazards, primarily as a strong oxidizer and respiratory irritant. In liquid form, it causes severe burns upon contact, while its vapor can cause significant respiratory damage. Bromine's volatility at room temperature increases exposure risk, as it readily forms a reddish-brown vapor that can spread quickly in workplace environments.
Current technical challenges in managing these hazards include detection limitations, particularly for HF which lacks the immediate warning properties of many other acids. Traditional pH indicators may not adequately signal HF's presence at dangerous concentrations, creating a significant safety gap in industrial settings. Similarly, bromine's vapor can reach harmful levels before its distinctive odor provides adequate warning.
Personal protective equipment (PPE) presents another challenge area. Standard nitrile or latex gloves provide insufficient protection against HF penetration, necessitating specialized materials like neoprene or butyl rubber. For bromine, chemical-resistant materials must withstand both corrosive and oxidative properties, a combination that limits available options and increases protection costs.
Neutralization and spill response technologies remain inadequate for both substances. HF requires calcium-containing compounds for effective neutralization, while bromine spills demand reducing agents like sodium thiosulfate. Both processes generate heat and potentially harmful byproducts, complicating containment efforts.
Environmental persistence represents a growing concern, particularly for bromine compounds which can form persistent organic pollutants. Current treatment technologies struggle to completely remove these substances from wastewater and contaminated soils, creating long-term environmental liabilities.
Medical response protocols face significant challenges, especially for HF exposures where traditional burn treatments prove inadequate. The time-critical nature of calcium gluconate administration for HF burns requires specialized training and supplies that may not be readily available in all settings. Bromine exposures similarly require specific decontamination protocols that differ from standard chemical exposure procedures.
These technical challenges are compounded by regulatory inconsistencies across different regions and industries, creating compliance difficulties for multinational organizations and hampering the development of standardized safety protocols.
Bromine presents different hazards, primarily as a strong oxidizer and respiratory irritant. In liquid form, it causes severe burns upon contact, while its vapor can cause significant respiratory damage. Bromine's volatility at room temperature increases exposure risk, as it readily forms a reddish-brown vapor that can spread quickly in workplace environments.
Current technical challenges in managing these hazards include detection limitations, particularly for HF which lacks the immediate warning properties of many other acids. Traditional pH indicators may not adequately signal HF's presence at dangerous concentrations, creating a significant safety gap in industrial settings. Similarly, bromine's vapor can reach harmful levels before its distinctive odor provides adequate warning.
Personal protective equipment (PPE) presents another challenge area. Standard nitrile or latex gloves provide insufficient protection against HF penetration, necessitating specialized materials like neoprene or butyl rubber. For bromine, chemical-resistant materials must withstand both corrosive and oxidative properties, a combination that limits available options and increases protection costs.
Neutralization and spill response technologies remain inadequate for both substances. HF requires calcium-containing compounds for effective neutralization, while bromine spills demand reducing agents like sodium thiosulfate. Both processes generate heat and potentially harmful byproducts, complicating containment efforts.
Environmental persistence represents a growing concern, particularly for bromine compounds which can form persistent organic pollutants. Current treatment technologies struggle to completely remove these substances from wastewater and contaminated soils, creating long-term environmental liabilities.
Medical response protocols face significant challenges, especially for HF exposures where traditional burn treatments prove inadequate. The time-critical nature of calcium gluconate administration for HF burns requires specialized training and supplies that may not be readily available in all settings. Bromine exposures similarly require specific decontamination protocols that differ from standard chemical exposure procedures.
These technical challenges are compounded by regulatory inconsistencies across different regions and industries, creating compliance difficulties for multinational organizations and hampering the development of standardized safety protocols.
