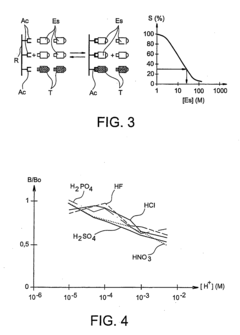
How to Utilize Hydrofluoric Acid Safely in Scientific Research
AUG 26, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrofluoric Acid Safety Background and Objectives
Hydrofluoric acid (HF) represents one of the most hazardous chemicals routinely used in scientific research and industrial applications. Unlike other strong acids, HF presents unique dangers due to its ability to penetrate skin and tissues rapidly, causing deep tissue damage and potentially fatal systemic toxicity through calcium sequestration. The historical development of HF safety protocols has evolved significantly since its first industrial production in the 1700s, with major advancements occurring following serious laboratory and industrial incidents throughout the 20th century.
The evolution of HF safety practices has been marked by increasingly stringent protocols, specialized equipment development, and enhanced training requirements. Early approaches focused primarily on physical barriers, while modern comprehensive safety systems integrate multiple layers of protection including specialized ventilation systems, specific neutralizing agents, and real-time monitoring technologies. This progression reflects growing understanding of HF's unique hazard profile and the inadequacy of standard acid safety measures when handling this particular substance.
Current global research trends indicate a dual approach to HF safety: improving protective measures for necessary applications while simultaneously developing alternative processes that eliminate or reduce HF usage where possible. This reflects the scientific community's recognition that while HF remains indispensable for certain applications, its inherent risks warrant continuous safety innovation and, where feasible, replacement.
The primary objective of this technical research is to establish a comprehensive framework for safe HF utilization in research environments that balances practical usability with maximum safety assurance. Specifically, we aim to identify best practices for HF handling across diverse research applications, evaluate emerging technologies for exposure prevention and monitoring, and develop decision matrices for determining when HF use is justified versus when alternatives should be employed.
Secondary objectives include assessing the effectiveness of current emergency response protocols for HF exposure incidents, identifying gaps in existing safety standards across different regulatory environments, and exploring the potential for developing standardized international safety protocols that could be implemented regardless of regional regulatory variations.
The technological trajectory suggests increasing integration of digital monitoring systems, advanced personal protective equipment materials, and automated handling systems to minimize direct human contact with HF. Additionally, there is growing interest in developing more effective topical treatments and systemic therapies for HF exposure that could significantly improve outcomes following accidental exposure incidents.
This research acknowledges the tension between HF's continued utility in numerous scientific fields and its exceptional hazard profile, seeking to define a path forward that neither unnecessarily restricts valuable research nor compromises on fundamental safety requirements for researchers and laboratory personnel.
The evolution of HF safety practices has been marked by increasingly stringent protocols, specialized equipment development, and enhanced training requirements. Early approaches focused primarily on physical barriers, while modern comprehensive safety systems integrate multiple layers of protection including specialized ventilation systems, specific neutralizing agents, and real-time monitoring technologies. This progression reflects growing understanding of HF's unique hazard profile and the inadequacy of standard acid safety measures when handling this particular substance.
Current global research trends indicate a dual approach to HF safety: improving protective measures for necessary applications while simultaneously developing alternative processes that eliminate or reduce HF usage where possible. This reflects the scientific community's recognition that while HF remains indispensable for certain applications, its inherent risks warrant continuous safety innovation and, where feasible, replacement.
The primary objective of this technical research is to establish a comprehensive framework for safe HF utilization in research environments that balances practical usability with maximum safety assurance. Specifically, we aim to identify best practices for HF handling across diverse research applications, evaluate emerging technologies for exposure prevention and monitoring, and develop decision matrices for determining when HF use is justified versus when alternatives should be employed.
Secondary objectives include assessing the effectiveness of current emergency response protocols for HF exposure incidents, identifying gaps in existing safety standards across different regulatory environments, and exploring the potential for developing standardized international safety protocols that could be implemented regardless of regional regulatory variations.
The technological trajectory suggests increasing integration of digital monitoring systems, advanced personal protective equipment materials, and automated handling systems to minimize direct human contact with HF. Additionally, there is growing interest in developing more effective topical treatments and systemic therapies for HF exposure that could significantly improve outcomes following accidental exposure incidents.
