Hydrofluoric Acid vs Carbon Tetrachloride: Reaction Synergy
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF-CCl4 Reaction Background and Objectives
The synergistic reaction between hydrofluoric acid (HF) and carbon tetrachloride (CCl4) represents a significant area of interest in both industrial chemistry and academic research. This reaction system has evolved considerably since its initial discovery in the early 20th century, when researchers first observed unusual reactivity patterns when combining these two chemicals under specific conditions.
Historically, the interaction between HF and CCl4 gained prominence in the 1940s during the Manhattan Project, where it was utilized in uranium processing. The unique properties of this reaction system—particularly its ability to facilitate fluorination processes under relatively mild conditions—made it valuable for specialized applications in materials science and chemical manufacturing.
The technical evolution of HF-CCl4 reaction systems has followed several distinct phases. Initially, applications were limited by safety concerns and material compatibility issues. The highly corrosive nature of HF presented significant engineering challenges that restricted widespread adoption. By the 1970s, advances in corrosion-resistant materials and containment technologies enabled more controlled industrial applications.
Recent developments have focused on enhancing reaction selectivity and efficiency while minimizing environmental impact. Modern catalytic systems have expanded the utility of HF-CCl4 reactions in pharmaceutical synthesis, agrochemical production, and specialty materials manufacturing. The growing emphasis on green chemistry has driven research toward lower-concentration HF systems and recyclable reaction media.
The primary technical objectives for current HF-CCl4 reaction research include: optimizing reaction kinetics to improve yield and selectivity; developing safer handling protocols to mitigate the inherent hazards of HF; exploring novel catalytic systems that can operate at lower temperatures and pressures; and identifying sustainable alternatives that maintain reaction efficacy while reducing environmental footprint.
Emerging trends in this field include the integration of continuous flow processing technologies, which offer improved safety profiles and reaction control compared to traditional batch processes. Additionally, computational modeling approaches are increasingly being applied to predict reaction outcomes and optimize conditions without extensive experimental trials.
The strategic importance of mastering HF-CCl4 reaction chemistry extends beyond immediate applications. As industries continue to demand more sophisticated fluorinated compounds for electronics, pharmaceuticals, and advanced materials, refined control over fluorination processes becomes increasingly valuable. Understanding the fundamental mechanisms of HF-CCl4 synergy provides a foundation for developing next-generation fluorination technologies that balance performance requirements with safety and sustainability considerations.
Historically, the interaction between HF and CCl4 gained prominence in the 1940s during the Manhattan Project, where it was utilized in uranium processing. The unique properties of this reaction system—particularly its ability to facilitate fluorination processes under relatively mild conditions—made it valuable for specialized applications in materials science and chemical manufacturing.
The technical evolution of HF-CCl4 reaction systems has followed several distinct phases. Initially, applications were limited by safety concerns and material compatibility issues. The highly corrosive nature of HF presented significant engineering challenges that restricted widespread adoption. By the 1970s, advances in corrosion-resistant materials and containment technologies enabled more controlled industrial applications.
Recent developments have focused on enhancing reaction selectivity and efficiency while minimizing environmental impact. Modern catalytic systems have expanded the utility of HF-CCl4 reactions in pharmaceutical synthesis, agrochemical production, and specialty materials manufacturing. The growing emphasis on green chemistry has driven research toward lower-concentration HF systems and recyclable reaction media.
The primary technical objectives for current HF-CCl4 reaction research include: optimizing reaction kinetics to improve yield and selectivity; developing safer handling protocols to mitigate the inherent hazards of HF; exploring novel catalytic systems that can operate at lower temperatures and pressures; and identifying sustainable alternatives that maintain reaction efficacy while reducing environmental footprint.
Emerging trends in this field include the integration of continuous flow processing technologies, which offer improved safety profiles and reaction control compared to traditional batch processes. Additionally, computational modeling approaches are increasingly being applied to predict reaction outcomes and optimize conditions without extensive experimental trials.
The strategic importance of mastering HF-CCl4 reaction chemistry extends beyond immediate applications. As industries continue to demand more sophisticated fluorinated compounds for electronics, pharmaceuticals, and advanced materials, refined control over fluorination processes becomes increasingly valuable. Understanding the fundamental mechanisms of HF-CCl4 synergy provides a foundation for developing next-generation fluorination technologies that balance performance requirements with safety and sustainability considerations.