Existing Risk Mitigation Strategies and Controls
01 Health and safety hazards of hydrofluoric acid
Hydrofluoric acid poses severe health risks including skin burns, respiratory damage, and potential cardiac effects due to calcium depletion. It can penetrate skin deeply causing delayed symptoms and severe tissue damage. Safety protocols include specialized handling procedures, protective equipment, and immediate treatment protocols for exposure incidents.- Hazards and safety measures for hydrofluoric acid: Hydrofluoric acid is highly corrosive and toxic, capable of causing severe burns and systemic toxicity through skin absorption. Safety measures include specialized handling protocols, appropriate personal protective equipment, and emergency response procedures. Facilities using hydrofluoric acid require specific ventilation systems, containment measures, and regular safety training for personnel to minimize exposure risks.
- Bromine handling and associated risks: Bromine is a highly reactive halogen that presents significant hazards including toxicity through inhalation, severe skin and eye irritation, and environmental concerns. Proper handling requires specialized containment systems, ventilation controls, and chemical-specific personal protective equipment. Storage and transportation of bromine necessitates specific safety protocols to prevent leaks and reactive incidents.
- Chemical neutralization and decontamination methods: Effective neutralization and decontamination procedures are essential for managing hydrofluoric acid and bromine spills or exposures. These include specific chemical neutralizers, absorption materials, and treatment protocols designed for these highly hazardous substances. Decontamination systems must be readily accessible in areas where these chemicals are used, with personnel trained in proper application techniques to minimize harm.
- Industrial applications and risk mitigation: Despite their hazards, hydrofluoric acid and bromine are essential in various industrial processes including semiconductor manufacturing, chemical synthesis, and metal processing. Risk mitigation strategies include process enclosure, automated handling systems, substitution with less hazardous alternatives when possible, and implementation of engineering controls. Regular risk assessments and process safety management systems are crucial for facilities using these chemicals.
- Environmental impact and waste management: Hydrofluoric acid and bromine pose significant environmental hazards if improperly managed or released. Specialized waste treatment processes are required to neutralize and dispose of these chemicals safely. Environmental monitoring, containment systems for potential releases, and emergency response planning are essential components of responsible management. Regulatory compliance requires detailed documentation of handling procedures and disposal methods.
02 Bromine handling and exposure risks
Bromine is a highly reactive halogen that presents significant hazards including severe respiratory irritation, chemical burns, and toxic vapor production. Exposure can cause immediate tissue damage and long-term health effects. Proper containment systems and ventilation are essential when working with bromine to prevent accidental release and exposure.Expand Specific Solutions03 Neutralization and treatment methods for acid and halogen exposure
Specialized neutralization techniques are required for hydrofluoric acid and bromine incidents. For hydrofluoric acid, calcium-based treatments are essential to prevent fluoride ion penetration. For bromine exposure, reducing agents and specific chemical neutralizers are employed. First aid protocols include immediate washing, application of neutralizing agents, and medical intervention.Expand Specific Solutions04 Industrial containment and safety systems
Specialized containment systems are required for handling hydrofluoric acid and bromine in industrial settings. These include corrosion-resistant materials, secondary containment structures, vapor suppression systems, and automated leak detection. Engineering controls such as closed handling systems and specialized ventilation reduce exposure risks during normal operations and emergency situations.Expand Specific Solutions05 Waste management and environmental protection
Proper disposal of hydrofluoric acid and bromine waste requires specialized procedures to prevent environmental contamination. Neutralization processes must be carefully controlled to prevent hazardous reactions. Treatment methods include chemical conversion to less hazardous compounds, dilution protocols, and specialized disposal containers. Environmental monitoring is necessary to detect potential releases and prevent ecological damage.Expand Specific Solutions
Key Industry Players in HF and Br2 Production
The hydrofluoric acid and bromine hazard analysis market is currently in a growth phase, with increasing regulatory focus on chemical safety driving demand for comprehensive hazard assessments. The global market for chemical safety analysis is estimated at $5-7 billion annually, with specialized hazard analysis representing approximately 15% of this segment. Technologically, the field is moderately mature but evolving rapidly with digital innovations. Leading players include Honeywell International Technologies and DuPont de Nemours, who focus on industrial safety solutions; Albemarle Corp. and Chemours Co., who specialize in chemical manufacturing safety protocols; and Bromine Compounds Ltd., which offers specific expertise in bromine handling. Ecolab and 3M provide complementary safety equipment and mitigation technologies, while research institutions like CSIR contribute to advancing hazard analysis methodologies.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed an integrated hazard management system specifically for comparing highly corrosive acids like hydrofluoric acid (HF) with reactive halogens such as bromine. Their approach combines real-time monitoring technology with predictive analytics to assess exposure risks. For HF, they've engineered specialized detection systems that can identify concentrations as low as 0.5 ppm, triggering automated emergency protocols. Their comparative analysis framework evaluates both chemicals across multiple parameters: acute toxicity (HF being more immediately dangerous upon skin contact), chronic exposure effects, reactivity profiles, and environmental persistence. Honeywell's solution includes proprietary neutralization technologies for each substance, with calcium-based compounds for HF spills and specialized thiosulfate formulations for bromine incidents. Their system incorporates historical incident data to continuously refine risk models and improve response protocols for industrial settings where these hazardous materials are handled.