This research acknowledges the tension between HF's continued utility in numerous scientific fields and its exceptional hazard profile, seeking to define a path forward that neither unnecessarily restricts valuable research nor compromises on fundamental safety requirements for researchers and laboratory personnel.
Research Applications and Demand Analysis
Hydrofluoric acid (HF) has established itself as an indispensable reagent across multiple scientific and industrial domains, with demand continuing to grow despite its hazardous properties. In semiconductor manufacturing, HF plays a critical role in silicon wafer cleaning, etching, and surface preparation processes. The global semiconductor industry, valued at approximately $600 billion, relies heavily on HF for producing high-performance microchips, with the demand for ultra-pure HF grades increasing as chip architectures become more sophisticated.
In materials science research, HF serves as a primary reagent for glass etching, mineral dissolution studies, and metal surface treatments. Academic and industrial laboratories conducting research in materials characterization consistently require HF for sample preparation and analysis. The growing focus on advanced materials development for renewable energy technologies has further expanded this application area.
Analytical chemistry represents another significant demand sector, where HF is essential for sample digestion in elemental analysis, particularly for silicate-based materials and certain metal alloys. Environmental monitoring laboratories utilize HF in their analytical protocols for soil, sediment, and geological sample preparation, driving steady consumption in this sector.
The petrochemical industry employs HF as a catalyst in alkylation processes for producing high-octane gasoline components. While this represents a bulk industrial application rather than research use, it influences the overall market dynamics and availability of HF for scientific purposes.
Market analysis indicates that research-grade HF consumption is growing at approximately 4.5% annually, driven primarily by expansion in semiconductor research, nanotechnology, and materials science. This growth has prompted increased attention to safety protocols and handling technologies, creating a parallel market for specialized HF safety equipment and neutralization systems.
The pharmaceutical and life sciences sectors show emerging applications for HF in specialized synthesis pathways and analytical procedures. While these applications currently represent a smaller market segment, they are expected to expand as new methodologies are developed.
Regional demand patterns reveal that East Asia, particularly Taiwan, South Korea, and China, leads in HF consumption due to concentrated semiconductor manufacturing activities. North America and Europe maintain significant research-based consumption, primarily in academic institutions and R&D facilities.
The critical nature of HF in these applications, coupled with its inherent hazards, has created a distinct market dynamic where users are increasingly willing to invest in advanced safety technologies and training. This has led to the development of specialized HF handling systems, including automated dispensing equipment, enhanced personal protective equipment, and sophisticated neutralization technologies, all designed to minimize exposure risks while maintaining research capabilities.
In materials science research, HF serves as a primary reagent for glass etching, mineral dissolution studies, and metal surface treatments. Academic and industrial laboratories conducting research in materials characterization consistently require HF for sample preparation and analysis. The growing focus on advanced materials development for renewable energy technologies has further expanded this application area.
Analytical chemistry represents another significant demand sector, where HF is essential for sample digestion in elemental analysis, particularly for silicate-based materials and certain metal alloys. Environmental monitoring laboratories utilize HF in their analytical protocols for soil, sediment, and geological sample preparation, driving steady consumption in this sector.
The petrochemical industry employs HF as a catalyst in alkylation processes for producing high-octane gasoline components. While this represents a bulk industrial application rather than research use, it influences the overall market dynamics and availability of HF for scientific purposes.
Market analysis indicates that research-grade HF consumption is growing at approximately 4.5% annually, driven primarily by expansion in semiconductor research, nanotechnology, and materials science. This growth has prompted increased attention to safety protocols and handling technologies, creating a parallel market for specialized HF safety equipment and neutralization systems.
The pharmaceutical and life sciences sectors show emerging applications for HF in specialized synthesis pathways and analytical procedures. While these applications currently represent a smaller market segment, they are expected to expand as new methodologies are developed.
Regional demand patterns reveal that East Asia, particularly Taiwan, South Korea, and China, leads in HF consumption due to concentrated semiconductor manufacturing activities. North America and Europe maintain significant research-based consumption, primarily in academic institutions and R&D facilities.