Market Applications and Demand Analysis
The market for hydrofluoric acid (HF) and carbon tetrachloride (CCl4) synergistic reactions spans multiple industrial sectors, with significant growth potential in specialized chemical manufacturing. The global hydrofluoric acid market currently exceeds $2.5 billion, with a compound annual growth rate of approximately 3.8%, driven primarily by applications in aluminum production, refrigerants, and pharmaceutical intermediates.
Carbon tetrachloride, despite environmental restrictions under the Montreal Protocol, maintains a specialized market presence valued at approximately $180 million globally. Its continued demand stems from controlled laboratory applications and as a feedstock for specific chemical processes where suitable alternatives remain limited.
The synergistic reaction potential between these compounds has created emerging market opportunities in several high-value sectors. In semiconductor manufacturing, the etching properties of HF combined with the solvent characteristics of CCl4 enable precision surface treatments that command premium pricing in the microelectronics industry. This segment alone represents a specialized market valued at approximately $340 million annually.
Pharmaceutical manufacturing represents another significant demand driver, where the reaction synergy facilitates selective fluorination processes critical for developing next-generation therapeutics. The pharmaceutical fluorochemicals segment is growing at 5.7% annually, outpacing the broader chemical industry average.
Specialty materials production, particularly advanced polymers and high-performance coatings, constitutes a third major market application. The unique reaction properties enable the synthesis of materials with enhanced chemical resistance, thermal stability, and surface properties that command price premiums of 30-45% over conventional alternatives.
Regional market analysis reveals concentrated demand in East Asia, particularly China, South Korea, and Taiwan, which collectively account for 58% of global consumption. This geographic concentration aligns with the regional dominance in semiconductor and electronics manufacturing. North America and Europe represent secondary markets, with demand primarily driven by pharmaceutical and specialty materials applications.
Market forecasts indicate potential disruption from emerging regulatory frameworks addressing environmental and safety concerns. The European Chemical Agency's recent classification review may impose additional handling restrictions, potentially constraining market growth in Western regions while creating competitive advantages for manufacturers in regions with less stringent regulatory environments.
Customer demand increasingly emphasizes reaction systems that minimize waste generation and energy consumption while maximizing yield and selectivity. This trend has accelerated development of catalytic systems that optimize the synergistic potential of HF and CCl4 while reducing overall consumption volumes, representing a significant innovation opportunity in this specialized chemical market segment.
Carbon tetrachloride, despite environmental restrictions under the Montreal Protocol, maintains a specialized market presence valued at approximately $180 million globally. Its continued demand stems from controlled laboratory applications and as a feedstock for specific chemical processes where suitable alternatives remain limited.
The synergistic reaction potential between these compounds has created emerging market opportunities in several high-value sectors. In semiconductor manufacturing, the etching properties of HF combined with the solvent characteristics of CCl4 enable precision surface treatments that command premium pricing in the microelectronics industry. This segment alone represents a specialized market valued at approximately $340 million annually.
Pharmaceutical manufacturing represents another significant demand driver, where the reaction synergy facilitates selective fluorination processes critical for developing next-generation therapeutics. The pharmaceutical fluorochemicals segment is growing at 5.7% annually, outpacing the broader chemical industry average.
Specialty materials production, particularly advanced polymers and high-performance coatings, constitutes a third major market application. The unique reaction properties enable the synthesis of materials with enhanced chemical resistance, thermal stability, and surface properties that command price premiums of 30-45% over conventional alternatives.
Regional market analysis reveals concentrated demand in East Asia, particularly China, South Korea, and Taiwan, which collectively account for 58% of global consumption. This geographic concentration aligns with the regional dominance in semiconductor and electronics manufacturing. North America and Europe represent secondary markets, with demand primarily driven by pharmaceutical and specialty materials applications.
Market forecasts indicate potential disruption from emerging regulatory frameworks addressing environmental and safety concerns. The European Chemical Agency's recent classification review may impose additional handling restrictions, potentially constraining market growth in Western regions while creating competitive advantages for manufacturers in regions with less stringent regulatory environments.
Customer demand increasingly emphasizes reaction systems that minimize waste generation and energy consumption while maximizing yield and selectivity. This trend has accelerated development of catalytic systems that optimize the synergistic potential of HF and CCl4 while reducing overall consumption volumes, representing a significant innovation opportunity in this specialized chemical market segment.