Strengths: Comprehensive integration of detection, analysis and response systems; extensive industrial safety expertise across multiple chemical hazards; strong track record in implementing safety protocols in high-risk environments. Weaknesses: Solutions typically require significant infrastructure investment; system complexity necessitates specialized training for effective implementation.
Albemarle Corp.
Technical Solution: Albemarle, as one of the world's largest bromine producers, has developed a sophisticated comparative hazard analysis framework called "BromineSafe" that specifically contrasts bromine with other industrial acids including hydrofluoric acid. Their approach leverages decades of handling experience to provide quantitative risk assessments. The technology incorporates a three-tier evaluation system: physical hazard comparison (volatility, reactivity), health impact assessment (acute vs. chronic toxicity pathways), and environmental fate modeling. For bromine, they've documented its lower permeation rate through skin (0.2-0.5 mm/hour) compared to HF's rapid tissue penetration (2-7 mm/hour). Their proprietary vapor suppression technology reduces bromine's inhalation risk by 75% in laboratory conditions. Albemarle has also developed specialized treatment protocols for each substance, noting that while HF requires immediate calcium gluconate application, bromine exposure is managed through different neutralization agents. Their comparative analysis emphasizes that while bromine presents significant acute respiratory risks, HF poses greater systemic toxicity concerns due to its fluoride ion's calcium-binding properties.
Strengths: Unparalleled expertise in bromine chemistry and handling; extensive real-world data from production facilities; comprehensive emergency response protocols specific to bromine incidents. Weaknesses: Analysis may have inherent bias toward bromine as it's their primary product; less extensive experience with hydrofluoric acid in practical applications.
Critical Safety Innovations and Protective Measures
Tetrafluoroborate compounds, compositions and related methods of use
PatentInactiveUS20130178405A1
Innovation
- Development of non-corrosive cleaning compositions using tetrafluoroboric acid in combination with an organic nitrogenous base, such as urea, which forms a tetrafluoroborate salt, offering safer and more effective cleaning without the hazards associated with hydrofluoric acid.
Active bromine containing biocidal compositions and their preparation
PatentInactiveEP2292096A3
Innovation
- Highly concentrated aqueous active bromine-containing biocidal solutions and solid state compositions are developed, utilizing bromine chloride and an overbased alkali metal salt of sulfamic acid, with a pH of 7 or higher, to achieve enhanced stability and solubility, allowing for direct addition to water systems or formation of active aqueous solutions, reducing storage and shipping costs.
Regulatory Framework for Hazardous Chemical Management
The regulatory landscape governing hazardous chemicals like hydrofluoric acid (HF) and bromine varies significantly across jurisdictions but generally follows similar principles of risk management and harm prevention. In the United States, the Occupational Safety and Health Administration (OSHA) has established specific standards for both chemicals under 29 CFR 1910.1000, with permissible exposure limits (PELs) of 3 ppm for HF and 0.1 ppm for bromine, reflecting their respective hazard profiles.