The critical nature of HF in these applications, coupled with its inherent hazards, has created a distinct market dynamic where users are increasingly willing to invest in advanced safety technologies and training. This has led to the development of specialized HF handling systems, including automated dispensing equipment, enhanced personal protective equipment, and sophisticated neutralization technologies, all designed to minimize exposure risks while maintaining research capabilities.
Current Safety Protocols and Technical Challenges
The current safety protocols for hydrofluoric acid (HF) in scientific research settings are comprehensive but face significant implementation challenges. Standard protocols mandate specialized personal protective equipment (PPE) including chemical-resistant gloves (typically neoprene or butyl rubber), face shields, acid-resistant lab coats, and closed-toe shoes. Many institutions have implemented a "buddy system" requirement, prohibiting solo work with HF regardless of concentration.
Engineering controls represent another critical safety layer, with properly functioning fume hoods being mandatory for all HF operations. These must maintain face velocities of 80-120 feet per minute and undergo regular certification. Some advanced facilities have implemented specialized HF handling areas with dedicated ventilation systems and acid-resistant work surfaces.
Emergency response protocols are particularly stringent for HF compared to other laboratory acids. All laboratories using HF must maintain calcium gluconate gel (2.5%) for immediate application to exposure sites, and clear evacuation procedures must be established. Many institutions require specialized HF spill kits containing calcium-based neutralizing agents rather than standard acid spill materials.
Despite these protocols, significant technical challenges persist. The detection of HF exposure presents a major difficulty as initial contact may not produce immediate pain or visible tissue damage, potentially delaying critical treatment. Current detection methods for workplace monitoring lack the sensitivity and response time needed for optimal safety.
The varying concentration requirements across different research applications complicate standardization efforts. While some applications require only dilute solutions (below 5%), others demand concentrated HF (up to 70%), necessitating different handling protocols that may create confusion in shared laboratory environments.
Material compatibility issues present ongoing challenges, as HF attacks glass, many metals, and certain plastics. Developing storage and handling equipment that balances chemical resistance with functionality and transparency remains problematic. The current polyethylene containers are resistant but opaque, making visual monitoring of contents difficult.
Training effectiveness represents another significant challenge. Despite mandatory training programs, research indicates that retention of emergency procedures remains suboptimal, particularly for researchers who use HF infrequently. Virtual reality and simulation-based training shows promise but has not been widely implemented.
Cross-disciplinary communication gaps further complicate safety efforts, as HF is used across diverse fields including chemistry, materials science, geology, and semiconductor research, each with different usage patterns and safety cultures. Establishing consistent protocols across these varied research environments remains an ongoing challenge.
Engineering controls represent another critical safety layer, with properly functioning fume hoods being mandatory for all HF operations. These must maintain face velocities of 80-120 feet per minute and undergo regular certification. Some advanced facilities have implemented specialized HF handling areas with dedicated ventilation systems and acid-resistant work surfaces.
Emergency response protocols are particularly stringent for HF compared to other laboratory acids. All laboratories using HF must maintain calcium gluconate gel (2.5%) for immediate application to exposure sites, and clear evacuation procedures must be established. Many institutions require specialized HF spill kits containing calcium-based neutralizing agents rather than standard acid spill materials.
Despite these protocols, significant technical challenges persist. The detection of HF exposure presents a major difficulty as initial contact may not produce immediate pain or visible tissue damage, potentially delaying critical treatment. Current detection methods for workplace monitoring lack the sensitivity and response time needed for optimal safety.
The varying concentration requirements across different research applications complicate standardization efforts. While some applications require only dilute solutions (below 5%), others demand concentrated HF (up to 70%), necessitating different handling protocols that may create confusion in shared laboratory environments.
Material compatibility issues present ongoing challenges, as HF attacks glass, many metals, and certain plastics. Developing storage and handling equipment that balances chemical resistance with functionality and transparency remains problematic. The current polyethylene containers are resistant but opaque, making visual monitoring of contents difficult.
Training effectiveness represents another significant challenge. Despite mandatory training programs, research indicates that retention of emergency procedures remains suboptimal, particularly for researchers who use HF infrequently. Virtual reality and simulation-based training shows promise but has not been widely implemented.