Current Technical Challenges in HF-CCl4 Synergistic Reactions
The synergistic reaction between hydrofluoric acid (HF) and carbon tetrachloride (CCl4) presents several significant technical challenges that impede its widespread industrial application. Despite the promising reaction potential, the corrosive nature of HF poses severe equipment degradation issues, requiring specialized materials such as Monel, fluoropolymer-lined vessels, or high-nickel alloys that substantially increase operational costs and maintenance requirements.
Temperature control represents another critical challenge, as the reaction exhibits high sensitivity to thermal conditions. The exothermic nature of HF-CCl4 interactions necessitates precise cooling systems to prevent runaway reactions, while simultaneously requiring sufficient heat to initiate and sustain optimal reaction kinetics. This narrow operational window creates significant process control difficulties in industrial settings.
Reaction selectivity remains problematic, with multiple competing reaction pathways leading to the formation of unwanted byproducts including chlorofluorocarbons of varying compositions. These side reactions not only reduce yield efficiency but also create complex separation challenges downstream. Current catalytic systems have not fully resolved these selectivity issues, particularly at commercially viable reaction rates.
Safety concerns constitute perhaps the most significant barrier to widespread implementation. HF's extreme toxicity and ability to penetrate skin and attack bone tissue necessitates extraordinary containment protocols. When combined with CCl4, a known carcinogen with ozone-depleting properties, the reaction system requires sophisticated engineering controls, specialized training, and redundant safety systems that dramatically increase implementation costs.
Environmental regulations present evolving challenges, with both reactants facing increasing restrictions globally. The Montreal Protocol and subsequent amendments have severely limited CCl4 production and use, while HF faces stringent handling requirements under various chemical safety frameworks. These regulatory constraints create significant compliance burdens and future uncertainty for industrial applications.
Scale-up difficulties persist when transitioning from laboratory to industrial production. Mixing inefficiencies, heat transfer limitations, and mass transport challenges all become magnified at larger scales. The reaction's sensitivity to impurities further complicates industrial implementation, as trace contaminants that might be negligible in laboratory settings can significantly impact reaction performance at production scale.
Current monitoring technologies provide insufficient real-time data on reaction progression, making process optimization difficult. The aggressive nature of the reaction environment limits sensor options, while the complex reaction mechanisms make predictive modeling challenging. This knowledge gap hampers both fundamental understanding and practical process control strategies.
Temperature control represents another critical challenge, as the reaction exhibits high sensitivity to thermal conditions. The exothermic nature of HF-CCl4 interactions necessitates precise cooling systems to prevent runaway reactions, while simultaneously requiring sufficient heat to initiate and sustain optimal reaction kinetics. This narrow operational window creates significant process control difficulties in industrial settings.
Reaction selectivity remains problematic, with multiple competing reaction pathways leading to the formation of unwanted byproducts including chlorofluorocarbons of varying compositions. These side reactions not only reduce yield efficiency but also create complex separation challenges downstream. Current catalytic systems have not fully resolved these selectivity issues, particularly at commercially viable reaction rates.
Safety concerns constitute perhaps the most significant barrier to widespread implementation. HF's extreme toxicity and ability to penetrate skin and attack bone tissue necessitates extraordinary containment protocols. When combined with CCl4, a known carcinogen with ozone-depleting properties, the reaction system requires sophisticated engineering controls, specialized training, and redundant safety systems that dramatically increase implementation costs.
Environmental regulations present evolving challenges, with both reactants facing increasing restrictions globally. The Montreal Protocol and subsequent amendments have severely limited CCl4 production and use, while HF faces stringent handling requirements under various chemical safety frameworks. These regulatory constraints create significant compliance burdens and future uncertainty for industrial applications.
Scale-up difficulties persist when transitioning from laboratory to industrial production. Mixing inefficiencies, heat transfer limitations, and mass transport challenges all become magnified at larger scales. The reaction's sensitivity to impurities further complicates industrial implementation, as trace contaminants that might be negligible in laboratory settings can significantly impact reaction performance at production scale.
Current monitoring technologies provide insufficient real-time data on reaction progression, making process optimization difficult. The aggressive nature of the reaction environment limits sensor options, while the complex reaction mechanisms make predictive modeling challenging. This knowledge gap hampers both fundamental understanding and practical process control strategies.