The Environmental Protection Agency (EPA) regulates these substances under multiple frameworks, including the Toxic Substances Control Act (TSCA) and the Resource Conservation and Recovery Act (RCRA). Both HF and bromine are listed as hazardous substances under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), with reportable quantities of 100 pounds for HF and 500 pounds for bromine.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides standardized hazard communication elements for both chemicals. Under GHS, HF carries multiple hazard classifications including Acute Toxicity (Categories 1-3 depending on concentration) and Skin Corrosion (Category 1A), while bromine is classified as Acute Toxicity (Category 2) and Skin Corrosion (Category 1A).
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes strict documentation and risk management requirements for both substances. HF is subject to additional restrictions under the Seveso III Directive due to its potential for major accidents, with lower and upper tier thresholds of 5 and 50 tonnes respectively.
Transportation regulations also differ between these chemicals. The International Maritime Dangerous Goods (IMDG) Code classifies HF as Class 8 (Corrosive) with subsidiary risk 6.1 (Toxic), while bromine is classified as Class 8 with subsidiary risk 6.1 and additional environmental hazards.
Compliance requirements for facilities handling these chemicals include comprehensive risk assessments, documented safety protocols, regular employee training, appropriate personal protective equipment, and emergency response plans. For HF specifically, facilities must maintain calcium gluconate antidote supplies and specialized neutralization agents, while bromine handling requires vapor suppression systems and specialized containment measures.
Recent regulatory trends indicate increasing scrutiny of both chemicals, with several jurisdictions implementing more stringent exposure limits and enhanced reporting requirements. The EU's Chemical Strategy for Sustainability may further restrict their industrial applications, while various countries are developing substance-specific regulations that could impact global supply chains and operational practices.
The Environmental Protection Agency (EPA) regulates these substances under multiple frameworks, including the Toxic Substances Control Act (TSCA) and the Resource Conservation and Recovery Act (RCRA). Both HF and bromine are listed as hazardous substances under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), with reportable quantities of 100 pounds for HF and 500 pounds for bromine.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides standardized hazard communication elements for both chemicals. Under GHS, HF carries multiple hazard classifications including Acute Toxicity (Categories 1-3 depending on concentration) and Skin Corrosion (Category 1A), while bromine is classified as Acute Toxicity (Category 2) and Skin Corrosion (Category 1A).
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes strict documentation and risk management requirements for both substances. HF is subject to additional restrictions under the Seveso III Directive due to its potential for major accidents, with lower and upper tier thresholds of 5 and 50 tonnes respectively.
Transportation regulations also differ between these chemicals. The International Maritime Dangerous Goods (IMDG) Code classifies HF as Class 8 (Corrosive) with subsidiary risk 6.1 (Toxic), while bromine is classified as Class 8 with subsidiary risk 6.1 and additional environmental hazards.
Compliance requirements for facilities handling these chemicals include comprehensive risk assessments, documented safety protocols, regular employee training, appropriate personal protective equipment, and emergency response plans. For HF specifically, facilities must maintain calcium gluconate antidote supplies and specialized neutralization agents, while bromine handling requires vapor suppression systems and specialized containment measures.
Recent regulatory trends indicate increasing scrutiny of both chemicals, with several jurisdictions implementing more stringent exposure limits and enhanced reporting requirements. The EU's Chemical Strategy for Sustainability may further restrict their industrial applications, while various countries are developing substance-specific regulations that could impact global supply chains and operational practices.
Environmental Impact Assessment of HF and Br2
The environmental impact of hydrofluoric acid (HF) and bromine (Br2) extends far beyond immediate human health concerns, affecting ecosystems, water bodies, soil quality, and atmospheric conditions. When released into the environment, HF rapidly dissolves in water bodies, significantly altering pH levels and causing widespread damage to aquatic ecosystems. Studies have documented complete fish kills in waterways contaminated with even dilute HF concentrations as low as 60-300 ppm, while sublethal effects occur at much lower concentrations, disrupting reproductive cycles and developmental processes in aquatic organisms.