Cross-disciplinary communication gaps further complicate safety efforts, as HF is used across diverse fields including chemistry, materials science, geology, and semiconductor research, each with different usage patterns and safety cultures. Establishing consistent protocols across these varied research environments remains an ongoing challenge.
Contemporary HF Safety Management Solutions
01 Personal protective equipment and handling procedures
Hydrofluoric acid requires specialized personal protective equipment including acid-resistant gloves, face shields, and chemical-resistant clothing. Proper handling procedures involve working in well-ventilated areas, using appropriate containment systems, and following strict protocols for transfer and storage. These safety measures are critical due to HF's ability to penetrate skin and cause severe tissue damage without immediate pain sensation.- Personal protective equipment and handling procedures: Proper personal protective equipment (PPE) is essential when handling hydrofluoric acid, including chemical-resistant gloves, face shields, and acid-resistant clothing. Specific handling procedures must be followed, such as working in well-ventilated areas, using appropriate containment vessels, and implementing strict protocols for transferring and storing the acid to minimize exposure risks and prevent accidents.
- Emergency response and first aid measures: Immediate and appropriate emergency response is critical for hydrofluoric acid exposure. This includes specific first aid protocols such as calcium gluconate application for skin exposure, specialized eye washing procedures for ocular exposure, and emergency medical treatment guidelines. Rapid response systems and training for personnel working with hydrofluoric acid are essential to mitigate the severe health effects of exposure.
- Neutralization and disposal methods: Safe neutralization and disposal of hydrofluoric acid requires specialized techniques and materials. Methods include controlled neutralization with appropriate bases, precipitation of fluoride ions, and treatment with calcium compounds. Proper disposal procedures must comply with environmental regulations and include containment, treatment, and documentation to prevent environmental contamination and ensure worker safety.
- Facility design and engineering controls: Facilities handling hydrofluoric acid require specialized design features including corrosion-resistant materials, dedicated ventilation systems, containment structures, and safety showers. Engineering controls such as closed systems, automated handling equipment, leak detection systems, and specialized storage facilities are essential to minimize exposure risks and contain potential spills or releases.
- Safety monitoring and training programs: Comprehensive safety monitoring and training programs are vital for facilities using hydrofluoric acid. These include regular air quality monitoring, exposure assessment, medical surveillance of workers, and detailed training on hazard recognition, proper handling procedures, and emergency response. Documentation systems, regular safety audits, and continuous education ensure ongoing safety compliance and preparedness.
02 Emergency response and first aid protocols
Specific emergency response protocols for hydrofluoric acid exposure include immediate application of calcium gluconate gel for skin contact, specialized eye washing procedures for eye exposure, and respiratory support for inhalation incidents. First aid stations in facilities using HF must be equipped with calcium-containing neutralizing agents, as traditional bases are insufficient for neutralizing HF burns. Rapid medical intervention is essential due to HF's ability to cause progressive tissue damage and potential systemic toxicity.Expand Specific Solutions03 Containment systems and facility design
Facilities handling hydrofluoric acid require specialized containment systems including acid-resistant storage tanks, secondary containment structures, and dedicated ventilation systems with scrubbers. Facility design considerations include segregated storage areas, non-reactive flooring materials, emergency shower and eyewash stations, and specialized waste neutralization systems. These engineering controls help prevent accidental releases and minimize exposure risks.Expand Specific Solutions04 Neutralization and waste treatment methods
Safe disposal of hydrofluoric acid requires specialized neutralization processes using calcium or magnesium compounds rather than conventional bases. Waste treatment methods include precipitation of fluoride ions as insoluble salts, ion exchange techniques, and controlled dilution protocols. Advanced treatment systems incorporate monitoring to verify complete neutralization before discharge, as residual HF can cause environmental damage and pose ongoing safety risks.Expand Specific Solutions05 Detection systems and monitoring protocols
Safety management for hydrofluoric acid facilities includes continuous air monitoring systems capable of detecting HF at low concentrations, leak detection technologies for storage and transfer systems, and regular inspection protocols. Advanced monitoring includes automated alarm systems that trigger emergency ventilation and containment measures when HF is detected. Personal monitoring devices are also employed for workers in high-risk areas to provide early warning of exposure.Expand Specific Solutions
Leading Institutions and Safety Equipment Manufacturers
The hydrofluoric acid safety market is currently in a growth phase, with increasing demand driven by expanding semiconductor, electronics, and chemical research sectors. The competitive landscape features established chemical manufacturers like Do-Fluoride New Materials and Stella Chemifa Corp specializing in high-purity fluoride compounds, alongside industrial safety solution providers such as Honeywell International Technologies. Major chemical conglomerates including BASF, Arkema, and Mitsubishi Gas Chemical offer comprehensive safety protocols and specialized containment systems. The technology maturity varies significantly, with companies like Daikin Industries and Soitec leading in advanced handling techniques for semiconductor applications, while research institutions like Chinese Academy of Science and Shandong University continue developing next-generation safety protocols and neutralization technologies for this highly hazardous but scientifically valuable acid.