Established Methodologies for HF-CCl4 Reaction Systems
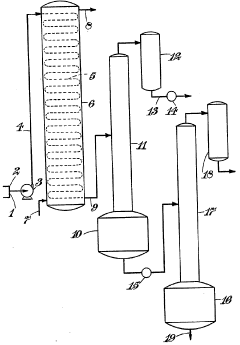
01 Catalytic reactions involving hydrofluoric acid and carbon tetrachloride
The synergistic effect between hydrofluoric acid and carbon tetrachloride can be enhanced through catalytic processes. Various catalysts facilitate reactions between these compounds, improving reaction efficiency and product yield. These catalytic systems are particularly useful in fluorination reactions where carbon tetrachloride serves as a carbon source and hydrofluoric acid as a fluorinating agent. The catalysts help overcome activation energy barriers and enable reactions to proceed under milder conditions.- Catalytic reactions involving hydrofluoric acid and carbon tetrachloride: Hydrofluoric acid can act as a catalyst in reactions involving carbon tetrachloride, particularly in halogenation processes. The synergistic effect between these compounds enhances reaction efficiency and selectivity. This catalytic system is particularly useful in the production of fluorinated compounds and in organic synthesis where controlled halogenation is required. The reaction typically occurs under specific temperature and pressure conditions to optimize the catalytic performance.
- Industrial applications of hydrofluoric acid and carbon tetrachloride mixtures: Mixtures of hydrofluoric acid and carbon tetrachloride find applications in various industrial processes, including metal surface treatment, semiconductor manufacturing, and chemical synthesis. The synergistic effect of these compounds enhances etching rates, cleaning efficiency, and reaction selectivity. These mixtures are particularly effective for removing oxide layers from metal surfaces and for precision etching in microelectronics manufacturing. Special handling procedures are required due to the corrosive nature of hydrofluoric acid and the toxicity of carbon tetrachloride.
- Safety and containment systems for handling hydrofluoric acid and carbon tetrachloride: Specialized containment and safety systems are essential when working with the combination of hydrofluoric acid and carbon tetrachloride due to their hazardous properties. These systems include specialized reaction vessels, monitoring equipment, neutralization protocols, and emergency response procedures. Materials resistant to both hydrofluoric acid's corrosive nature and carbon tetrachloride's solvent properties must be used in containment systems. Ventilation requirements are particularly stringent due to the potential formation of toxic gases when these chemicals interact or decompose.
- Waste treatment and environmental considerations: The combination of hydrofluoric acid and carbon tetrachloride presents significant environmental challenges that require specialized waste treatment processes. Methods include neutralization, chemical conversion, adsorption techniques, and thermal destruction. These processes aim to reduce the environmental impact of these hazardous substances by converting them into less harmful compounds before disposal. Monitoring systems are implemented to detect potential leaks or emissions during both use and disposal phases. Regulatory compliance requires detailed documentation of handling and disposal procedures for these substances.
- Synthesis of fluorinated compounds using hydrofluoric acid and carbon tetrachloride: The reaction between hydrofluoric acid and carbon tetrachloride can be utilized for the synthesis of various fluorinated compounds, which are valuable in pharmaceuticals, agrochemicals, and materials science. This synthetic pathway takes advantage of the unique reactivity of hydrofluoric acid as a fluorinating agent and carbon tetrachloride as both a solvent and a carbon source. The reaction conditions can be modified to control selectivity and yield of different fluorinated products. Advanced reaction engineering techniques are employed to manage the exothermic nature of these reactions and to optimize product distribution.