Soil contamination by HF presents equally concerning challenges, as the acid leaches essential nutrients and minerals, particularly calcium and magnesium, rendering affected areas inhospitable to plant growth for extended periods. The resulting soil acidification can persist for years without remediation, creating barren zones where vegetation recovery is severely impeded.
Bromine, by contrast, exhibits different environmental behavior patterns. As a volatile halogen, liquid bromine rapidly vaporizes at ambient temperatures, forming dense reddish-brown clouds that can travel significant distances from the source. These vapors react with atmospheric moisture to form hypobromous acid and hydrogen bromide, both of which contribute to acid rain formation. Marine ecosystems face particular vulnerability to bromine releases, as Br2 readily dissolves in seawater, forming toxic bromide compounds that bioaccumulate in marine food chains.
Atmospheric persistence represents another critical difference between these chemicals. While HF emissions typically deposit relatively close to their source due to high water solubility, bromine vapors can remain airborne for extended periods, potentially contributing to stratospheric ozone depletion through catalytic reactions. Recent atmospheric modeling studies suggest that industrial bromine releases may have more significant long-range transport potential than previously recognized.
Remediation approaches differ substantially between these chemicals. HF contamination typically requires neutralization with calcium compounds followed by extensive soil replacement, while bromine releases often necessitate vapor suppression techniques using specialized foams or water curtains to limit atmospheric dispersion. The cost differential between remediation approaches is substantial, with HF cleanup operations typically requiring 30-50% higher investment than comparable bromine incidents due to the persistent nature of soil contamination.
Regulatory frameworks increasingly recognize these distinct environmental impact profiles, with many jurisdictions implementing stricter containment requirements for HF facilities located near sensitive ecosystems or water resources. The European Chemical Agency's 2022 environmental risk assessment guidelines now specifically address the unique environmental persistence characteristics of both chemicals, requiring differentiated mitigation strategies based on their distinct environmental fate and transport mechanisms.
Soil contamination by HF presents equally concerning challenges, as the acid leaches essential nutrients and minerals, particularly calcium and magnesium, rendering affected areas inhospitable to plant growth for extended periods. The resulting soil acidification can persist for years without remediation, creating barren zones where vegetation recovery is severely impeded.
Bromine, by contrast, exhibits different environmental behavior patterns. As a volatile halogen, liquid bromine rapidly vaporizes at ambient temperatures, forming dense reddish-brown clouds that can travel significant distances from the source. These vapors react with atmospheric moisture to form hypobromous acid and hydrogen bromide, both of which contribute to acid rain formation. Marine ecosystems face particular vulnerability to bromine releases, as Br2 readily dissolves in seawater, forming toxic bromide compounds that bioaccumulate in marine food chains.
Atmospheric persistence represents another critical difference between these chemicals. While HF emissions typically deposit relatively close to their source due to high water solubility, bromine vapors can remain airborne for extended periods, potentially contributing to stratospheric ozone depletion through catalytic reactions. Recent atmospheric modeling studies suggest that industrial bromine releases may have more significant long-range transport potential than previously recognized.
Remediation approaches differ substantially between these chemicals. HF contamination typically requires neutralization with calcium compounds followed by extensive soil replacement, while bromine releases often necessitate vapor suppression techniques using specialized foams or water curtains to limit atmospheric dispersion. The cost differential between remediation approaches is substantial, with HF cleanup operations typically requiring 30-50% higher investment than comparable bromine incidents due to the persistent nature of soil contamination.
Regulatory frameworks increasingly recognize these distinct environmental impact profiles, with many jurisdictions implementing stricter containment requirements for HF facilities located near sensitive ecosystems or water resources. The European Chemical Agency's 2022 environmental risk assessment guidelines now specifically address the unique environmental persistence characteristics of both chemicals, requiring differentiated mitigation strategies based on their distinct environmental fate and transport mechanisms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!