Do-Fluoride New Materials Co., Ltd.
Technical Solution: Do-Fluoride has developed a comprehensive safety management system for hydrofluoric acid handling that integrates multiple layers of protection. Their approach includes specialized containment vessels with polytetrafluoroethylene (PTFE) linings resistant to HF corrosion, automated dispensing systems that minimize direct human contact, and real-time monitoring technology that detects HF vapor at concentrations as low as 0.5 ppm. The company has pioneered a neutralization protocol using calcium-based compounds that rapidly convert HF to non-hazardous calcium fluoride. Their laboratory safety stations feature specialized ventilation systems with scrubbers that can remove 99.9% of HF vapors before air is exhausted. Additionally, they've developed specialized training programs using virtual reality simulations to prepare researchers for emergency scenarios without exposure risk.
Strengths: Comprehensive end-to-end safety solutions specifically designed for fluoride chemistry; extensive experience in fluoride material handling; proprietary neutralization technologies. Weaknesses: Solutions may be costly for smaller research facilities; some technologies are optimized for industrial rather than laboratory scale; requires significant training investment.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed the HF Sentinel Safety System, an integrated approach to hydrofluoric acid safety in research environments. The system combines specialized HF-resistant PPE with their patented rapid-response calcium gluconate delivery mechanisms that can be deployed within seconds of exposure. Their engineering controls include negative pressure workstations with specialized filtration systems capable of capturing 99.8% of HF vapors. Honeywell's automated dispensing technology eliminates direct handling of concentrated HF through precision pumps accurate to within 0.01mL, significantly reducing spill risks. The company's safety protocols are supported by their HF Guardian software, which provides real-time monitoring of workspace HF levels, tracks inventory, documents usage patterns, and automatically alerts emergency response teams when anomalies are detected. Their system also incorporates specialized waste neutralization protocols using proprietary buffering agents that convert HF waste to safer compounds before disposal.
Strengths: Extensive industrial safety expertise; integrated hardware and software solutions; global support network for implementation and training. Weaknesses: Higher initial implementation costs; system components may require proprietary consumables; some features may be excessive for smaller research applications.
Critical Safety Technologies and Protocols Review
Method for producing diluted hydrofluoric acid
PatentActiveUS20180148332A1
Innovation
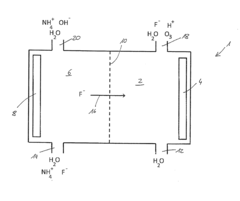
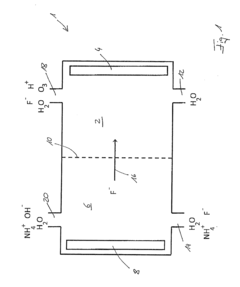
- A method using an electrode arrangement with an anode and cathode chambers separated by an anion exchange membrane, where pure water and an electrolyte solution containing fluoride ions are electrolyzed to produce dilute hydrofluoric acid with precise concentration control, and ozone can be simultaneously produced, with the ability to adjust concentrations independently using electrical current and electrolyte concentration.
Method for the detection of fluoride or hydrogen fluoride and detection kit
PatentInactiveUS20050227368A1
Innovation
- A method involving a silylated organic compound that undergoes desilylation in the presence of HF, allowing for separate detection of the silylated and desilylated forms, using reagents like BTSFA and MTBSTFA, and employing immunological tests for enhanced sensitivity and specificity.