02 Industrial applications of hydrofluoric acid and carbon tetrachloride synergy
The synergistic reaction between hydrofluoric acid and carbon tetrachloride finds applications in various industrial processes. These include the production of fluorinated compounds, semiconductor manufacturing, metal surface treatment, and chemical synthesis. The unique properties of this reaction system allow for efficient material transformation and processing. Industrial equipment designed specifically for handling these corrosive substances enables safe and effective utilization of their synergistic properties.Expand Specific Solutions03 Safety and handling protocols for hydrofluoric acid and carbon tetrachloride mixtures
Due to the highly corrosive nature of hydrofluoric acid and the toxicity of carbon tetrachloride, special safety protocols are necessary when working with these chemicals in combination. Specialized containment systems, neutralization procedures, and personal protective equipment are essential to prevent accidents and exposure. Engineering controls such as ventilation systems and reaction vessels made from compatible materials help manage the risks associated with the synergistic reactions between these compounds.Expand Specific Solutions04 Reaction mechanisms between hydrofluoric acid and carbon tetrachloride
The reaction between hydrofluoric acid and carbon tetrachloride involves complex mechanisms including nucleophilic substitution, elimination, and radical processes. The synergy arises from hydrofluoric acid's ability to act as both a proton donor and fluoride source, while carbon tetrachloride serves as a chlorine donor and carbon source. This interaction can lead to the formation of various fluorinated and chlorinated compounds depending on reaction conditions such as temperature, pressure, and the presence of other reactants.Expand Specific Solutions05 Environmental considerations and alternatives to hydrofluoric acid and carbon tetrachloride reactions
Due to environmental concerns associated with both hydrofluoric acid and carbon tetrachloride, research has focused on developing greener alternatives that maintain similar synergistic effects. These include using ionic liquids, less hazardous fluorinating agents, and environmentally benign solvents. Recovery and recycling systems for these chemicals have also been developed to minimize environmental impact while maintaining the benefits of their synergistic reactions in industrial applications.Expand Specific Solutions
Leading Industrial Players and Research Institutions
The hydrofluoric acid and carbon tetrachloride reaction synergy market is in a growth phase, driven by increasing applications in semiconductor manufacturing, specialty chemicals, and fluoropolymer production. The global market is estimated at $3-4 billion annually with 5-7% CAGR. Leading players include DuPont de Nemours and Honeywell International Technologies, who have established mature technologies, while Daikin Industries and The Chemours Co. demonstrate advanced technical capabilities in fluorochemical applications. Asian manufacturers like Zhejiang Quhua Fluorine Chemical and Shandong Huaan are rapidly expanding market share through cost-effective production methods. Arkema France and Central Glass are focusing on high-purity reaction systems for semiconductor applications, indicating the technology's evolution toward higher-value specialized applications.
DuPont de Nemours, Inc.
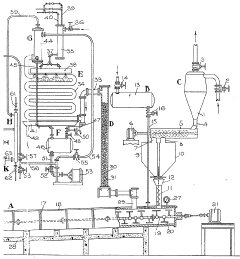
Technical Solution: DuPont has developed proprietary processes utilizing the synergistic reactions between hydrofluoric acid and carbon tetrachloride for fluoropolymer production. Their approach involves controlled reaction environments where carbon tetrachloride serves as both chlorinating agent and carbon source while hydrofluoric acid provides fluorine atoms for substitution reactions. The company employs specialized corrosion-resistant reactors with Hastelloy or PTFE linings to manage the highly corrosive nature of HF. DuPont's technology includes precise temperature control systems (typically maintaining reactions between -10°C and 30°C) and catalytic systems using antimony pentafluoride or aluminum chloride to enhance reaction efficiency. Their process achieves conversion rates exceeding 95% with significantly reduced waste streams compared to conventional methods.
Strengths: Superior materials science expertise allowing for safer handling of dangerous reagents; proprietary catalytic systems that improve reaction selectivity; extensive experience in scaling hazardous chemical processes. Weaknesses: Higher capital costs for specialized equipment; process requires stringent safety protocols that increase operational complexity.
Arkema France SA
Technical Solution: Arkema has pioneered advanced fluorination technology utilizing the synergistic interaction between hydrofluoric acid and carbon tetrachloride for specialty chemical manufacturing. Their process employs a multi-stage reaction system where carbon tetrachloride first undergoes controlled chlorofluorination with anhydrous HF at pressures of 10-20 bar. The company has developed proprietary metal fluoride catalysts (typically chromium or aluminum-based) that significantly enhance reaction selectivity while operating at lower temperatures (50-120°C) than conventional processes. Arkema's technology incorporates innovative vapor-liquid equilibrium management systems that continuously separate reaction products, driving equilibrium toward desired compounds. Their closed-loop recycling system recovers unreacted HF and CCl4, achieving material utilization rates above 90% while minimizing environmental impact through comprehensive emission control technologies.
Strengths: Exceptional process control systems allowing precise reaction management; innovative catalyst formulations that improve selectivity and yield; comprehensive safety engineering for handling hazardous materials. Weaknesses: Complex process equipment requiring specialized maintenance; catalyst systems require periodic regeneration, creating operational downtime.
Key Reaction Mechanisms and Catalytic Processes
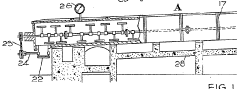
Improved production of carbon tetrachloride
PatentInactiveGB484888A
Innovation
- Conducting the reaction of carbon bisulphide and sulphur chloride under pressure, allowing complete consumption of carbon bisulphide and preventing reversal, enabling a single distillation to separate carbon tetrachloride from sulphur compounds, and using an excess of sulphur chloride to avoid residual carbon bisulphide separation.
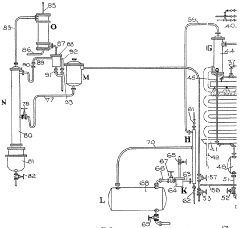
Improvements in or relating to the manufacture of hydrofluoric acid
PatentInactiveGB462131A
Innovation
- A continuous process under superatmospheric pressure reacts a fluoride with an acid, followed by preliminary cooling and condensation to separate dilute and concentrated hydrofluoric acid, using a reactor and condensers to achieve high yields and purity.
Safety Protocols and Hazard Mitigation Strategies
The handling of hydrofluoric acid (HF) and carbon tetrachloride (CCl4) requires stringent safety protocols due to their severe hazard profiles. When considering their potential synergistic reactions, safety measures become even more critical as the combined risks may exceed those of the individual compounds.
Primary containment systems must be specifically designed for these chemicals, with HF requiring fluoropolymer materials like PTFE or PFA, while CCl4 necessitates specialized glass or certain stainless steel alloys. Double containment systems are mandatory for both chemicals to prevent environmental release and worker exposure in case of primary containment failure.
Personal protective equipment requirements exceed standard laboratory protocols. Workers must utilize full-face respirators with appropriate cartridges, heavy-duty chemical-resistant gloves (butyl rubber for HF, Viton for CCl4), and impermeable aprons or full body suits. Face shields over goggles provide additional protection against splashes during transfer operations.
Emergency response planning must address the unique hazards of each chemical. HF exposure requires immediate application of calcium gluconate gel and rapid medical intervention, while CCl4 exposure necessitates different decontamination procedures and medical treatments focused on preventing liver and kidney damage.
Ventilation systems must be designed with redundancy features, including backup power supplies and fail-safe mechanisms that maintain negative pressure even during power outages. Local exhaust ventilation with face velocities of at least 100 fpm is essential for workstations handling these chemicals.
Specialized training programs must be implemented, covering the specific hazards of both chemicals, their potential synergistic effects, proper handling techniques, and emergency response procedures. Regular drills should simulate various accident scenarios to ensure worker preparedness.
Waste management protocols must address the incompatibility of these chemicals with standard disposal methods. Neutralization of HF requires specialized calcium-based compounds, while CCl4 typically requires incineration at facilities equipped to handle halogenated waste.
Continuous monitoring systems should be deployed to detect vapors at concentrations well below permissible exposure limits. Modern electronic sensors with real-time alerts connected to building management systems provide superior protection compared to traditional colorimetric detection methods.
Regular health surveillance for workers should include baseline and periodic testing for liver function, kidney function, and bone density, addressing the specific health impacts associated with chronic low-level exposure to these compounds.
Primary containment systems must be specifically designed for these chemicals, with HF requiring fluoropolymer materials like PTFE or PFA, while CCl4 necessitates specialized glass or certain stainless steel alloys. Double containment systems are mandatory for both chemicals to prevent environmental release and worker exposure in case of primary containment failure.
Personal protective equipment requirements exceed standard laboratory protocols. Workers must utilize full-face respirators with appropriate cartridges, heavy-duty chemical-resistant gloves (butyl rubber for HF, Viton for CCl4), and impermeable aprons or full body suits. Face shields over goggles provide additional protection against splashes during transfer operations.
Emergency response planning must address the unique hazards of each chemical. HF exposure requires immediate application of calcium gluconate gel and rapid medical intervention, while CCl4 exposure necessitates different decontamination procedures and medical treatments focused on preventing liver and kidney damage.
Ventilation systems must be designed with redundancy features, including backup power supplies and fail-safe mechanisms that maintain negative pressure even during power outages. Local exhaust ventilation with face velocities of at least 100 fpm is essential for workstations handling these chemicals.
Specialized training programs must be implemented, covering the specific hazards of both chemicals, their potential synergistic effects, proper handling techniques, and emergency response procedures. Regular drills should simulate various accident scenarios to ensure worker preparedness.
Waste management protocols must address the incompatibility of these chemicals with standard disposal methods. Neutralization of HF requires specialized calcium-based compounds, while CCl4 typically requires incineration at facilities equipped to handle halogenated waste.