Regulatory Compliance and International Standards
The regulatory landscape for hydrofluoric acid (HF) usage in scientific research is complex and varies significantly across different jurisdictions. In the United States, the Occupational Safety and Health Administration (OSHA) has established specific standards under 29 CFR 1910.1200 for hazard communication and 29 CFR 1910.132 for personal protective equipment requirements when handling HF. Additionally, the Environmental Protection Agency (EPA) regulates HF under the Toxic Substances Control Act (TSCA) and the Resource Conservation and Recovery Act (RCRA) for waste management.
The European Union implements stricter controls through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation and the CLP (Classification, Labeling and Packaging) regulation. These frameworks mandate comprehensive safety data sheets, appropriate labeling, and specific risk management measures for HF handling. The EU's approach emphasizes the precautionary principle, requiring substitution with less hazardous alternatives whenever technically feasible.
In Asia, Japan's Industrial Safety and Health Law and China's Regulations on Safe Management of Hazardous Chemicals provide comparable frameworks, though enforcement mechanisms vary considerably. Australia's model Work Health and Safety (WHS) regulations classify HF as a Schedule 10 hazardous chemical, requiring specific risk assessment protocols.
International standards such as ISO 45001 for occupational health and safety management systems provide globally recognized frameworks for HF handling. The American National Standards Institute (ANSI) and the American Society for Testing and Materials (ASTM) have developed specific protocols for HF handling in laboratory settings, including ANSI/AIHA Z9.5 for laboratory ventilation and ASTM E1132 for HF first aid procedures.
Compliance with these regulations requires comprehensive documentation, including detailed standard operating procedures (SOPs), regular risk assessments, and emergency response plans. Most jurisdictions mandate regular training for personnel, with certification requirements varying by region. Documentation must typically be maintained for 30 years in most European countries and 5-10 years in the United States, depending on the specific application.
Recent regulatory trends indicate movement toward harmonization of global standards through the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). This system aims to standardize hazard communication elements worldwide, though implementation timelines and specific requirements continue to vary by country. Research institutions must remain vigilant regarding regulatory updates, as non-compliance can result in significant penalties, including fines exceeding $100,000 per violation in some jurisdictions and potential criminal liability for willful violations resulting in harm.
The European Union implements stricter controls through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation and the CLP (Classification, Labeling and Packaging) regulation. These frameworks mandate comprehensive safety data sheets, appropriate labeling, and specific risk management measures for HF handling. The EU's approach emphasizes the precautionary principle, requiring substitution with less hazardous alternatives whenever technically feasible.
In Asia, Japan's Industrial Safety and Health Law and China's Regulations on Safe Management of Hazardous Chemicals provide comparable frameworks, though enforcement mechanisms vary considerably. Australia's model Work Health and Safety (WHS) regulations classify HF as a Schedule 10 hazardous chemical, requiring specific risk assessment protocols.
International standards such as ISO 45001 for occupational health and safety management systems provide globally recognized frameworks for HF handling. The American National Standards Institute (ANSI) and the American Society for Testing and Materials (ASTM) have developed specific protocols for HF handling in laboratory settings, including ANSI/AIHA Z9.5 for laboratory ventilation and ASTM E1132 for HF first aid procedures.
Compliance with these regulations requires comprehensive documentation, including detailed standard operating procedures (SOPs), regular risk assessments, and emergency response plans. Most jurisdictions mandate regular training for personnel, with certification requirements varying by region. Documentation must typically be maintained for 30 years in most European countries and 5-10 years in the United States, depending on the specific application.
Recent regulatory trends indicate movement toward harmonization of global standards through the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). This system aims to standardize hazard communication elements worldwide, though implementation timelines and specific requirements continue to vary by country. Research institutions must remain vigilant regarding regulatory updates, as non-compliance can result in significant penalties, including fines exceeding $100,000 per violation in some jurisdictions and potential criminal liability for willful violations resulting in harm.