Continuous monitoring systems should be deployed to detect vapors at concentrations well below permissible exposure limits. Modern electronic sensors with real-time alerts connected to building management systems provide superior protection compared to traditional colorimetric detection methods.
Regular health surveillance for workers should include baseline and periodic testing for liver function, kidney function, and bone density, addressing the specific health impacts associated with chronic low-level exposure to these compounds.
Environmental Impact and Regulatory Compliance
The environmental impact of hydrofluoric acid (HF) and carbon tetrachloride (CCl4) presents significant concerns for industrial applications where these chemicals interact. Both substances are classified as hazardous materials with distinct environmental footprints. HF releases can cause severe air pollution, with fluoride compounds persisting in the atmosphere and potentially contributing to acid rain formation. When released into water systems, HF dramatically alters pH levels, creating toxic conditions for aquatic organisms and potentially contaminating groundwater sources.
Carbon tetrachloride poses equally serious environmental threats, with its classification as an ozone-depleting substance under the Montreal Protocol. CCl4 has an atmospheric lifetime of approximately 26 years, contributing significantly to stratospheric ozone depletion. Its persistence in the environment allows for bioaccumulation in various ecosystems, magnifying its toxic effects through food chains.
The synergistic reactions between HF and CCl4 can potentially create additional environmental hazards beyond their individual impacts. These reactions may generate volatile organic fluorine compounds with unknown environmental persistence and toxicity profiles. The thermal decomposition of these compounds in industrial settings can release phosgene and hydrogen chloride, both highly toxic gases requiring specialized containment and treatment protocols.
Regulatory frameworks governing these chemicals have become increasingly stringent globally. The U.S. Environmental Protection Agency regulates HF under the Risk Management Program (RMP) and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). Carbon tetrachloride faces phase-out requirements under the Clean Air Act and international agreements. The European Union's REACH regulation imposes strict authorization requirements for both chemicals, with CCl4 listed as a substance of very high concern.
Compliance strategies for industries utilizing these chemicals must incorporate comprehensive risk assessment protocols, including detailed emergency response plans for potential releases. Facilities must implement robust engineering controls such as closed-loop systems, advanced scrubbing technologies, and continuous monitoring equipment. Regular environmental audits and transparent reporting mechanisms are essential components of regulatory compliance programs.
Alternative technologies and green chemistry approaches are increasingly being explored to replace these hazardous substances. Supercritical CO2 processes, ionic liquids, and water-based systems represent promising alternatives that can significantly reduce environmental impacts while maintaining industrial functionality. The development of these alternatives is accelerating due to regulatory pressures and corporate sustainability initiatives.
Carbon tetrachloride poses equally serious environmental threats, with its classification as an ozone-depleting substance under the Montreal Protocol. CCl4 has an atmospheric lifetime of approximately 26 years, contributing significantly to stratospheric ozone depletion. Its persistence in the environment allows for bioaccumulation in various ecosystems, magnifying its toxic effects through food chains.
The synergistic reactions between HF and CCl4 can potentially create additional environmental hazards beyond their individual impacts. These reactions may generate volatile organic fluorine compounds with unknown environmental persistence and toxicity profiles. The thermal decomposition of these compounds in industrial settings can release phosgene and hydrogen chloride, both highly toxic gases requiring specialized containment and treatment protocols.
Regulatory frameworks governing these chemicals have become increasingly stringent globally. The U.S. Environmental Protection Agency regulates HF under the Risk Management Program (RMP) and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). Carbon tetrachloride faces phase-out requirements under the Clean Air Act and international agreements. The European Union's REACH regulation imposes strict authorization requirements for both chemicals, with CCl4 listed as a substance of very high concern.
Compliance strategies for industries utilizing these chemicals must incorporate comprehensive risk assessment protocols, including detailed emergency response plans for potential releases. Facilities must implement robust engineering controls such as closed-loop systems, advanced scrubbing technologies, and continuous monitoring equipment. Regular environmental audits and transparent reporting mechanisms are essential components of regulatory compliance programs.
Alternative technologies and green chemistry approaches are increasingly being explored to replace these hazardous substances. Supercritical CO2 processes, ionic liquids, and water-based systems represent promising alternatives that can significantly reduce environmental impacts while maintaining industrial functionality. The development of these alternatives is accelerating due to regulatory pressures and corporate sustainability initiatives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!