Emergency Response and Incident Management Systems
Effective emergency response and incident management systems are critical components of any laboratory safety protocol when working with hydrofluoric acid (HF). Given the severe health risks associated with HF exposure, including potentially fatal systemic toxicity and painful burns that may not be immediately apparent, organizations must establish comprehensive emergency protocols that can be activated immediately when incidents occur.
A well-designed emergency response system for HF incidents should include clearly defined roles and responsibilities for all laboratory personnel. This includes designating specific individuals as emergency coordinators who are thoroughly trained in HF first aid procedures and can direct response efforts until medical professionals arrive. These coordinators should be available during all operational hours when HF is in use, ensuring continuous emergency coverage.
First aid supplies specifically designed for HF exposure must be readily accessible in multiple locations throughout facilities where HF is handled. These supplies should include calcium gluconate gel (2.5%), which is the primary treatment for dermal HF exposure, calcium gluconate solution for eye irrigation, and injectable calcium gluconate for severe cases when administered by medical professionals. Regular inventory checks of these supplies should be conducted to ensure they remain within expiration dates.
Communication systems form another crucial element of effective incident management. Laboratories should implement redundant notification systems including emergency phones with direct lines to security or emergency services, alarm buttons, and potentially mobile alert applications that can simultaneously notify all relevant personnel of an HF incident. These systems should be tested regularly to verify functionality.
Documentation and reporting procedures must be established to record all incidents involving HF, regardless of severity. This documentation serves multiple purposes: identifying patterns that might indicate systemic safety issues, providing information for improving safety protocols, and meeting regulatory compliance requirements. Post-incident analysis should be conducted after any HF-related emergency to identify root causes and implement corrective measures.
Regular drills simulating various HF exposure scenarios should be conducted at least quarterly. These drills help familiarize staff with emergency procedures, identify potential weaknesses in the response system, and reduce panic during actual emergencies. Scenarios should include different types of exposure (skin contact, inhalation, ingestion) and varying severity levels to ensure comprehensive preparedness.
Coordination with local emergency services is essential, as many first responders may have limited experience with HF-specific hazards. Facilities should provide information about their HF usage to local hospitals and emergency departments, including typical quantities stored, specific locations, and recommended treatment protocols. This proactive approach ensures that external emergency responders are prepared to provide appropriate care for HF exposure victims.
A well-designed emergency response system for HF incidents should include clearly defined roles and responsibilities for all laboratory personnel. This includes designating specific individuals as emergency coordinators who are thoroughly trained in HF first aid procedures and can direct response efforts until medical professionals arrive. These coordinators should be available during all operational hours when HF is in use, ensuring continuous emergency coverage.
First aid supplies specifically designed for HF exposure must be readily accessible in multiple locations throughout facilities where HF is handled. These supplies should include calcium gluconate gel (2.5%), which is the primary treatment for dermal HF exposure, calcium gluconate solution for eye irrigation, and injectable calcium gluconate for severe cases when administered by medical professionals. Regular inventory checks of these supplies should be conducted to ensure they remain within expiration dates.
Communication systems form another crucial element of effective incident management. Laboratories should implement redundant notification systems including emergency phones with direct lines to security or emergency services, alarm buttons, and potentially mobile alert applications that can simultaneously notify all relevant personnel of an HF incident. These systems should be tested regularly to verify functionality.
Documentation and reporting procedures must be established to record all incidents involving HF, regardless of severity. This documentation serves multiple purposes: identifying patterns that might indicate systemic safety issues, providing information for improving safety protocols, and meeting regulatory compliance requirements. Post-incident analysis should be conducted after any HF-related emergency to identify root causes and implement corrective measures.
Regular drills simulating various HF exposure scenarios should be conducted at least quarterly. These drills help familiarize staff with emergency procedures, identify potential weaknesses in the response system, and reduce panic during actual emergencies. Scenarios should include different types of exposure (skin contact, inhalation, ingestion) and varying severity levels to ensure comprehensive preparedness.
Coordination with local emergency services is essential, as many first responders may have limited experience with HF-specific hazards. Facilities should provide information about their HF usage to local hospitals and emergency departments, including typical quantities stored, specific locations, and recommended treatment protocols. This proactive approach ensures that external emergency responders are prepared to provide appropriate care for HF exposure victims.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!